
Review Article
J Endocr Disord. 2015; 2(1): 1018.
Hypoglycemia and PDX1 Targeted Therapy
Yu J¹, Liu SH¹, Sanchez R¹, Nemunaitis J² and Brunicardi FC¹*
¹Department of Surgery, University of California, USA
²Department of Surgery, Mary Crowley Cancer Research Center, USA
*Corresponding author: F Charles Brunicardi, Department of Surgery, University of California, Los Angeles, CA, USA
Received: November 07, 2015; Accepted: December 12, 2015; Published: December 15, 2015
Abstract
Hypoglycemia, which refers to dangerously low glucose level in blood, is a potential life-threatening condition. The causes of different types of hypoglycemia could vary. Multiple preventive and therapeutic managements for hypoglycemia are currently under investigations. Pancreatic and Duodenal homeobox 1 (PDX1), also known as insulin promoter factor 1, is one of the most important transcriptional factors for insulin and glucose regulation in pancreatic islet beta cells. Herein, this topic will review several aspects of hypoglycemia, including the causes of hypoglycemia, PDX1 function in insulin regulation, existing hypoglycemia animal models and the potentials of PDX1 targeted therapy in treating patients with hypoglycemia.
Keywords: Hypoglycemia; PDX1; Insulinoma; Diabetes
Abbreviations
DM: Diabetes Mellitus; PDAC: Pancreatic Ductal Adenocarcinoma; TK: Thymidine Kinase; GCV: Ganciclovir; RIP: Rat Insulin Promoter; Sstr: Somatostatin receptor
Introduction
Hypoglycemia, the technical term for low blood sugar (blood glucose), is a clinical syndrome defined by abnormally low blood glucose concentrations, usually less than 3.0 mmol/L (55 mg/dl) in adults, 3.9 mmol/L (70 mg/dl) in diabetic patients or 2.2 mmol/L (40 mg/dl) in infants. The symptoms caused by hypoglycemia usually come very quickly and include a feeling of hunger, shakiness, nervousness, sweating, confusion, sleepiness, dizziness, anxiety and weakness and can lead to unconsciousness and death [1-7]. In healthy human body, the concentration of blood glucose is closely controlled and normally maintained in a narrow range, approximately between 4.0 to 6.0 mmol/L (70 to 110 mg/dl). While glucose homeostasis is very complex, for the sake of this review, glucose homeostasis is mainly regulated by two hormones insulin and glucagon (Figure 1), which are both secreted by the islets of Langerhans within thepancreas [8-13]. The regulation of blood glucose homeostasis is involved in multiple layers of regulative mechanisms [12-20]. Insulin secretion from β cells of the pancreatic islets is stimulated by high glucose concentrations, which in turn, helps transport glucose from blood into cells for proper cellular function. Secondly, extra glucose can be stored either in liver or in skeletal muscle as glycogen to prevent high glucose concentrations in bloodstream. Thirdly, when blood glucose concentration falls after a meal or during exercise, insulin secretion decreases and glucagon, produced by alpha cells in the pancreas, signals the liver to break down glycogen and release glucose back into the bloodstream. In this case, stored glycogen can be used for energy between meals and blood glucose will rise to normal levels. β cells constitutively secrete a small amount of insulin into blood stream throughout the day and night, which is also essential to maintain blood glucose concentration and prevent the liver from over secreting glucose. In general, all these protective mechanisms for regulating blood glucose hemostasis prevent the human body from hypoglycemia [21-23]. Derangements in these mechanisms can lead to hypoglycemia.
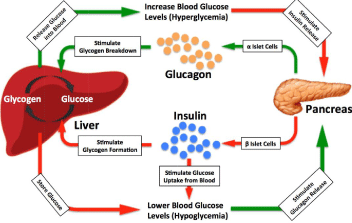
Figure 1: Regulation of glucose homeostasis by islet hormones.
Derangements of these mechanisms can lead to hypoglycemia.
Hypoglycemia
Hypoglycemia occurs in people of almost all ages, although the causes of hypoglycemia for infants, adults and the elderly may vary. Hypoglycemia can be classified as fasting, reactive, surreptitious, and artifactual [2,24-30]. Common causes of hypoglycemia include prolonged fasting, excessive effects of diabetic medicines, such as insulin, strenuous physical activity, or alcohol overconsumption [2,26-30].
Fasting hypoglycemia
Fasting hypoglycemia, which is also called post absorptive hypoglycemia, is diagnosed when a patient has low blood glucose concentration after physical activity, an overnight fast, between meals, usually 8 hours or longer in a patient after a meal. Common causes of fasting hypoglycemia include excessive effects of diabetic medications, strenuous physical activity, or alcohol overconsumption; Most cases of fasting hypoglycemia are believed to have underlying diseases (Table 1).
Types of non-diabetic hypoglycemia
Potential causes
References
Reactive hypoglycemia
Pre-diabetes
[33,34]
Enzyme deficiency
[35-37]
Carbohydrate-rich meal
[38-40]
Nesidioblastosis
[41-43]
Fasting hypoglycemia
Medications
[44-46]
Alcohol
[47-49]
Exercise
[50-52]
Liver disease
[53,54]
Insulinoma
[55,56]
Table 1: Types of non-diabetic hypoglycemia.
Insulinoma is another cause for fasting hypoglycemia and affects relatively young patients. Insulinoma is a rare neuroendocrine tumor of the pancreas. The diagnosis of insulinoma relies on the demonstration of Whipple’s triad, consisting of symptomatic hypoglycemia, documentation of hypoglycemia and resolution of symptoms upon glucose administration as well as imaging using CT scanning, endoscopic ultrasound and octreotide scanning. Insulinoma are benign in 90% of cases, however can cause severe and even lethal hypoglycemia. The treatment is surgical resection for benign insulinoma, however 10% of insulinoma are malignant; the patients suffer horribly from uncontrollable hypoglycemia for which there is no effective treatment.
Nesidioblastosis can cause hyperinsulinemic hypoglycemia because of neoformation of Langerhans islets, and it has been recognized in both adults and infants [31,32]. In adults, nesidioblastosisis associated with Roux-en-Y gastric bypass surgery requiring recurrent hospitalizations for hypoglycemia [33-45]. Congenital nesidioblastosis is also commonly referred to as persistent hyperinsulinemic hypoglycemia of infancy. It is usually caused by a number of genetic mutations, and symptoms can range from mild to severe. Severe cases can manifest as cyanosis or seizures, which can then lead to developmental delay if brain damage occurs. Although the clinical presentation between hypoglycemia induced byinsulinoma and nesidioblastosis might be similar, the symptoms in patients with nesidioblastosis occur mainly postprandially (reactive hypoglycemia) and only rarely while fasting. In contrast, most patients with insulinoma have fasting hypoglycemia.
Reactive hypoglycemia
Reactive hypoglycemia, also called postprandial hypoglycemia, usually happens when blood glucose levels become dangerously low within 4 hours after a meal. Although the symptoms of reactive hypoglycemia are similar to that of fasting hypoglycemia, these two types of hypoglycemia have different causes (Table 1). It is believed that reactive hypoglycemia results from excess insulin secretion by islets following a large carbohydrate-rich meal. However, the mechanism of continuous elevated insulin secretion is still unclear. Possible causes include a pre-diabetic condition that results in improper regulation of insulin secretion or rare enzyme deficiencies that remain food undigested [46-56]. It is important to note that the symptoms of reactive hypoglycemia can occur without low blood glucose levels and therefore no immediate medical treatment is required for these cases of reactive hypoglycemia.
Hypoglycemia and diabetes
Diabetic hypoglycemia is diagnosed when low blood glucose concentrations of 3.9 mmol/L (70 mg/dl) or lower happen in patients with diabetes mellitus. It usually occurs as a consequence of diabetic therapies, especially exogenous insulin. Remarkably, of all the causes listed in (Table 1), hypoglycemia occurs most frequently in patients with diabetes as a result of diabetic therapies [57-59]. However, diagnosis and management of hypoglycemia in diabetic patients are quite different from non-diabetic ones [57,58,60-62].
Diabetes mellitus: It has been cited as one of the most challenging health problems in the US. It is a group of metabolic disorders characterized by hyperglycemia, in which the patients have very high blood glucose over a prolonged period of time, because of either inadequate insulin secretion, or improper responses to insulin, or both [63-65]. For patients with diabetes, insulin therapy and other diabetes medications are designed to decrease the high blood glucose levels back to a normal range. Over-dose of insulin or other diabetic medications can cause blood glucose level to drop too low, resulting in hypoglycemia. Therefore, diabetic hypoglycemia usually occurs as a consequence of anti-diabetic therapies in these patients. Other causes of diabetic hypoglycemia include prolonged fasting and excessive body activity without proper adjustment of food and medications.
Hypoglycemia and T1DM: T1DM, also known as juvenile-onset or insulin-dependent diabetes, is a type of diabetes where the auto immune attack destroys the insulin-producing β cells of the islets of Langerhans in pancreas, leading to insulin deficiency [64]. Patients with T1DM do not produce insulin, and must receive frequent insulin injections or reply on insulin pumps for continuous insulin supply [66,67]. Insulin injection for T1DM is the most common cause of severe hypoglycemia in young adults. These patients have to carefully monitor their blood glucose level and regulate insulin supply accordingly; however blood glucose levels can easily fall and result in severe hypoglycemia. Islet transplantation can potentially restore the function of islet cells and successfully stabilize glycemic control in these patients.
Hypoglycemia and T2DM: T2DM, also known as noninsulindependent diabetes mellitus or adult-onset diabetes [68], is the most common form of diabetes mellitus worldwide, accounting for more than 90% of cases. Different from to an absolute deficiency of insulin secretion from islets of pancreas in patients with T1DM, the T2DM is mainly characterized by hyperglycemia in the context of insulin resistance and relatively insufficient insulin secretion. Comparing to T1DM, patients with T2DM tend less likely to be hypoglycemic. The most common cause of hypoglycemia in T2DM is iatrogenic, which occurs when insulin analogues, insulin secret gouge drugs, or combined therapy cause blood glucose levels to fall below normal [69,70]. A retrospective review of 102 patients with diabetes reported that drug-induced hypoglycemic coma occurred in 97% patients out of the hospital. The annual prevalence of severe hypoglycemia caused by sulphonyl ureas in T2DM is 7% [71].
Hypoglycemia and T3cDM: The T3cDM is associated with exocrine pancreas disorders [72,73]. Chronic pancreatitis (approximately 78.5% of all T3cDM) is the most common cause for T3cDM, while pancreatic cancer (approximately 8% of all T3cDM) is the second most common cause according to recent studies [74,75]. The glycemic control for patients with total pancreatectomyis challenging, because of complete lack of both glucagon and insulin secretions, as well as lack of a third islet hormone, pancreatic polypeptide. Hypoglycemia frequently happens in these patients due to the brittleness of their glucose regulation [72,74,76]. Islet transplantation restores functioning islet and can successfully stabilize glucose homeostasis in post-pancreatitis my patients [77].
PDX1 and Insulin Regulation
PDX1 regulates insulin expression
Pancreatic and Duodenal homeobox 1 (PDX1), also known as Insulin Promoter Factor 1 (IPF1), is a homeobox domain-containing transcription factor specific for pancreatic islet β cells. PDX1 functions as a master regulator for a variety of essential cellular events including embryonic pancreas development, maturation and maintenance of postnatal β cell functions [78-82]. PDX1 expression is firstly detected at embryonic day 8.5 in the dorsal and ventral buds that eventually fuse and give rise to pancreas. During embryonic development of pancreas, PDX1 is expressed in all precursor cells; while in the adult stage, PDX1 expression is more restricted to the nuclei of β cells and a small subpopulation of delta and PP cells in the islet [83]. It is known that PDX1 plays a central role in maintaining the mature β cell function and glucose metabolism through regulating the expression of multiple key endocrine β-cell-specific genes including insulin, glucokinase, islet amyloid polypeptide and the glucose transporter type 2 [84-89]. Deletion or homozygous inactive mutations in Pdx1 is lethal in mice due to whole pancreatic agenesis. Conditional knockout of Pdx1 gene in β cells of mice leads to overt diabetes, whereas knockingdown of Pdx1 expression results in decreased insulin secretion [90]. Pdx1 mutant zebra fish have the key diabetic features of reduced β cells, decreased insulin and elevated glucose levels [91]. In humans, heterozygous mutations in PDX1 have been associated with diabetes, including type 4 maturity-onset diabetes of the young (MODY IV) and non-MODY type 2 diabetes. In addition to diabetes, abnormal PDX1 is also involved in other Pathophysiologic conditions, such as chronic pancreatitis with decreased PDX1 expression and others. Overall, PDX1 has been demonstrated to directly regulate glucosedependent insulin expression and secretion.
PDX1 is associated with PDAC and insulinomas
PDX1 is mainly expressed and functions in islet β cells in adults. However, aberrant elevated PDX1 expression in pancreatic cancer and neuroendocrine tumors, including insulinoma, strongly suggested that PDX1 as a fundamental transcriptional factor for pancreas development may play an important role in tumorigenesis, especially in PDAC [92-94] and insulinoma [95,96]. PDX1 is able to promote Kras G12D oncogenic protein-induced development of PanIN, metaplasia and pancreatic ductal adenocarcinoma [97-99]. In addition, over expression of PDX1 in both benign cells (HEK293 and Human Pancreatic Ductal Cells), as well as pancreatic cancer cells (PANC1 and MiaPaca 2) and insulinoma cells (Min6 and βTC6 cells) lead to significant increases in cell proliferation, invasion and colony formation in vitro, as well as promotion of tumor growth in xenograft SCID mice [92,93,95]. Therefore, these studies suggest that PDX1 is involved in tumorigenesis of both PDAC and insulinoma.
A special type of pancreatic neuroendocrine tumor insulinomais a very rare benign tumor, raised from islet β cells that produce insulin, occurring in only 3-4 per million people [100-102], affect relatively young patients, mean patient age at diagnosis being 50 years old. These patients with insulinoma suffer horribly from uncontrollable hypoglycemia for which there is no effective treatment, because the expanded β cell mass continues to secrete insulin and disrupt the glucose regulation, causing severe hyperinsulinemic hypoglycemia.
Nesidioblastosis in adults is a complication of Roux-en-Y gastric bypass surgery requiring recurrent hospitalizations for hyper insulinemic hypoglycemia. Congenital nesidioblastosis is also commonly referred to as persistent hyperinsulinemic hypoglycemia of infancy. It is caused by a number of genetic mutations, and symptoms can range from mild to severe. Severe cases can manifest as cyanosis or seizures, which can then lead to developmental delay if brain damage occurs. The abnormal histologic aspects of the tissue included the presence of islet cell enlargement, islet cell dysplasia, β cells budding from ductal epithelium, and islets in opposition to ducts. Most congenital hyperinsulinism is caused by different mechanisms than excessive proliferation of β cells in a fetal pattern and the term fell into disfavor after it was recognized in the late 1980s that the characteristic tissue features were sometimes seen in pancreatic tissue from normal infants and even adults, and is not consistently associated with hyperinsulinemic hypoglycemia. As an important islet β cell specific transcription factor, PDX1 exerts its cellular functions under normal conditions by regulating expression of genes critical for insulin synthesis. In insulinoma cells, PDX1 was significantly over expressed in both human insulinoma specimens and mouse insulinoma cell line [95]. Knocking down of Pdx1 expression using bi-functional shRNA resulted in significant inhibition of insulin expression, Pdx1 expression and glucosestimulated insulin secretion, as well as cell proliferation in mouse β TC6 cells. Nanoparticle capsulated bash RNA-mPDX1 prevented death from severe hyperinsulinemic hypoglycemia in insulinoma SCID mice (Figure 2) [95]. Overall, PDX1 plays a significant role in regulating insulin synthesis and the expression level correlates with its insulinoma. A novel therapeutic strategy targeting PDX1 gene has been proved effective in preventing mortality for hyperinsulinemic hypoglycemia in an insulinoma mouse model.
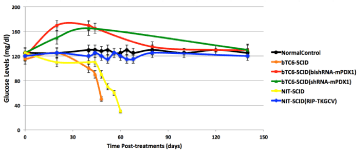
Figure 2: Duration of PDX1 targeted gene therapy in β TC6 and NIT1
insulinoma-induced hypoglycemia mouse models. Liposomal delivered
shRNA-mPDX1 and bishRNA-mPDX1 therapies successfully prevented
the risk of hypoglycemia and hypoglycemic death in two mouse models of
hypoglycemia.
Hypoglycemia Animal Models
Severe hypoglycemia is a life-threatening disease; therefore hypoglycemic animal models are essential for designing and testing preventive and therapeutic treatments. So far, drug-induced hypoglycemic effects have been observed on multiple animal models, including mouse, rat, rabbit, dog and pig. Insulin administration induced hypoglycemia remains the most frequently use approach in animal models. Fasting-induced hypoglycemia rat models have been generated and studied since 1970s [103-105]. It has been reported that other medications including metformin, GLP1 analogs, DPP4 inhibitors, methadone [106], sulfonylurea [107], and sunitinib [108] are known to trigger hypoglycemia in various animal models. Moreover, exacts from herbal medicines exhibit hypoglycemic effects, including Cordycepsmilitaris [109], Camellia sinensis [110]. Besides, there were genetic engineered animal models reported to have moderate to severe hypoglycemia. Sstr5gene knockout mice and Sstr1/5 double gene knockout mice demonstrated hyperinsulinemic hypoglycemia and improved glucose in tolerance associated with elevated islet PDX1 expression [111,112], suggesting that Sstr genes are negative regulators for PDX1 and controlling PDX1 expression level in islets is critical for prevention of hyperinsulinemic hypoglycemia [96,113-115]. Ubiquitous expression of the constitutively active form of Pik3ca (H1047R) leads to hypoglycemia and hypoinsulinemia in mice [116]. MiR-378/378* microRNA gene knockout display hypoglycemia and increased hepatic triglyceride level with enhanced insulin sensitivity in mice [117]. RIP-Tag2 transgenic mice develop pancreatic β cell tumors leading to progressive autonomous insulin secretion and hypoglycemia, which is lethal when these transgenic mice reach the age of 3 to 4 months [118]. Mouse insulinoma cell line NIT-1 and β TC6 were used to generate insulinoma SCID mouse models; when implanted within the peritoneal cavity, these mice uniformly succumb to hypoglycemia within a mean of 60 days and are therefore a mouse model to study the preventive and therapeutic strategies for severe hypoglycemia [113,119].
PDX1 targeted therapy for hypoglycemia
Unfortunately, there are no effective therapies for severe hypoglycemia caused by insulinoma and nesidioblastosis. Surgical removal of malignancy continues to be the treatment of choice because these tumors generally respond poorly to chemotherapeutic agent regimens (fluorouracil, doxorubicin, and streptozocin) [120]. PDX1-targeted therapies could provide an alternative strategy to conventional therapies [12,13]. For example insulinoma-specific cytotoxicity using the suicide gene Thymidine Kinase (TK) driven by Rat Insulin Promoter (RIP), which is activated by PDX1,and antiviral drug Ganciclovir (GCV) delivered by a nontoxic, non-inflammatory liposomal delivery system, was successfully prevent hypoglycemic death in an insulinoma SCID mouse model [119]. Using liposomal delivery of RIP-TK resulted in euglycemia of the mice, whereas adenoviral delivery of RIP-TK causes significant hyperglycemia due to damage to the islets. More recently, a novel bi-functional shRNA nanoparticle targeting PDX1 significantly and effectively abated PDX1 in insulinoma and in pancreas islets, therefore reversed hypoglycemia in vivo in two mouse models [95,96,121,122]. Bifunctional shRNA nanoparticle prevented death from hypoglycemia in an insulinoma mouse model; glucose levels rose to 170mg/dl after three biweekly treatments then returned to normal by 90 days after treatment. In Sstr1/5-/- mice, fasting hypoglycemia was reversed by three biweekly treatments of bi-functional shRNA nanoparticles; glucose levels rose to slightly greater than 200mg/dl after treatment, the remarkably returned to euglycemia 90 days after therapy. Glucose levels before and after bi-shRNAmPDX1, shRNAmPDX1 and RIP RIPTK (GCV) treatments in two insulinoma-induced hypoglycemia mouse models are summarized in (Figure 2). These studies suggest that PDX1 targeted therapies could represent a promising therapy for severe hypoglycemia induced by insulinomas.
Conclusion
Severe hypoglycemia with extremely low blood glucose level is dangerous and a potential life-threatening condition. There are very limited therapeutic management options for multiple types of hypoglycemia and there are no effective therapies for hyperinsulinemic hypoglycemia due to malignant insulinoma and nesidioblastosis. PDX1 targeted therapies successfully restored glucose regulation in two mouse models of hypoglycemia and suggest a promising preventive and therapeutic strategy for severe hypoglycemia.
References
- Haymond MW. Hypoglycemia in infants and children. Endocrinol Metab Clin North Am. 1989; 18: 211-252.
- Hofeldt FD. Reactive hypoglycemia. Endocrinology and metabolism clinics of North America. 1986; 18: 185-201.
- Lteif AN, Schwenk WF. Hypoglycemia in infants and children. Endocrinol Metab Clin North Am. 1999; 28: 619-646.
- Pourmotabbed G, Kitabchi AE. Hypoglycemia. Obstet Gynecol Clin North Am. 2001; 28: 383-400.
- Service FJ. Hypoglycemia. Endocrinol Metab Clin North Am. 1988; 17: 601-616.
- Service FJ. Hypoglycemia. Endocrinology and metabolism clinics of North America. 1977; 26: 937-955.
- Yealy DM, Wolfson AB. Hypoglycemia. Emerg Med Clin North Am. 1989; 7: 837-848.
- Andrali SS, Sampley ML, Vanderford NL, Ozcan S. Glucose regulation of insulin gene expression in pancreatic beta-cells. Biochem J. 2008; 415: 1-10.
- Owerbach D, Billesbolle P, Poulsen S, Nerup J. Glucose regulation and the insulin gene. Lancet. 1982; 1: 1304.
- Ralston SL. Insulin and glucose regulation. Vet Clin North Am Equine Pract. 2002; 18: 295-304.
- Brunicardi FC, Dyen Y, Brostrom L, Kleinman R, Colonna J, Gelabert H. The circulating hormonal milieu of the endocrine pancreas in healthy individuals, organ donors, and the isolated perfused human pancreas. Pancreas. 2000; 21: 203-211.
- Brunicardi FC. Pancreatic surgery and glucose regulation: introduction. World journal of surgery. 2001; 25: 451.
- Tirone TA, Brunicardi FC. Overview of glucose regulation. World J Surg. 2001; 25: 461-467.
- Campbell JE, Drucker DJ. Islet α cells and glucagon--critical regulators of energy homeostasis. Nat Rev Endocrinol. 2015; 11: 329-338.
- Shieh JJ, Pan CJ, Mansfield BC, Chou JY. A potential new role for muscle in blood glucose homeostasis. J Biol Chem. 2004; 279: 26215-26219.
- Kanauchi M, Yamano S, Kanauchi K, Saito Y. Homeostasis model assessment of insulin resistance, quantitative insulin sensitivity check index, and oral glucose insulin sensitivity index in no obese, nondiabetic subjects with high-normal blood pressure. The Journal of clinical endocrinology and metabolism. 2003; 88: 3444-3446.
- Hers HG. Mechanisms of blood glucose homeostasis. J Inherit Metab Dis. 1990; 13: 395-410.
- Beck B, Villaume C. Nutrient homeostasis: long-term interrelations between pancreatic hormones, blood glucose and dietary wheat bran in men. J Nutr. 1987; 117: 153-158.
- Unger RH, Ohneda A, Aguilar-Parada E, Eisentraut AM. The role of aminogenic glucagon secretion in blood glucose homeostasis. The Journal of clinical investigation. 1969; 48: 810-822.
- Weinhouse S. Role of the liver in blood glucose homeostasis. The Journal of the Medical Society of New Jersey. 1962; 59: 535-540.
- Rozance PJ, Hay WW. Describing hypoglycemia--definition or operational threshold? Early human development. 2010; 86: 275-280.
- Perlmuter LC, Flanagan BP, Shah PH, Singh SP. Glycemic control and hypoglycemia: is the loser the winner? Diabetes Care. 2008; 31: 2072-2076.
- Herbel G, Boyle PJ. Hypoglycemia. Pathophysiology and treatment. Endocrinol Metab Clin North Am. 2000; 29: 725-743.
- Field JB. Hypoglycemia. Definition, clinical presentations, classification, and laboratory tests. Endocrinology and metabolism clinics of North America. 1989; 18: 27-43.
- Horwitz DL. Factitious and artifactual hypoglycemia. Endocrinol Metab Clin North Am. 1989; 18: 203-210.
- Baruh S, Sherman L, Kolodny HD, Singh AJ. Fasting hypoglycemia. Med Clin North Am. 1973; 57: 1441-1462.
- Kogut MD. Hypoglycemia: pathogenesis, diagnosis and treatment. Curr Probl Pediatr. 1974; 4: 1-59.
- Permutt MA. Postprandiol hypoglycemia. Diabetes. 1976; 25: 719-733.
- Bailey CJ, Flatt PR, Marks V. Drugs inducing hypoglycemia. Pharmacol Ther. 1989; 42: 361-384.
- Marks V, Teale JD. Drug-induced hypoglycemia. Endocrinology and metabolism clinics of North America. 1999; 28: 555-577.
- Maeda Y, Yokoyama K, Takeda K, Takada J, Hamada H, Hujioka Y, et al. Adult-onset diffuse nesidioblastosis causing hypoglycemia. Clinical journal of gastroenterology. 2013; 6: 50-54.
- Qintar M, Sibai F, Taha M. Hypoglycemia due to an adult-onset nesidioblastosis, a diagnostic and management dilemma. Avicenna J Med. 2012; 2: 45-47.
- Solter M, Sekso M. Glucose-insulin interaction in obese individuals with asymptomatic reactive hypoglycemia. Acta diabetologica latina. 1979; 16: 119-127.
- Chutorian AM, Nicholson JF, Killian P. Reactive hypoglycemia in children. Transactions of the American Neurological Association. 1973; 98: 188-192.
- Martin J, Maurhofer O, Bellance N, Benard G, Graber F, Hahn D, et al. Disruption of the histidine triad nucleotide-binding hint2 gene in mice affects glycemic control and mitochondrial function. Hepatology. 2013; 57: 2037-2048.
- Fyfe JC, Kurzhals RL, Hawkins MG, Wang P, Yuhki N, Giger U, et al. A complex rearrangement in GBE1 causes both perinatal hypoglycemic collapse and late-juvenile-onset neuromuscular degeneration in glycogen storage disease type IV of Norwegian forest cats. Molecular genetics and metabolism. 2007; 90: 383-392.
- Berry GT, Fukao T, Mitchell GA, Mazur A, Ciafre M, Gibson J, et al. Neonatal hypoglycemia in severe succinyl-CoA: 3-oxoacid CoA-transferase deficiency. Journal of inherited metabolic disease. 2001; 24: 587-595.
- Spring B, Chiodo J, Harden M, Bourgeois MJ, Mason JD, Lutherer L. Psychobiological effects of carbohydrates. J Clin Psychiatry. 1989; 50 Suppl: 27-33.
- Hopman WP, Houben PG, Speth PA, Lamers CB. Glucomannan prevents postprandial hypoglycemia in patients with previous gastric surgery. Gut. 1988; 29: 930-934.
- Speth PA, Jansen JB, Lamers CB. Effect of acarbose, pectin, a combination of acarbose with pectin, and placebo on postprandial reactive hypoglycemia after gastric surgery. Gut. 1983; 24: 798-802.
- Dravecka I, Lazurova I. Nesidioblastosis in adults. Neoplasma. 2014; 61: 252-256.
- Ceppa EP, Ceppa DP, Omotosho PA, Dickerson JA, Park CW, Portenier DD. Algorithm to diagnose etiology of hypoglycemia after Roux-en-Y gastric bypass for morbid obesity: case series and review of the literature. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery. 2012; 8: 641-647.
- Ueda Y, Kurihara K, Kondoh T, Okanoue T, Chiba T. Islet-cell hyperplasia causing hyperinsulinemic hypoglycemia in an adult. J Gastroenterol. 1998; 33: 125-128.
- Melachuri S, Gandrud L, Bostrom B. The association between fasting hypoglycemia and methylated mercaptopurine metabolites in children with acute lymphoblastic leukemia. Pediatric blood & cancer. 2014; 61: 1003-1006.
- Halonen P, Salo MK, Mäkipernaa A. Fasting hypoglycemia is common during maintenance therapy for childhood acute lymphoblastic leukemia. J Pediatr. 2001; 138: 428-431.
- Semel JD, Wortham E, Karl DM. Fasting hypoglycemia associated with disopyramide. Am Heart J. 1983; 106: 1160-1161.
- Liu C1, Yu Z, Li H, Wang J, Sun L, Qi Q, et al. Associations of alcohol consumption with diabetes mellitus and impaired fasting glycemia among middle-aged and elderly Chinese. BMC Public Health. 2010; 10: 713.
- Diabetes and alcohol: distinctive interactions. Prescrire Int. 2008; 17: 118-120.
- Jain H, Beriwal S, Singh S. Alcohol induced ketoacidosis, severe hypoglycemia and irreversible encephalopathy. Medical science monitor: international medical journal of experimental and clinical research. 2002; 8: 77-79.
- Mundy HR, Georgiadou P, Davies LC, Cousins A, Leonard JV, Lee PJ. Exercise capacity and biochemical profile during exercise in patients with glycogen storage disease type I. The Journal of clinical endocrinology and metabolism. 2005; 90: 2675-2680.
- Tuominen JA, Karonen SL, Melamies L, Bolli G, Koivisto VA. Exercise-induced hypoglycemia in IDDM patients treated with a short-acting insulin analogue. Diabetologia. 1995; 38: 106-111.
- Arogyasami J, Conlee RK, Booth CL, Diaz R, Gregory T, Sephton S, et al. Effects of exercise on insulin-induced hypoglycemia. J Appl Physiol. 1990; 69: 686-693.
- Houten SM, Herrema H, Te Brinke H, Denis S, Ruiter JP, Van Dijk TH, et al. Impaired amino acid metabolism contributes to fasting-induced hypoglycemia in fatty acid oxidation defects. Human molecular genetics. 2013; 22: 5249-5261.
- De Pra M, Laudanna E. [Baker-Winegrad disease (hepatomegaly, hypoglycemia during fasting, hyperlactacidemic metabolic acidosis, hepatic fructose-1-6-diphosphatase deficiency). Presentation of the 1st Italian case and pathogenetic hypothesis]. Minerva pediatrica. 1987; 30: 1973-1986.
- Del Prato S, Riccio A, Vigili de Kreutzenberg S, Dorella M, Avogaro A, et al. Mechanisms of fasting hypoglycemia and concomitant insulin resistance in insulinoma patients. Metabolism. 1993; 42: 24-29.
- Brodows RG, Campbell RG. Control of refractory fasting hypoglycemia in a patient with suspected insulinoma with diphenylhydantoin. J Clin Endocrinol Metab. 1974; 38: 159-162.
- Banarer S, Cryer PE. Hypoglycemia in type 2 diabetes. Med Clin North Am. 2004; 88: 1107-1116,
- Frier BM. Morbidity of hypoglycemia in type 1 diabetes. Diabetes Res Clin Pract. 2004; 65 Suppl 1: S47-52.
- Kong AP, Chan JC. Hypoglycemia and Comorbidities in Type 2 Diabetes. Curr Diab Rep. 2015; 15: 80.
- Whiteman VE, Homko CJ, Reece EA. Management of hypoglycemia and diabetic ketoacidosis in pregnancy. Obstet Gynecol Clin North Am. 1996; 23: 87-107.
- Cryer PE. Hypoglycemia risk reduction in type 1 diabetes. Experimental and clinical endocrinology & diabetes: official journal, German Society of Endocrinology [and] German Diabetes Association. 2013; 2: 412-423.
- Mendoza A, Kim YN, Chernoff A. Hypoglycemia in hospitalized adult patients without diabetes. Endocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2005; 11: 91-96.
- Kerner W, Bruckel J, Diabetes AG. Definition, classification and diagnosis of diabetes mellitus. Experimental and clinical endocrinology & diabetes: official journal, German Society of Endocrinology German Diabetes Association. 2014; 122: 384-386.
- Canivell S, Gomis R. Diagnosis and classification of autoimmune diabetes mellitus. Autoimmun Rev. 2014; 13: 403-407.
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014; 37: 81-90.
- Matteucci E, Giampietro O, Covolan V, Giustarini D, Fanti P, Rossi R. Insulin administration: present strategies and future directions for a noninvasive (possibly more physiological) delivery. Drug design, development and therapy. 2015; 9: 3109-3118.
- In Diabetes (Type 1 and Type 2) in Children and Young People: Diagnosis and Management, London, in press. 2015.
- Nathan DM. Diabetes: Advances in Diagnosis and Treatment. JAMA. 2015; 314: 1052-1062.
- Rutter GA, Hodson DJ. Minireview: intraislet regulation of insulin secretion in humans. Mol Endocrinol. 2013; 27: 1984-1995.
- Leech CA, Dzhura I, Chepurny OG, Kang G, Schwede F, Genieser HG. Molecular physiology of glucagon-like peptide-1 insulin secretagogue action in pancreatic beta cells. Progress in biophysics and molecular biology. 2011; 107: 236-247.
- UK Hypoglycaemia Study Group. Risk of hypoglycemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007; 50: 1140-1147.
- Ewald N, Bretzel RG. Diabetes mellitus secondary to pancreatic diseases (Type 3c)--are we neglecting an important disease? Eur J Intern Med. 2013; 24: 203-206.
- Cui Y, Andersen DK. Pancreatogenic diabetes: special considerations for management. Pancreatology: official journal of the International Association of Pancreatology. 2011; 11: 279-294.
- Ewald N, Kaufmann C, Raspe A, Kloer HU, Bretzel RG, Hardt PD. Prevalence of diabetes mellitus secondary to pancreatic diseases (type 3c). Diabetes Metab Res Rev. 2012; 28: 338-342.
- Price S, Cole D, Alcolado JC. Diabetes due to exocrine pancreatic disease--a review of patients attending a hospital-based diabetes clinic. QJM. 2010; 103: 759-763.
- Hardt PD, Brendel MD, Kloer HU, Bretzel RG. Is pancreatic diabetes (type 3c diabetes) under diagnosed and misdiagnosed? Diabetes Care. 2008; 31 Suppl 2: S165-169.
- Koh A, Imes S, Shapiro AM, Senior PA. Successful treatment of brittle diabetes following total pancreatectomyis by islet allotransplantation: a case report. JOP. 2013; 14: 428-431.
- Holland AM, Gonez LJ, Naselli G, Macdonald RJ, Harrison LC. Conditional expression demonstrates the role of the homeodomain transcription factor Pdx1 in maintenance and regeneration of beta-cells in the adult pancreas. Diabetes. 2005; 54: 2586-2595.
- Hale MA, Kagami H, Shi L, Holland AM, Elsasser HP, Hammer RE. The homeodomain protein PDX1 is required at mid-pancreatic development for the formation of the exocrine pancreas. Developmental biology. 2005; 286: 225-237.
- Yokoi N, Serikawa T, Walther R. Pdx1, a homeodomain transcription factor required for pancreas development, maps to rat chromosome 12. Experimental animals / Japanese Association for Laboratory Animal Science. 1977; 46: 323-324.
- McKinnon CM, Docherty K. Pancreatic duodenal homeobox-1, PDX-1, a major regulator of beta cell identity and function. Diabetologia. 2001; 44: 1203-1214.
- Pedica F, Beccari S, Pedron S, Montagna L, Piccoli P, Doglioni C, et al. PDX-1 (pancreatic/duodenal homeobox-1 protein 1). Pathologica. 2014; 106: 315-321.
- Oliver-Krasinski JM, Stoffers DA. On the origin of the beta cell. Genes Dev. 2008; 22: 1998-2021.
- Bretherton-Watt D, Gore N, Boam DS. Insulin upstream factor 1 and a novel ubiquitous factor bind to the human islet amyloid polypeptide/amylin gene promoter. Biochem J. 1996; 313: 495-502.
- Serup P, Jensen J, Andersen FG, Jorgensen MC, Blume N, Holst JJ. Induction of insulin and islet amyloid polypeptide production in pancreatic islet glucagonoma cells by insulin promoter factor 1. Proceedings of the National Academy of Sciences of the United States of America. 1996; 93: 9015-9020.
- Waeber G, Thompson N, Nicod P, Bonny C. Transcriptional activation of the GLUT2 gene by the IPF-1/STF-1/IDX-1 home box factor. Mol Endocrinol. 1996; 10: 1327-1334.
- Watada H, Kajimoto Y, Kaneto H, Matsuoka T, Fujitani Y, Miyazaki J. Involvement of the homeodomain-containing transcription factor PDX-1 in islet amyloid polypeptide gene transcription. Biochemical and biophysical research communications. 1996; 229: 746-751.
- Watada H, Kajimoto Y, Miyagawa J, Hanafusa T, Hamaguchi K, Matsuoka T, et al. PDX-1 induces insulin and glucokinase gene expressions in alphaTC1 clone 6 cells in the presence of betacellulin. Diabetes. 1996; 45: 1826-1831.
- Macfarlane WM, Campbell SC, Elrick LJ, Oates V, Bermano G, Lindley KJ, et al. Glucose regulates islet amyloid polypeptide gene transcription in a PDX1- and calcium-dependent manner. The Journal of biological chemistry. 2006; 275: 15330-15335.
- Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994; 371: 606-609.
- Yee NS, Yusuff S, Pack M. Zebrafish pdx1 morphant displays defects in pancreas development and digestive organ chirality, and potentially identifies a multipotent pancreas progenitor cell. Genesis. 2001; 30: 137-140.
- Liu SH, Patel S, Gingras MC, Nemunaitis J, Zhou G, Chen C, et al. PDX-1: demonstration of oncogenic properties in pancreatic cancer. Cancer. 2011; 117: 723-733.
- Liu S, Ballian N, Belaguli NS, Patel S, Li M, Templeton NS, et al. PDX-1 acts as a potential molecular target for treatment of human pancreatic cancer. Pancreas. 2008; 37: 210-220.
- Wang XP, Li ZJ, Magnusson J, Brunicardi FC. Tissue microarray analyses of pancreatic duodenal homeobox-1 in human cancers. World journal of surgery. 2005; 29: 334-338.
- Liu SH, Rao DD, Nemunaitis J, Senzer N, Zhou G, Dawson D. PDX-1 is a therapeutic target for pancreatic cancer, insulinoma and islet neoplasia using a novel RNA interference platform. PloS one. 2012; 7: e40452.
- Feanny MA, Fagan SP, Ballian N, Liu SH, Li Z, Wang X, et al. PDX-1 expression is associated with islet proliferation in vitro and in vivo. J Surg Res. 2008; 144: 8-16.
- Bournet B, Dufresne M, Selves J, Torrisani J, Cordelier P, Buscail L. [Kras oncogene and pancreatic cancer: thirty years after]. Medicine sciences: M/S. 2013; 29: 991-997.
- Whipple CA, Young AL, Korc M. A KrasG12D-driven genetic mouse model of pancreatic cancer requires glypican-1 for efficient proliferation and angiogenesis. Oncogene. 2012; 31: 2535-2544.
- Bai H1, Li H, Zhang W, Matkowskyj KA, Liao J, Srivastava SK, et al. Inhibition of chronic pancreatitis and pancreatic intraepithelial neoplasia (PanIN) by capsaicin in LSL-KrasG12D/Pdx1-Cre mice. Carcinogenesis. 2011; 32: 1689-1696.
- Rossi RE, Massironi S, Conte D, Peracchi M. Therapy for metastatic pancreatic neuroendocrine tumors. Annals of translational medicine. 2014; 2: 8.
- Reid MD, Balci S, Saka B, Adsay NV. Neuroendocrine tumors of the pancreas: current concepts and controversies. Endocrine pathology. 2014; 25: 65-79.
- Milan SA, Yeo CJ. Neuroendocrine tumors of the pancreas. Curr Opin Oncol. 2012; 24: 46-55.
- Wapnir RA, Lifshitz F. Fasting-induced hypoglycemia in experimentally malnourished rats. The Journal of nutrition. 1977; 107: 383-390.
- Minick MC. Induction of fasting hypoglycemia and hyperinsulinemic in the mouse by pituitary and urinary peptides. Comp Biochem Physiol. 1970; 37: 39-48.
- Muller EE, Miedico D, Giustina G, Cocchi D. Ineffectiveness of hypoglycemia, cold exposure and fasting in stimulating GH secretion in the mouse. Endocrinology. 1971; 88: 345-350.
- Faskowitz AJ, Kramskiy VN, Pasternak GW. Methadone-induced hypoglycemia. Cell Mol Neurobiol. 2013; 33: 537-542.
- Schmid HA. Pasireotide (SOM230) prevents sulfonylurea-induced hypoglycemia in rats. Experimental and clinical endocrinology & diabetes: official journal, German Society of Endocrinology [and] German Diabetes Association. 2015; 123: 193-197.
- Szaaek E, Karbownik A, Sobaaska K, Grabowski T, Poaom W, Lewandowska M, et al. The pharmacokinetics and hypoglycemic effect of sunitinib in the diabetic rabbits. Pharmacol Rep. 2014; 66: 892-896.
- Cheng YW, Chen YI, Tzeng CY, Chang CH, Lee YC, Chen HC, et al. Aqueous extracts of Cordyceps militaris (Ascomycetes) lower the levels of plasma glucose by activating the cholinergic nerve in streptozotocin-induced diabetic rats. International journal of medicinal mushrooms. 2013; 15: 277-286.
- Abeywickrama KR, Ratnasooriya WD, Amarakoon AM. Oral hypoglycemic, antihyperglycaemic and antidiabetic activities of Sri Lankan Broken Orange Pekoe Fannings (BOPF) grade black tea (Camellia sinensis L.) in rats. Journal of ethnopharmacology. 2011; 135: 278-286.
- Wang XP, Norman MA, Yang J, Cheung A, Moldovan S, Demayo FJ, et al. Double-gene ablation of SSTR1 and SSTR5 results in hyperinsulinemic and improved glucose tolerance in mice. Surgery. 2004; 136: 585-592.
- Ramirez JL, Grant M, Norman M, Wang XP, Moldovan S, De Mayo FJ, et al. Deficiency of Somatostatin (SST) receptor type 5 (SSTR5) is associated with sexually dimorphic changes in the expression of SST and SST receptors in brain and pancreas. Molecular and cellular endocrinology. 2004; 221: 105-119.
- Zhou G, Liu SH, Shahi KM, Wang H, Duan X, Lin X, et al. Negative regulation of pancreatic and duodenal homeobox-1 by Somatostatin receptor subtype 5. Mol Endocrinol. 2012; 26: 1225-1234.
- Tirone TA, Norman MA, Moldovan S, DeMayo FJ, Wang XP, Brunicardi FC. Pancreatic Somatostatin inhibits insulin secretion via SSTR-5 in the isolated perfused mouse pancreas model. Pancreas. 2003; 26: 67-73.
- Fagan SP, Azizzadeh A, Moldovan S, Ray MK, Adrian TE, Ding X, et al. Insulin secretion is inhibited by subtype five Somatostatin receptor in the mouse. Surgery. 1998; 124: 254-258.
- Kinross KM, Montgomery KG, Mangiafico SP, Hare LM, Kleinschmidt M, Bywater MJ, et al. Ubiquitous expression of the Pik3caH1047R mutation promotes hypoglycemia, hypoinsulinemia, and organomegaly. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2015; 29: 1426-1434.
- Liu W, Cao H, Ye C, Chang C, Lu M, Jing Y, et al. Hepatic miR-378 targets p110alpha and controls glucose and lipid homeostasis by modulating hepatic insulin signalling. Nature communications. 2014; 5: 5684.
- Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985; 315: 115-122.
- Tirone TA, Fagan SP, Templeton NS, Wang X, Brunicardi FC. Insulinoma-induced hypoglycemic death in mice is prevented with beta cell-specific gene therapy. Annals of surgery. 2011; 233: 603-611.
- Kulke MH, Bergsland EK, Yao JC. Glycemic control in patients with insulinoma treated with everolimus. N Engl J Med. 2009; 360: 195-197.
- Jay CM, Ruoff C, Kumar P, Maass H, Spanhel B, Miller M, et al. Assessment of intravenous pbi-shRNA PDX1 nanoparticle (OFHIRNA-PDX1) in yucatan swine. Cancer Gene Ther. 2013; 20: 683-689.
- Ballian N, Hu M, Liu SH, Brunicardi FC. Proliferation, hyperplasia, neogenesis, and neoplasia in the islets of Langerhans. Pancreas. 2007; 35: 199-206.