Abstract
We found that inert fluoro-carbon skeletons of Perfluorooctanoic Acid (PFOA), Perfluorooctane Sulfonate (PFOS) and 1H, 1H, 2H, 2H-perfluorooctanesulfonic acid (6:2FTS) could be broken down by potassium permanganate as oxidant in acidic liquid phase at room temperature. This opened a new Approach to the Remediation of Aqueous Film Forming Foams (AFFFs). The breakdown was confirmed from the HPLC-MS and Ion Chromatography (IC) data. Due the oxidization’s contribution, those fluoro-carbon skeletons’ half-life was estimated to be approximately 3 months, much shorter than the several decades that occur in nature.
Keywords: PFOA; PFOS; 6:2FTS; Breakdown; Oxidization; Potassium permanganate
Introduction
Poly- and Perfluoroalkyl Substances (PFASs) exhibit unique physical and chemical properties, such as hydrophobicity and oleophobicity, which are not evident in other components, and also extreme stability with respect to thermal, chemical and biodegradation [1]. Due to their important anti-wetting and anti-staining properties, they have been used widely and domestically in such activities as clothing, upholstery, carpeting, painted surfaces, food containers, cookware, etc [2]. However, since their fluoro-carbon skeletons are inert and resistant to biodegradation under natural environmental conditions (CF3-CF3 of 99 kcal/mol vs CH3-CH3 of 89 kcal/mol) [3,4] this has led to their global accumulation and distribution in the environment. This has in turn raised serious concerns about their impact on the environment and public health [5-7].
Aqueous Film Forming Foam (AFFF) is a good example that has been widely used to extinguish fires [8,9]. Its main ingredients are anionic fluoro surfactants such as Perfluorooctane Sulfonate (PFOS) and perfluorooctanoic acid (PFOA). Due to serious misgivings about their biological and environmental impact and their persistent nature, PFOS was phased out in the early 2000s. Lots of alternatives were found on the market. These include, for example, 1H, 1H, 2H, 2H-perfluorooctanesulfonic acid (6:2FTS)-, and 1H, 1H, 2H, 2H-perfluorodecane sulfonic acid (8:2FTS)-based fluoro surfactants [10]. Those fluorotelomers were synthesised via telomerisation with linear structures that differ from the products of electrochemical fluorination, such as PFOS containing linear and branched isomers [6,11]. Although they are re-ported to be environmentally safe their fluoro-carbon skeletons still raise concerns about their biodegradability in the natural environment [12]. For example, the half-life of 6:2FTS is estimated to be >10 years 12, which is shorter but still similar to >41 years for PFOS, and >92 years for PFOA (USEPA 505-F-14-001), respectively. It should be noted that those values depend on estimating approach, initial concentration, degradation conditions, etc., and consequently they have varied in the literature [12-14]. New ingredients thus include short chains of the fluoro- carbon (C3-C6) [15,16] and fluorine-free surfactants [17].
Previously we used chemical oxidant (potassium permanganate, KMnO4) to break down the derived groups of fluoro surfactants because the non-fluoro-carbon can be broken down much more easily than fluoro-carbon skeleton [18]. However, we found that the fluorocarbon skeleton could potentially be broken down, although the process was slow. This particular phenomenon is interesting because it might lead to a new degradation approach that is different from previous ones, for example Fenton reaction of hydrogen peroxide [19], persulphate [4], advanced electrochemical oxidization [20], Sonolytic conversion [21] etc. Compared to the heat-up approach [22], this mild condition (occurred at room temperature) offers the promise to scale-up its application. The additional advantages of this oxidant include cost-effectiveness, stability, environmentally safe, easy to operate etc [23,24]. Here we selected 3 common fluoro surfactants - PFOA, PFOS and 6:2FTS - to verify the possibility of breaking down these ingredients using KMnO4.
Materials and Methods
All chemicals including PFOA, PFOS and 6:2FTS, potassium permanganate (KMnO4, ACS reagent, =99.0%), hydrogen chloride (HCl, 37%, w/w, AR), methanol and ammonium acetate (NH4Ac) were purchased from Sigma-Aldrich (Australia). Only polypropylene containers/pipette tips were used throughout to avoid any potential interference from Teflon containers/caps. Milli-Q water was used (> 18 MΩ•cm) in the present study.
All samples were diluted in Milli-Q water in centrifuge tubes (polypropylene) without pre-treatment. 0.1% KMnO4 + 0.36% HCl (w/w) was placed in the tubes for the oxidization process [4,23,24]. The tubes were kept at room temperature (~24°C) and not shielded from the laboratory fluorescent lamp for the purposes of domestic lighting. The tubes were occasionally shaken (once per day) during oxidization. Samples were filtered with nylon syringe filters (0.2μm) prior to HPLC-MS analysis HPLC-MS (Agilent 1260 + Quadrupole 6130) before and after the oxidization [25,26].
For HPLC-MS analysis, we followed the standard method (EPA/600/R-08/092) [27]. In general, 10μL sample solution was injected into Agilent 1260 high-performance liquid chromatography fitted with an XDB-C18 column kept at 40°C. Its dimensions were 2.1 mm internal diameter, 100 mm length and 5μm particle size. The flow rate was 0.5mL/min for gradient mobile phase of methanol: 5mM aqueous NH4Ac for separation. Quadrupole 6130 detector was maintained at 70 V under negative mode for scanning. Extraction of the molecular ions was conducted at m/z 413 for PFOA, 499 for PFOS and 427 for 6:2FTS, respectively. Quantification was done by producing a calibration curve using external standard solutions of PFOA, PFOS (only linear isomers were quantified) and 6:2FTS with correlation coefficients higher than 0.99 and limit of detection being ~0.2 ppb (signal: noise > 3). Blank samples of Milli-Q water and methanol were run prior to each set of test to minimize any background contamination that could have originated from the Teflon components of the HPLC instrument itself. The nebulizer gas (nitrogen) pressure was set at 40 psi, drying gas flow rate was 9L/min and temperature 325 °C, capillary voltage was + 3500 V and skimmer voltage was – 15 V. More details are listed in Ref [21].
Free fluoride (F-) and sulphate (SO4 2-) were detected using Ion Chromatography (IC), which was conducted using DIONEX (ICS- 2000, RFIC, and Thermo Scientific). The ion exchange column was IonPacTM AS18, 2 × 250 mm and kept at 35 °C under 2230 psi pump pressure. Following 25μL sample injection, 10 mM KOH was gradually flow at 0.25mL/min. Conductivity detector was employed with a suppressor of 43mA.
Note that each time we ran at least 6 samples in parallel (3 samples without addition of oxidant as controls and the other 3 samples with oxidant) for quality assurance and quality control (QA/QC) [28].
Results and Discussion
Figure 1 indicates that the colour change depends on the oxidization process. At the beginning, the purple solution confirms the existence of KMnO4. The colour became increasingly darker and changed to brown after 3 months, suggesting the decomposition of oxidant KMnO4. With this decomposition some targets have been oxidized, such as PFOA, PFOS or 6:2FTS in the solution, although the nature of the decomposition of KMnO4 should not be ignored [23,24].

Figure 1: Images depicting the oxidization process subjected to 0.1% KMnO4
+ 0.36% HCl (w/w) at 3 months and 6 months. All samples were diluted to
100 ppm (w/w).
After 6 months, the solution became transparent and some precipitates were observed on the bottom of the containers, these being the products of the oxidization, such as MnSO4, MnO2, etc. Those precipitates should not be injected into the HPLC column so that they need to be filtered off for HPLC analysis.
Figure 2 shows the HPLC-MS results when fluoro surfactants were subjected to the oxidization using 0.1% KMnO4 + 0.36% HCl (w/w). All 3 samples, including PFOA (a), PFOS (b) and 6:2FTS (c), feature the decreased peak heights after the oxidization (marked as “after”) compared to the absence of oxidant in the solution as controls (marked as “before”), suggesting the breakdown of the fluoro surfactants. (d) high lights the quantitative data where PFOA, PFOS and 6:2FTS were calibrated using external standards. Here the multipeaks in (b) are assigned to its isomers. We just analysed the linear isomer using its standard. Basically, after 3 months’ oxidization, a significant decrease in the 3 samples’ concentration, from 62% to 45%, was observed.
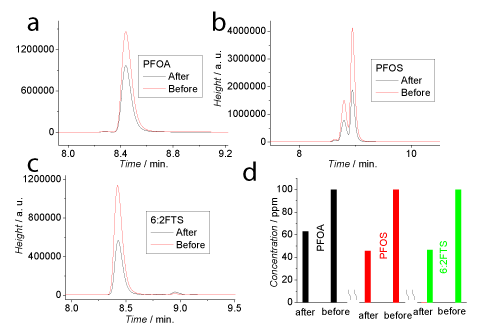
Figure 2: HPLC-MS data showing the breakdown of PFOA (a), PFOS (b) and
6:2FTS (c) subjected to oxidization with 0.1% KMnO4 + 0.36% HCl (w/w) for
3 months. (d) Illustrates a comparison of the fluoro surfactant concentration.
All samples were diluted to 100 ppm (w/w).
Figure 3 shows the HPLC-MS data and IC data after 6 months’ oxidization. In Figure 3 (a), we can see the concentrations of fluorosurfactants declined further compared to the results of 3 months’ oxidization, suggesting that the oxidization process was gradual. The kinetics information was unclear from the limited data points. However, we can estimate the half-life to be approximately 3 months for all 3 samples, which is much shorter than the several decades that occur in the natural environment [1,29], suggesting that the oxidant does make a contribution. We should also note that the oxidization capacity of 0.1% KMnO4 + 0.36% HCl faded with time and was not as strong as the fresh solution, as observed in Figure 1. In other words, the breakdown process can be accelerated if the oxidant solution can be refreshed continuously. Another option is the temperature increase that warrants further research.
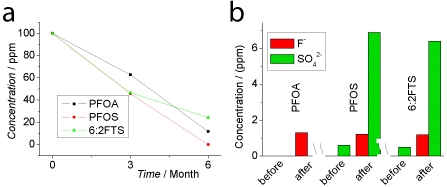
Figure 3: HPLC-MS data (a) and ion chromatograph data (b) showing the
breakdown of PFOA, PFOS and 6:2FTS subjected to the oxidization with
0.1% KMnO4 + 0.36% HCl (w/w) for 3-6 months. (b) Compares the free
concentrations of F- and SO4
2- before and after oxidization for 6 months. All
samples were diluted to 100 ppm (w/w).
Figure 3 (b) shows the IC data for the free ions of F- and SO4 2- . We can see the concentration of F- significantly increased after 6 months’ oxidization when compared to almost zero (less than the limit of detection, 0.5 ppm) for all 3 samples in the absence of oxidization. It confirmed the breakdown of the fluoro-carbon skeleton of the fluorosurfactants because the free ion of F- was released from the fluoro-carbon skeleton. For PFOS and 6:2FTS, we also observed the increased concentration of SO4 2- after oxidization, which originated from their sulfonic groups. Conversely, there is no detectable SO4 2- before or after the oxidization of PFOA, supporting the above assumption that sulfonic-containing groups were broken down and converted into SO4 2- because PFOA does not contain this kind of group. Note that the SO4 2- was detected before oxidization commenced, which may be due to the impurity from the original sample, or due to the partial breakdown by oxygen from air because we kept the control samples (without oxidant) in parallel including dilution and storage in air for 6 months.
The concentrations of F- were estimated to be ~1.2 ppm (~0.6 mM) for all 3 samples and SO4 2- 6.5 ppm (~0.068 mM) for PFOS and 6:2FTS, respectively. Considering the samples have been diluted to 100 ppm (0.24 mM for PFOA, 0.20mM for PFOS and 0.23mM for 6:2FTS), the measured concentration of F- and SO4 2- indicated the breakdown of fluoro-carbon is not 100% but 17-20% in terms of F-, 25-30% in terms of SO4 2- (taking out the amount before the oxidization and only for PFOA and 6:2FTS), respectively. This agrees with the results in Figure 3 (a). Another possible reason is the fluoro-carbon skeleton were mainly broken down into fragments, but not completely into free inorganic ions, which requires more experimental research. The leakage of HF gas into air should also be considered.
In summary, we successfully demonstrated the degradation of PFOA, PFOS and 6:2FTS using KMnO4 as oxidant at the room temperature. Although the kinetics information is absent, the halflife for all 3 fluorosurfactants was estimated to be ~3 months, which represents a rapid approach compared to natural degradation of 870- 1400 years or 10-17 years for surface-mediation [12]. Compared to 30-60 min for advanced electrochemical oxidization 30 and catalyzed H2O2 propagation reactions [19], this approach is simple and costeffective for breaking down fluoro surfactant samples. Admittedly, the concentrations of PFOA, PFOS and 6:2FTS in this test are higher than the ones in nature, such as in contaminated groundwater [31]. Therefore, a pre-concentration module might be needed to improve the breakdown efficiency for the practical remediation.
Acknowledgment
The authors kindly acknowledge funding support provided by CRC CARE and the Australian Government Department of Defence.
References
- Vierke L, Staude C, Biegel-Engler A, Drost W, Schulte C. Perfluorooctanoic acid (PFOA)—main concerns and regulatory developments in Europe from an environmental point of view. Environ Sci Eur. 2012.
- McKenzie ER, Siegrist RL, McCray JE, Higgins CP. Effects of Chemical Oxidants on Perfluoroalkyl Acid Transport in One-Dimensional Porous Media Columns. Environmental Science & Technology. 2015; 49: 1681-1689.
- Carter KE, Farrell J. Oxidative Destruction of Perfluorooctane Sulfonate Using Boron-Doped Diamond Film Electrodes. Environmental Science & Technology. 2008; 42: 6111-6115.
- Vecitis CD, Park H, Cheng J, Mader BT, Hoffmann MR. Treatment technologies for aqueous Perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA). Frontiers of Environmental Science & Engineering in China. 2009; 3: 129-151.
- Naidu R. in 5th International Contaminated Site Remediation Conference. 2011.
- Kärrman A, Elgh-Dalgren K, Lafossas C, Møskeland T. Environmental levels and distribution of structural isomers of Perfluoroalkyl Acids After Aqueous Fire-Fighting Foam (AFFF) contamination. Environmental Chemistry. 2011; 8: 372-380.
- Place BJ, Field JA. Identification of Novel Fluor chemicals in Aqueous Film-Forming Foams Used by the US Military. Environmental Science & Technology. 2012; 46: 7120-7127.
- Moody CA, Field JA. Per fluorinated surfactants and the environmental implications of their use in fire-fighting foams. Environmental Science & Technology. 2000; 34: 3864-3870.
- Anderson RH, Long GC, Porter RC, Anderson JK. Occurrence of select perfluoroalkyl substances at U.S. Air Force aqueous film-forming foam release sites other than fire-training areas: Field-validation of critical fate and transport properties. Chemosphere. 2016; 150: 678-685.
- Cortina T and Korzeniowski S. Firefighting foams–Reebok redux. Industrial Fire J. 2008.
- Schultz MM, Barofsky DF, Field JA. Quantitative Determination of Fluorotelomer Sulfonates in Groundwater by LC MS/MS. Environmental Science & Technology. 2004; 38: 1828-1835.
- Washington JW, Ellington JJ, Jenkins JM, Evans JJ, Yoo H and Hafner SC. Degradability of an Acrylate-Linked, Fluorotelomer Polymer in Soil. Environmental Science & Technology. 2009; 43: 6617-6623.
- Washington JW, Naile JE, Jenkins TM, Lynch DG. Characterizing fluorotelomer and polyfluoroalkyl substances in new and aged fluorotelomerbased polymers for degradation studies with GC/MS and LC/MS/MS. Environmental Science & Technology. 2014; 48: 5762-5769.
- Washington JW, Jenkins TM, Rankin K, Naile JE. Decades-Scale Degradation of Commercial, Side-Chain, Fluorotelomer-based Polymers in Soils & Water. Environmental Science & Technology. 2015; 49: 915-923.
- Wang Z, Cousins IT, Scheringer M, Hungerbuehler K. Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: status quo, ongoing challenges and possible solutions. Environment international. 2015; 75: 172-179.
- Houtz EF, Sutton R, Park JS, Sedlak M. Poly and perfluoroalkyl substances in wastewater: Significance of unknown precursors, manufacturing shifts, and likely AFFF impacts. Water Research. 2016; 95: 142-149.
- Hetzer R, Kümmerlen F, Wirz K, Blunk D. Fire Testing a New Fluorine-free AFFF Based on a Novel Class of Environmentally Sound High Performance Siloxane Surfactants. Fire Safety Science. 2014; 11: 1261-1270.
- Fang, C, Megharaj M, Naidu R. Chemical oxidization of some AFFFs leads to the formation of 6:2FTS and 8:2FTS. Environmental Toxicology and Chemistry. 2015; 34: 2625-2628.
- Mitchell SM, Ahmad M, Teel AL, Watts RJ. Degradation of Perfluorooctanoic Acid by Reactive Species Generated through Catalyzed H2O2 Propagation Reactions. Environmental Science & Technology Letters. 2014; 1: 117-121.
- Zhuo Q, Deng s, yang B, Yu G. Degradation of perfluorinated compounds on a boron-doped diamond electrode. Electrochimica Acta. 2012; 77: 17-22.
- Vecitis CD, Park H, Cheng J, Mader BT, Hoffmann MR. Kinetics and Mechanism of the Sonolytic Conversion of the Aqueous Per fluorinated Surfactants, Perfluorooctanoate (PFOA), and Perfluorooctane Sulfonate (PFOS) into Inorganic Products. The Journal of Physical Chemistry A. 2008; 112: 4261-4270.
- Liu CS, Shih K, Wang F. Oxidative decomposition of perfluorooctanesulfonate in water by permanganate. Separation and Purification Technology. 2012; 87: 95-100.
- Li Z, Hanlie H. Combination of surfactant solubilization with permanganate oxidation for DNAPL remediation. Water research. 2008; 42: 605-614.
- Yan YE, Schwartz FW. Kinetics and mechanisms for TCE oxidation by permanganate. Environmental Science & Technology. 2000; 34: 2535-2541.
- Frost RL, Zhou Q, He H, Xi Y. An infrared study of adsorption of paranitrophenol on mono-, di- and tri-alkyl surfactant intercalated organoclays. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2008; 69: 239-244.
- Boulanger B, Vargo J, Schnoor JL, Hornbuckle, KC. Detection of Perfluorooctane Surfactants in Great Lakes Water. Environmental Science & Technology. 2004; 38: 4064-4070.
- Park, H, Vecitis CD, Cheng J, Choi W, Mader BT, Hoffmann MR. Reductive defluorination of aqueous perfluorinated alkyl surfactants: effects of ionic head group and chain length. The Journal of Physical Chemistry A. 2009; 113: 690-696.
- Wang, P. Lu Y, Wang T, Fu Y, Zhu Z, Liu Z, Xie S et al. Occurrence and transport of 17 perfluoroalkyl acids in 12 coastal rivers in south Bohai coastal region of China with concentrated fluoropolymer facilities. Environmental Pollution. 2014; 190: 115-122.
- Seow, J. Fire fighting foams with fluorochemicals: environemental reivew. 2013.
- Niu J, Lin H, Xu J, Wu H, Li Y. Electrochemical Mineralization of Perfluorocarboxylic Acids (PFCAs) by Ce-Doped Modified Porous Nanocrystalline PbO2 Film Electrode. Environmental Science & Technology. 2012; 46: 10191-10198.
- Fang C, Megharaj M, Naidu R. Surface-Enhanced Raman Scattering (SERS) detection of fluorosurfactants in firefighting foams. RSC Advances. 2016; 14: 11140-11145.
