Abstract
Crystal Violet (CV) enters in water bodies through the effluents of textile, medical, paint, biotechnology industries. CV considered as mutagenic and mitotic in nature and also causes skin irritation, eye damage and other severe effects on human as well as aquatic lives. Due to its toxicity and harmful effects, environmental agencies have put strict regulations on discharge of CV dye contaminated water into natural water bodies. In the past decades, several processes have been cited in the literature for the decontamination of CV dye; however they have their own limitations for purification. Most of the cited processes require sophisticated tools to separate adsorbent from water after purification. For the proposed adsorbent, there is no need of such filtration processes as the proposed chitosan beads can be easily removed from water after purification without any tools. The same beads can be utilized several times just simple chemical reactivation. In the proposed process, we have utilized the same beads up to eight times and the removal efficiency was found to be more than 90% within 90 minutes. The material is cost effective, eco-friendly and easy to synthesize. To understand the adsorption rate and the adsorption mechanism of CV over chitosan beads, the kinetics and adsorption isotherms models were analyzed. The obtained experimental adsorption data was found to be best fitted to pseudo second order kinetics and Langmuir adsorption isotherm.
Keywords: Crystal Violet; Industrial waste water; Chitosan Beads; Adsorption; Re-usable
Introduction
Synthetic dyes are routinely used in the paper, textile, food, printing, etc. industries. The major amounts of these dyes are released into the environment and surface water from industries, which deteriorate the quality of surface water. Due to their nonbiodegradable nature and having complex molecular structure, these dyes are considered to be carcinogenic and mutagenic [1]. Out of the other synthetic dye CV (C25N3H30Cl) commonly used in several industrial process. The chemical structure of CV has been given Figure 1a, which shows triphenylmethane as chromophore group responsible for intense color. Although CV being considered as harmful on inhalation and causes skin irritation, conjunctiva and permanent blindness. However, it is commonly used in pharmaceutical industries, textile industry for violet coloration and also used as antibacterial, antifungal and anthelmintic in patients. The undesirable and illegal release of CV in the environment causes significant threat to human health [2]. So, to keep the health of mankind and aquatic life it is important to separate the dye from contaminated water before discharging in to water stream. The separation of dyes from contaminated water generally being carried out by several reported technique [3] like precipitation ozonation, electrochemical oxidation, membrane filtration, coagulation/ flocculation, advanced oxidation, reverse osmosis.
However, these techniques are expensive, time consuming, and generates toxic sludge. In due course, the adsorption technique has been espousing as an appropriate and advanced treatment technique for dye contaminated water owing to its many advantages such as simplicity, ability to treat minute concentration of dyes as well as regeneration capability. Activated carbon has shown good adsorption capacity with enormous surface reactivity. However, it’s difficult to regenerate; as on regeneration almost 15% is lost resulting in decreased uptake capacity also hampers the large-scale application [3].
Consequently, research has been attracted towards the development of an effective adsorbent with high regeneration capability. In this regard several adsorbents like de-oiled soya ash [4], bamboo tree leaf [5], clays, surfactant modified alumina, acid activated bentonite composite beads, powdered mycelia, activated carbon prepared by rice husk, banana peel, charcoal, iron oxide (Fe3O4)-coated biochar etc. have been explored for the removal of CV from water. According to the literature review, many other different materials also have been used for the removal of dyes like amberlite resin, alganic acid, Mn-doped ZnO nanocrystals, manganese ferrite, alumina-silica composite aerogels, ZnO-Fe3O4, nanocomposite [6].
Chitosan is also considered as one of such adsorbents [7] which have been widely used in various fields of adsorption due to its excellent adsorption affinity, and non-toxicity. Chitosan, a linear polysaccharide consisting of randomly dispersed N-acetyl-Dglucosamine as acetylated unit and β-(1→4)-linked D-glucosamine as a deacetylated unit. Its chemical structure is shown in Figure 1b. In the present work, chitosan beads activated by NaOH have been used due to its significant adsorption properties compared to several other adsorbents. The adsorbent can be easily synthesized without requiring any complicated experimental setup and serves as a potent sorbent for decontamination of CV dye. The batch adsorption experiments were carried out as a function of pH, sorbent dose, contact time, and concentration of adsorbate i.e. CV dye. The experimental sorption data obtained from the varying time and concentration were fitted to kinetics and isotherm models to understand the adsorption mechanism.
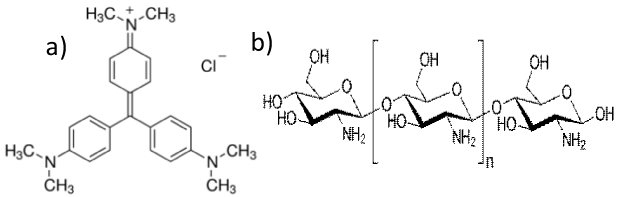
Figure 1: (a) Structure of Crystal Violet and (b) Structure of Chitosan.
Materials and Methods
All reagents were of analytical purity. Acetic acid, sodium hydroxide, and Chitosan flakes were procured from Merck, India and used without further purification. The crystal violet (CV) was obtained as the commercial grade. De-ionized (DI) water having 18.2 MO-cm resistivity was used throughout the experiments which were obtained from Millipore milli-Q element water purification system, USA. A stock solution containing 1000mgL-1of CV dye was prepared by dissolving required amount of dye in DI water. Further stock solution was diluted to the desired working range. The pH of the solution was adjusted by 0.1M NaOH and HCl and the concentration of the dye in the solution was analyzed at an optimized wavelength (570nm) using Hitachi; UV-VIS spectrophotometer (model U-3900H)
Preparation of chitosan beads
To synthesize Chitosan beads 3.0g of chitosan was taken in flask. To this, 100mL of acetic acid (3% w/v) was added. The solution was left for overnight to ensure complete dissolution. In the Chitosan solution, 2 M NaOH solutions was poured drop wise. The beads of varying sizes were obtained. The size of the beads depends on the diameter of the dispenser, through which the solution is poured. The detailed synthesis of the beads via ionic gelation technique [8]. The beads were collected from the solution after 24 hours and were used for further experiments. The detailed spectroscopic and microscopic studies of chitosan beads were carried out after air-dried.
Batch adsorption experiments for optimization of adsorption parameters
Adsorption of CV by Chitosan beads were investigated by batch adsorption. The adsorption parameters like pH, contact time and dose of adsorbent and adsorbate was optimized by carrying several experiments. Each of the adsorption study was conducted at room temperature (20°C-30°C). Initially, the known amounts of Chitosan beads were added to the 25mL of CV dye solution having 1mgL-1 concentration. The solution was then stirred and left for 60 minutes and the chitosan beads was then separated out manually. The remaining concentration of CV in the solution after adsorption was analyzed using UV-Vis spectrophotometer. The schematic for synthesis and adsorption of CV dye by chitosan beads have been presented in Figure 2.
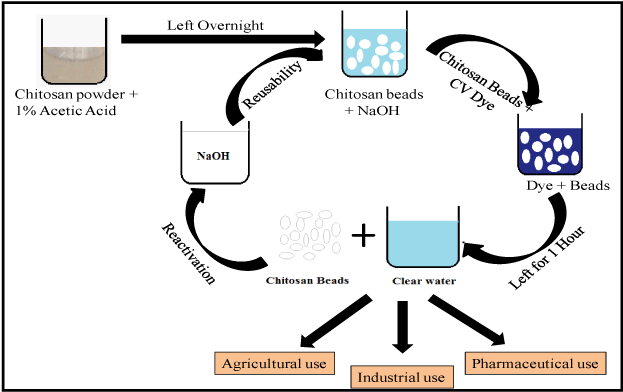
Figure 2: Schematic presentation of synthesis and adsorption of CV dye over Chitosan beads.
In order to evaluate the maximum sorption of CV dye, an entire set of experiments were performed at varying pH (2-10), adsorbent dose (1-15 beads), time interval (10-90 min) at room temperature. The percent removal of CV in batch sorption experiment was calculated using Equation 1:
Percent Removal of CV= (Ci-Cf) × 100/Ci (1)
Where Ci and Cf represents the initial and final concentration (mgL-1) of CV dye solution.
Desorption and recycling of adsorbent
The desorption and reusable efficiency of the Chitosan beads were investigated. The CV adsorbed chitosan beads were immersed in the 1M NaOH solution for 24 hours to reactivate the chitosan beads. Several sorption-desorption cycles were performed at optimized parameters. The remaining concentration of CV was determined after each cycle, and the percentages desorption and adsorption was calculated.
Characterization of the beads
The infrared spectra of fresh and CV loaded Chitosan beads were analyzed using Fourier transform infrared (FTIR) spectroscopy (Agilent make cary 630). The phase analysis of chitosan beads was carried out by diffraction patterns obtained from X-ray diffractometer (Bruker make AXS D8) using CuKa radiation and diffract plus software. The crystallite size of Chitosan beads was calculated with the help of Debye Scherer’s equation. The surface morphology of fresh as well as CV loaded chitosan beads were analyzed using JEOL JSM 6701F make scanning electron microscope operated at emission current of 3.0kV.
Results and Discussion
Characterization
XRD analysis: The phase analysis of air-dried chitosan beads was carried out having the 20 range from 10° to 90°. The XRD pattern of dried chitosan beads is shown in Figure 3. The Chitosan beads shows sharp diffraction peak at 19.9° [9]. The crystallite size of Chitosan beads calculates using Scherer’s equation [10] was found to be 5.39nm respectively.
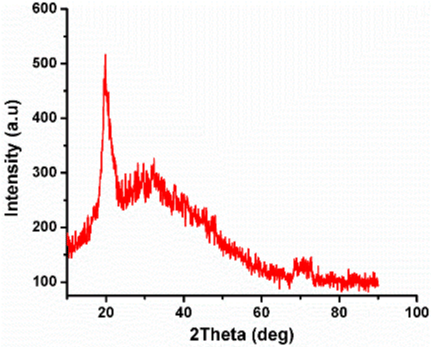
Figure 3: XRD Pattern of Chitosan.
FTIR analysis: The FTIR Spectra of fresh as well as CV loaded Chitosan beads are shown in Figure 4. The functional groups on air dried chitosan beads before and after CV dye adsorption were characterized by FTIR in the range of 4000-430 cm-1 as shown in Figure 4. In pure chitosan beads, the absorption peaks at 3340cm- 1 originated from N-H stretching vibration mode, the peak at 2860cm-1 shows C-H alkyl group, as Chitosan is a polysaccharide (a hydrocarbon). The peak at 1660cm-1 indicates presence of C=N group. Another peak at 1566cm-1 indicates the deformation band of the primary amine (-NH) responsible for protonation of dye and the peak at 1400cm-1 reveals the band arises due to axial deformation of the C-N amide and band at 1316cm-1 indicates the presence of CH-OH and CH2-OH stretching. The band at 1020cm-1 indicates the stretching of the C-OH of the primary alcohol group [11]. The additional peak at 875cm-1 in FTIR spectra of CV loaded chitosan beads indicates the peaks corresponding to CV adsorption. From the spectrum it has been concluded that CV binds over Chitosan beads.
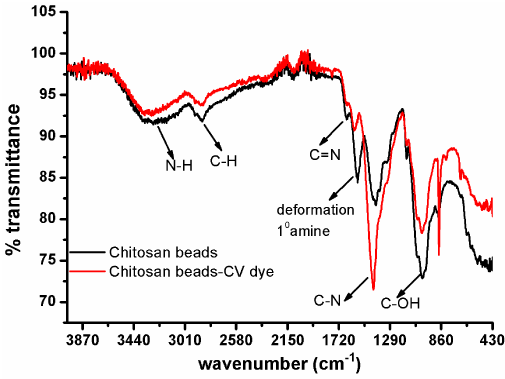
Figure 4: FTIR spectra of fresh as well as CV dye loaded Chitosan beads.
SEM-EDS
The surface morphology and elemental composition of chitosan beads before and after CV dye adsorption is shown in Figure 5a and 5b respectively. It has been observed that drop or oval like chitosan beads seems to have macro porous structure having interconnected pores. These pores may be responsible for adsorption of CV. Further, the presence of C, O and N elements in EDS spectra confirms the synthesis of chitosan beads. In the elemental composition of CV adsorbed chitosan beads, the increased percentage of C and N elements confirms the adsorption of CV over chitosan beads.
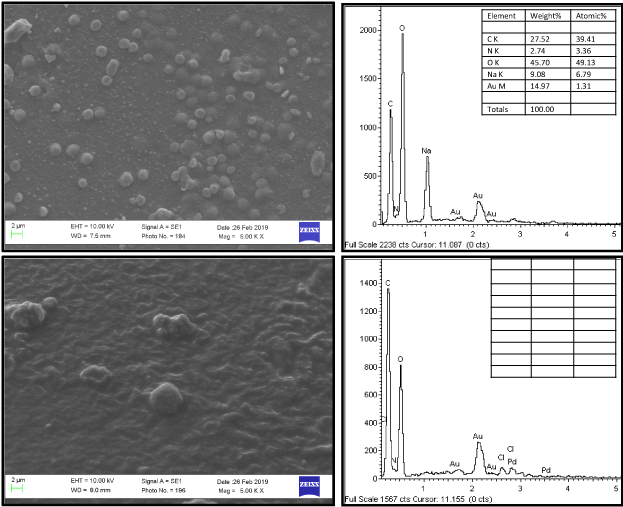
Figure 5: SEM-EDS of air-dried chitosan beads (a) pure Chitosan and (b) after adsorption of CV.
Optimization of adsorption parameters
Optimization of pH: pH is one of the significant factors affecting adsorption of pollutant having its prominent effect on the surface feature of sorbent as well as ionization of adsorbate [12]. The percent removal of CV by Chitosan beads was studied by varying the solution pH from 2 to 10 in 25mL of CV dye solution (10mgL-1 concentrations). To each solution 7 beads (weight around 1.5g/bead) of Chitosan was added. From Figure 6a, the percent removal of CV by chitosan was found to be increases as pH increase and almost completely removed at 9.5-10 pH range. Further all the experiments were done at optimized pH (9.5-10).
Optimization of adsorbent dose: In order to investigate the effect of adsorbent dose on the percent removal of CV, various number of beads (1-15 beads) were utilized at constant concentration and volume of CV as performed in the pH study. The respective results are shown in Figure 6b which reveals that percent removal of CV increases with number of beads. It is due to the increase in active sites available on chitosan beads which enhance the adsorption capacity [13]. The maximum removal of CV was observed at 9beads whereas further increase in the adsorbent dose results in a plateau.
Optimization of contact time: Contact time between CV and chitosan was optimized by varying the reaction time from 15 to 90 min using fixed number of chitosan beads, volume and dye concentration as shown in Figure 6c. It was found that percent removal of CV by Chitosan beads increases with contact time. It is due to the fact that an optimized contact time allows maximum interaction between the adsorbent and the dye. The highest removal was found at 90min beyond which it became constant.
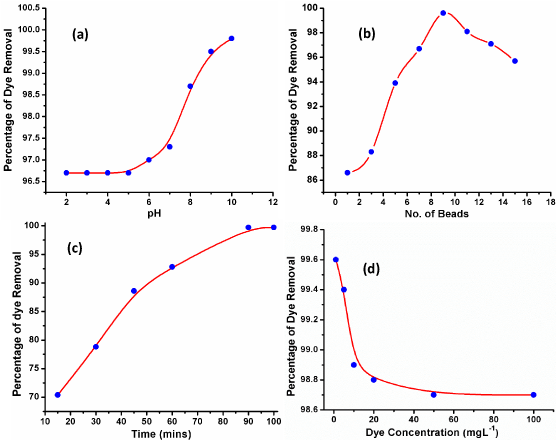
Figure 6: Optimization of adsorption parameters for adsorption of CV over chitosan beads.
Optimization of CV dye concentration: The CV dye concentration was varied to evaluate the maximum sorption capacity of Chitosan. The initial concentration of CV dye was varied from 1-300 mgL-1 at earlier optimized pH, dose and time and the percent removal was studies for each concentration. The results are shown in Figure 6d which reveals the percent removal of CV dye decreases with increase in concentration. The percent decrease is due to the complete occupation of active sites of Chitosan once it adsorbs certain concentration of CV.
Kinetics study
To evaluate the mechanism and kinetics for the adsorption of CV, characteristic adsorption constants were determined using pseudo first order and pseudo second order models. The Lagergren’s pseudo first-order plot [14] and Ho’s Pseudo second-order kinetics plot [15] along with their correlation coefficients are shown in Figure 7a and 7b. The lower value of linear correlation coefficient of pseudo first order in comparison to pseudo second-order kinetics model conclude that the adsorption of CV onto the chitosan beads is not better explained by the pseudo first-order kinetics mechanism. Higher value of the correlation coefficient for pseudo second-order equation suggested that CV could be speedily sequestered by carbon functional groups which results in quickly reaching the system in equilibrium. The confirmation of pseudo second-order kinetics indicates that the adsorbent and adsorbate are both involved in rate-determining step, which may be chemisorption.
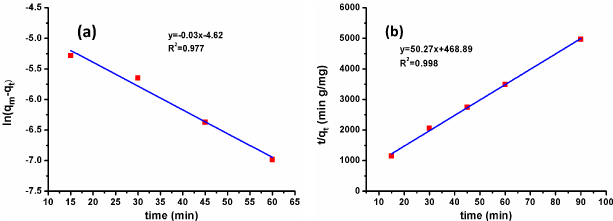
Figure 7: (a) Pseudo first order and (b) Pseudo second order kinetics for the adsorption of CV.
Adsorption isotherm
Adsorption isotherm is required to understand the adsorption process and to limit the adsorption efficiency for the synthesized chitosan beads. In the present investigation, Langmuir and Freundlich isotherm equations [16] are used to explain the adsorption ways of the chitosan beads. The graphs obtained by plotting Langmuir and Freundlich equations are shown in Figure 8a and 8b. The CV experiment adsorption data on the chitosan beads were found to be best fitted to Langmuir model since its correlation coefficient, R²=1 is higher in comparison to that for the Freundlich adsorption isotherm.
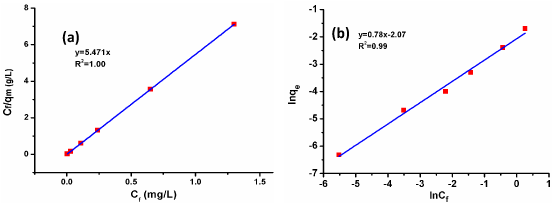
Figure 8: (a) Langmuir and (b) Fruendlich adsorption isotherm for the adsorption of CV.
Mechanism
The amine group in chitosan gets protonated after addition of acetic acid which further gets neutralized after adding NaOH [17]. From pH study, it was found the highest sorption of CV takes place between pH 9.5-10 which results in incorporation of negatively charged -OH groups over the surface of spherical chitosan beads. Further, the structure of CV possesses positively charged ‘N’ atom, so an electrostatic interaction between the negatively charged chitosan beads and the positively charged CV dye facilitates the adsorption of CV over chitosan beads. The probable mechanism for interaction between CV and chitosan has been presented in Figure 9.
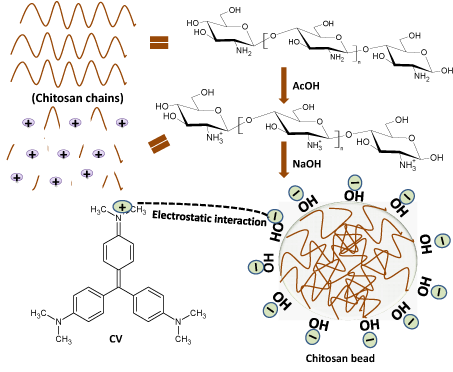
Figure 9: Probable mechanism for interaction between CV and chitosan beads.
Reusability
Reusability study makes the adsorbent practically cost effective and reusable for further adsorption cycles. In reusability study, the Chitosan-CV after adsorption experiments was kept in 1M NaOH solution for reactivation upto 24 hours and then used for further adsorption of CV. Initially 7 beads of Chitosan-CV were used for adsorption of 1mgL-1 to 100mgL-1 CV dye solution (25mL). Interestingly Chitosan-CV beads almost completely remove CV at first cycle. Then the reusable efficiency of Chitosan-CV was tested for higher concentrations and found to have almost good removal efficiency. The reusability efficiency of chitosan beads for multiple adsorption of CV has been presented in Figure 10.
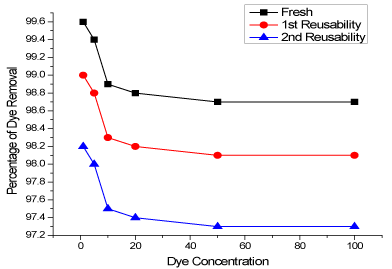
Figure 10: Reusable efficiency of chitosan beads for multiple adsorption of CV.
Conclusion
In this study, the low-cost chitosan beads were prepared and used as an effective sorbent for the multi cycle adsorption of CV dye from water. The synthesized chitosan beads are potentially non-toxic and could be easily separated after adsorption of CV dye. The optimization of adsorption parameters for percentage uptake of CV in aqueous solution was conducted. A set of optimal reaction conditions for CV uptake was obtained as contact time (90min), number of beads [9] and pH (9.5-10). Under optimal values of operating adsorption parameters, highest percentage uptake of dye was achieved to be 99.6%.The CV adsorption experimental data was applied to kinetic and isotherm modelling at various process parameters to analyze the adsorption behavior. The obtained results indicated that the experimental sorption data was found to be best fitted to pseudosecond order kinetics model and Langmuir adsorption isotherm. Overall, Chitosan beads were found to exhibit the potential as an effective sorbent for the CV dye adsorption from wastewater.
Acknowledgement
The authors thank Dr. V. Achanta, Director CSIR-NPL and Dr. Nahar Singh, Head of Division for continuous encouragement and permission to publish the proposed work. The authors also thank Dr. N. Vijyan, Dr. S.P. Singh and Dr. Sukhvir Singh for providing XRD, FT-IR and TEM characterization.
Funding Source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Highlights
• Synthesis of Chitosan Beads in alkaline medium by simple wet chemical route.
• The synthesized beads exhibit strong adsorption affinity for crystal violet dye
• The proposed Chitosan beads can be reactivated several times by keeping beads in NaOH solution for 15 minutes.
• The activated beads can be reutilized upto 8 times for more than 90% removal efficiency.
References
- Padhi B. Pollution due to synthetic dyes toxicity & carcinogenicity studies and remediation. International Journal of Environmental Sciences. 2012; 3: 940.
- Au W, Pathak S, Collie CJ, Hsu T. Cytogenetic toxicity of gentian violet and crystal violet on mammalian cells in vitro. Mutation Research/Genetic Toxicology. 1978; 58: 269-276.
- Mohan SV, Karthikeyan J. Removal of lignin and tannin colour from aqueous solution by adsorption onto activated charcoal. Environmental pollution. 1997; 97: 183-187.
- Mittal A, Gupta V, Malviya A, Mittal J. Process development for the batch and bulk removal and recovery of a hazardous, water-soluble azo dye (Metanil Yellow) by adsorption over waste materials (Bottom Ash and De-Oiled Soya). Journal of hazardous materials. 2008; 151: 821-832.
- Rafatullah M, Sulaiman O, Hashim R, Ahmad A. Adsorption of methylene blue on low-cost adsorbents: a review. Journal of hazardous materials. 2010; 177: 70-80.
- Ramakrishna KR, Viraraghavan T. Dye removal using low cost adsorbents. Water Science and Technology. 1997; 36: 189-196.
- Crini G, Gimbert F, Robert C, Martel B, Adam O, Morin-Crini N, et al. The removal of Basic Blue 3 from aqueous solutions by chitosan-based adsorbent: batch studies. Journal of hazardous materials. 2008; 153: 96-106.
- Rorrer GL, Hsien TY, Way JD. Synthesis of porous-magnetic chitosan beads for removal of cadmium ions from wastewater. Industrial & Engineering Chemistry Research. 1993; 32: 2170-2178.
- Yadollahi M, Farhoudian S, Barkhordari S, Gholamali I, Farhadnejad H, Motasadizadeh H. Facile synthesis of chitosan/ZnO bio-nanocomposite hydrogel beads as drug delivery systems. International journal of biological macromolecules. 2016; 82: 273-278.
- Monshi A, Foroughi MR, Monshi MR. Modified Scherrer equation to estimate more accurately nano-crystallite size using XRD. World Journal of Nano Science and Engineering. 2012; 2: 154-160.
- Guo T-Y, Xia Y-Q, Wang J, Song M-D, Zhang B-H. Chitosan beads as molecularly imprinted polymer matrix for selective separation of proteins. Biomaterials. 2005; 26: 5737-5745.
- Frost R, Griffin R. Effect of pH on Adsorption of Arsenic and Selenium from Landfill Leachate by Clay Minerals 1. Soil Science Society of America Journal. 1977; 41: 53-57.
- Ali I, Gupta V. Advances in water treatment by adsorption technology. Nature protocols. 2006; 1: 2661-2667.
- Yuh-Shan H. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics. 2004; 59: 171-177.
- Ho Y-S, McKay G. Pseudo-second order model for sorption processes. Process biochemistry. 1999; 34: 451-465.
- Dada A, Olalekan A, Olatunya A, Dada O. Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR Journal of Applied Chemistry. 2012; 3: 38-45.
- Ngah WW, Kamari A, Koay Y. Equilibrium and kinetics studies of adsorption of copper (II) on chitosan and chitosan/PVA beads. International journal of biological macromolecules. 2004; 34: 155-161.
