Abstract
Aims: Under the background of carbon peaking and carbon neutrality goals, co-composting kitchen and garden waste using microbial formulations allows the production of high-quality organic fertilizer, which is important for resource recycling.
Methods: The sources of bacteria used in this study were well-fermented chicken manure and garden soil. Enrichment cultures of highly efficient degrading bacteria were carried out under high-temperature conditions. The conditions for the cultivation of the bacterial preparations were optimized.
Results: The activity and number of efficient degrading bacteria increased significantly with increasing enrichment time. After the addition of the bacterial agent, the time it took for the pile to reach a high temperature was reduced from 8 days in the control group to 2 days. More importantly, the rest of the physical and chemical indicators are in line with the relevant standards. The high-throughput sequencing results showed that the number of high-temperature bacteria was 162.5% higher in the high-temperature phase and 81.2% higher at the end of composting than in the control group.
Conclusions: This study addresses the problems of a lack of efficient decomposing bacteria and an insufficient number of high-temperature bacteria in kitchen and garden waste, as well as insufficient research on the preparation of efficient decomposing bacteria through independent screening, and preliminary results were obtained.
Keywords: Co-composting; Kitchen waste; Garden waste; Physiochemical properties; Microbial community
Introduction
Organic solid wastes are organic solid and semisolid wastes generated by humans in production and construction, daily life and other activities, which cannot be used at a certain time and place and are discarded to pollute the environment. The production of organic solid waste has increased substantially in recent years due to the growing population and the rising standard of living in our country. When such a large amount of waste is disposed of using the landfill method, it not only encroaches on land resources; in the event of a leachate leak, the remaining harmful toxins are not broken down naturally and can also upset the balance of the soil ecosystem. This hinders the growth and development of plant roots in the surrounding area. If toxic substances accumulate in plants and enter the human body through the food chain, they can cause diseases such as cancer, which can seriously affect the healthy development of human beings.
Kitchen waste and garden waste are two common types of typical organic waste. Kitchen waste refers to food that is considered fit for consumption but wasted or lost in family or restaurant routines, enriched with organic matter such as starch, protein, fibre and fat [4,17]. The consumption and disposal of kitchen waste has become a widely debated global issue in the 21st century [5]. Based on this situation, many developed countries have attached great importance to the treatment of kitchen waste. They conducted early research on this issue to develop comprehensive kitchen waste treatment systems [9]. In May 2019, the Ministry of Ecology and Environment of the People's Republic of China launched the "Zero Waste City" campaign to promote waste reduction, improve the resourceful use of solid waste, and minimize the amount of solid waste going to landfills.
Kitchen waste is difficult to recycle because of its high-water content, organic content and high nutrient content [32]. The high C/N content and loose structure of garden waste can solve the problem of the sticky nature of kitchen waste and the need for additional carbon sources in composting [30]. By mixing kitchen waste with garden waste, the composted product can be used as organic fertilizer for garden and agricultural production [11]. However, there are currently few reports on the combined composting of kitchen waste and garden plant waste [3,15].
This is because at all stages of the composting process, the microbial community has a strong influence on the composting process [21,27]. Therefore, the aim of this study was to investigate effective microbial formulations for the co-composting of kitchen and garden waste and to determine the evolution of the microbial composition during the composting process and the physicochemical characteristics of the compost quality.
Materials and Methods
Material Source and Composting Operation
Fresh kitchen and garden waste was used as the input substrate in this experiment. The kitchen waste was collected from an aerobic composting plant in the district of the cotton stream in Qingdao, China. Before composting trials were carried out, garden waste was produced from yard trimmings, wood chips and tree branches, which were then cut to the appropriate size.
A co-composting trial was carried out with a mixture of kitchen and garden waste. The kitchen waste was mixed with garden waste, the moisture content was set at 60%, and the initial temperature was set at 55 °C before composting began. The composting process was repeated over a period of several days until the Germination Index (GI) of the composted samples exceeded 60%. During the composting process, its physicochemical characteristics were determined, and microbiological analysis was carried out.
Physicochemical Analyses
Temperature was measured by inserting a thermometer 25 cm below the material and measuring the temperature of the compost as well as the ambient temperature. The moisture content was determined by taking a 5 g sample of compost in a crucible and drying it in an oven at 105°C for 24 h. The moisture content of the compost sample was calculated from the difference in mass between the before and after samples. Total Kjeldahl nitrogen (TKN) was determined by steam distillation of a 0.5 g sample according to the AFNOR T90-1110 standard using the classical Kjeldahl method.
Microbial Community Analyses
Following the description of the method in previous studies, all compost samples were preprocessed [2]. The DNA samples were stored at -20°C. The purity and concentration of the extracted DNA samples were determined using a NanoPhoto metre with purity values between 1.8 and 2.0. In addition, the primer sets= 338F (5'-ACTCCTACGGGAGGCAGCAG-3') and 806R (5'-GGACTACHVGGGTWTCTAAT-3') was used to amplify the V3 and V4 regions of the bacteria.
Results
Enrichment of Bacteria from Co-composting of Kitchen and Garden Waste
To obtain native microorganisms for the development of a microbial agent for co-composting, samples from kitchen waste and garden waste composting were used as a source of beneficial bacteria [16]. In addition, enrichment cultures of high temperature-degrading bacteria were carried out at a temperature of 50°C and 160 r/min, and the amount of organic matter in the culture solution was measured every 24 hours. As shown in Figure 1, the enrichment process lasted for 4 cycles, 34 days in total. The CODCr content in the culture solution was approximately 18,000 mg/L at the start of the enrichment process and stabilized at approximately 3,500 mg/L at the end of the degradation process. As the enrichment time increased, the degradation rate of organic matter gradually increased. By the fourth cycle, the highest degradation rate of organic matter was 6840 mg/L/d, indicating that the activity and number of microorganisms efficiently degrading organic matter in the culture solution increased significantly.
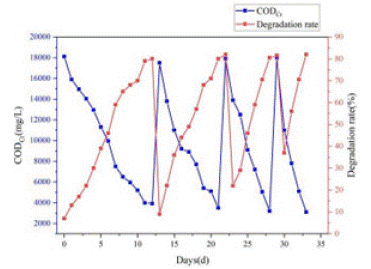
Figure 1: Changes of organic matter in the cultured fluid.
High-throughput sequencing of a mixture of the final enrichment broth and the seven selected high-efficiency degraders was carried out. As shown in Table 1, the dominant genera in the agent included Aneurinibacillus_sp., uncultured_bacterium, Paenibacillus_dendritiformis, Luteimonas compost and uncultured_prokaryote. Of these, Aneurinibacillus_sp. was the genus with the highest relative abundance at 22.0%. This was consistent with the performance of the screened agents, indicating that the experimentally screened strains can still show high activity when mixed with the original enrichment solution.
Genus
Percentage
Aneurinibacillus_sp
22.0%
Paenibacillus_dendritiformis
12.8%
uncultured_bacterium
11.4%
Luteimonas compost
9.7%
uncultured_prokaryote
8.9%
Table 1: The relative abundance of dominant bacteria in microbial preparation samples.
Optimization of Culture Conditions for High-Efficiency Microbial Agents
The effect of nitrogen sources on microbial agent activity: Composites have different uptake rates of different nitrogen sources. The addition of all four organic nitrogen sources was effective in increasing the number of cells in the culture medium compared to the control group without a nitrogen source. The experimental group in which tryptone was added had the highest number of cells. This indicates that tryptone has the strongest ability to promote an increase in cell numbers.
Moreover, the effect of different nitrogen sources on the utilization of organic matter by microorganisms varied. Using tryptone as the nitrogen source, the highest rates of organic matter degradation were observed during incubation. Over 50% of the organic matter was degraded after 12 hours of incubation compared to 15.3% for the control group. The organic matter degradation rates of the four nitrogen sources (tryptone (T1), peptone (T2), yeast powder (T3) and maize pulp powder (T4)) after 48 h of incubation were 65.8%, 58.3%, 55.5% and 41.3%, respectively, as shown in Figure 2. Therefore, tryptone was chosen as the best nitrogen source for the subsequent experiments. Different concentrations of tryptone were set, and the number of cells in the culture was measured after 48 h of incubation to determine the optimum concentration of tryptone. As shown in Figure 2, the number of cells in the culture solution increased and then decreased as the concentration of tryptone increased. The highest cell count was observed when the tryptone concentration was 12 g/L. Therefore, the optimum concentration of tryptone was 12 g/L.
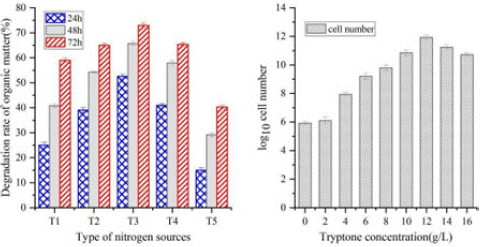
Figure 2: (a) Effect of different nitrogen sources on the rate of organic matter degradation by microorganisms; (b) The effect of different tryptone concentrations on bacterial concentrations (tryptone (T1), peptone (T2), yeast powder (T3), maize pulp powder (T4) and control group (T5)).
The effect of carbon sources on microbial agent activity: Carbon is one of the main components of the culture medium, as it is the main component of the cells that make up the bacteria. It provides the energy needed for the microorganisms to grow, which affects the number of microorganisms in the culture medium. Glucose, lactose, sucrose, maltose and starch are all carbon sources that are readily available to microorganisms. As shown in Figure 3, the experimental group with the addition of glucose had the best promotion of cell numbers, with a 5.9% increase over the highest value in the control group and a 16.4% increase over the number of cells before optimization. Therefore, glucose was chosen as the optimal carbon source for the subsequent experiments.
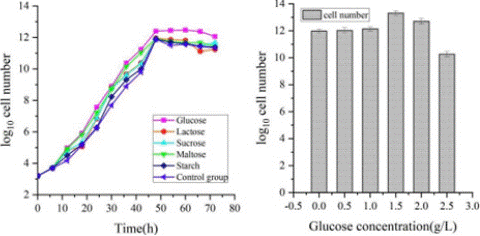
Figure 3: (a) Effect of different carbon source to cell number; (b) Effect of different glucose concentration to cell number.
Different concentrations of glucose were set, and the number of cells in the culture solution was measured after 48 hours of incubation to determine the optimal glucose concentration. As shown in Figure 3, as the glucose concentration increased, the number of cells in the culture solution increased and then decreased. When the glucose concentration was 1.5 g/L, the number of cells in the culture solution increased by 12.3% compared to the control group. Therefore, the optimal glucose concentration was 1.5 g/L.
Physicochemical Parameters of Materials in the Co-composting Process
In the experimental group, the temperature inside the pile was 14.3 on Day 1 and reached 50 on Day 6, which was maintained until Day 14. After Day 14, the pile temperature was below 35 and close to room temperature, indicating that the co-composting process was almost complete. In the control group, the maximum fermentation temperature was only 29.3 throughout the co-composting process, which was much lower than in the experimental group, and the temperature exceeded 25 for only 5 days, which is shown in Figure 4.
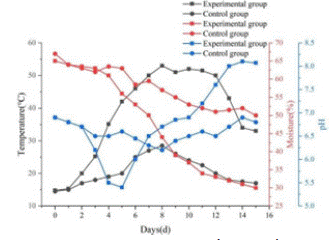
Figure 4: Temperature, moisture, pH changes during co-composting of kitchen waste and garden waste.
The initial pH value was 6.8, which then decreased to 5.4 on the 3rd day and subsequently gradually increased. Finally, the pH was stable between 8.0 and 8.5, which is in line with the standard for organic fertilizer pH.
Moisture is a carrier of soluble nutrients required for the metabolic and physiological activities of microorganisms. The initial moisture content of the co-composted material was approximately 65%. As the composting time increased, it gradually decreased to 27.6%, which was the optimum moisture content for microbial decomposition. Compared to the control group, the moisture content of the composted material in the experimental group decreased more.
As shown in Table 2, during this process, the EC increased from 3.21 to 3.78 mS·cm-1, both of which were less than 4.0 mS·cm-1, and the compost products all reached a basic level of decomposition. During the co-composting process, the GI value gradually increased, eventually reaching 67.3%. This indicates that the co-composting of kitchen and garden waste has finally reached maturity.
Days (d)
EC (mS·cm-1)
GI (%)
0
3.21
4.7
2
2.94
10.9
4
3.03
19.3
6
3.15
27.2
8
3.21
40.5
10
3.39
58.6
12
3.45
60.9
14
3.52
62.1
16
3.67
64.5
18
3.73
66.2
20
3.78
67.3
Table 2: EC, GI changes during co-composting of kitchen waste and garden waste.
Microbial Community Profile in Co-composting Systems
Fresh kitchen waste was first used as the raw material for composting under optimized culture conditions. The developed compound microbial agent was mixed with kitchen waste in a fermentation reactor for a small-scale trial. The organoleptic changes in the compost material were observed, and the temperature, moisture and pH of the fermentation process were measured. As shown in Figure 5, a pilot composting trial was carried out at the aerobic composting plant in the district of the cotton stream in Qingdao, China, where the compost was mechanically turned and stacked. Compound bacterial agents were added to the kitchen waste, and changes in temperature, organic matter, total nutrient content and microbial indicators during composting were measured. Based on the results of the two experimental phases, the small and pilot tests, the microbial agent prepared in this study can effectively promote the aerobic composting process of kitchen waste.

Figure 5: Strip composting diagram of a sanitation company in Qingdao, with kitchen and garden waste co-composting on site.
The diversity of the bacterial communities was studied using the Ace, Chao1, coverage, Shannon's and Simpson's diversity indices to better understand the changes in the microbial communities in the co-composting treatments. A total of 127698 sequences were obtained from the sequenced samples. The results showed that the Chao1 and Ace indices of the bacteria increased from D1 to D3 (Table 3). The bacterial Shannon indices increased gradually after the start of the co-composting, and the change in Simpson diversity indices was small. Furthermore, the coverage index for all three compost samples exceeded 0.99, indicating high community coverage in the samples.
Sample number
Shannon
Simpson
Ace
Chao1
D1
3.20
0.09
210.52
223.46
D2
4.10
0.10
1101.26
1088.01
D3
5.32
0.01
1139.04
1124.33
Table 3: The a diversity index of bacteria of different compost samples.
The relative abundances of the bacterial phyla during the co-composting processes are shown in Figure 6. The two major phyla showing the greatest relative abundance were Firmicutes and Proteobacteria, with relative abundances of 52.67% and 17.69%, respectively. Moreover, it was clear that Chloroflexi was the most abundant bacterial genus in the D1 sample at the start of co-composting, with a relative abundance of 24.13%. After the start of co-composting, there was a significant change in the bacterial composition. From D1 to D3 of co-composting, the dominant group in the samples changed to Firmicutes.
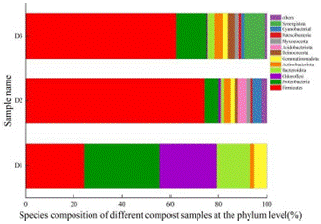
Figure 6: Relative abundance of bacteria at phylum level in different compost samples.
At the species level, the bacterial relative abundance in the samples contained significantly more efficient bacteria in the high-temperature phase than in the precomposting phase. As shown in Table 4, in the high-temperature phase, Aneurinibacillus_sp., Ureibacillus_thermosphaericus, Paenibacillus_dendritiformis and Trueperaradiovictrix were abundant in both groups, accounting for 35.41%, 11.2%, 5.6% and 5.2% of the total genera, respectively, all of which were efficient degrading bacteria screened in this experiment. The proportion of species with a relative abundance less than 1% in the high-temperature phase was 12.5%, indicating that the proportion of dominant bacteria in the associated material could be effectively increased by inoculation with exogenous microorganisms. At the end of co-composting, the dominant bacterial groups of D3 were thoroughly different from those of D1.
Sample stage Genus
Percentage
unclassified_g norank_fA4
6.50%
nclassified_g norank_fnorank_oSBR1031
5.80%
unclassified_g Prevotella
4.80%
uncultured_bacterium_gTruepera
4.60%
Aneurinibacillus_sp
35.41%
Ureibacillus_thermosphaericus
11.20%
Paenibacillus_dendritiformis
5.60%
Trueperaradiovictrix
5.20%
Aneurinibacillus_sp
11.30%
Sam3 nclassified_gnorank_fnorank_oSBR1031
6.30%
Ureibacillus_thermosphaericu
5.40%
Table 4: Relative abundance of bacteria at species level in different compost samples.
Discussion
Enrichment of Bacteria from Co-composting of Kitchen and Garden Waste
Due to insufficient numbers of efficient degrading bacteria in kitchen waste, traditional aerobic composting is slow to heat up, and the composting effect is poor. The addition of microbial agents to composting material can effectively increase the proportion of efficient degrading bacteria in the composting material, thus shortening the composting time and improving the quality of the composted product.
High-temperature bacteria are microorganisms that can grow under high-temperature conditions, and their suitable growth temperature is 40-60°C. The high-temperature bacteria can grow well in the temperature range of 50-65°C, which is higher than the majority of strains studied by López MJ et al. that grew well at 40-50°C [14] Therefore, the inoculation of high-temperature bacteria in compost can increase the reaction rate, promote the degradation of organic matter, extend the high-temperature phase of compost and promote the decomposition of materials.
Aneurinibacillus_sp. is a gram-positive microbial agent among the highly efficient degrading bacteria obtained from this experimental enrichment. This is consistent with Aneurinibacillus sp. isolated and screened by Pernicova I et al. from soil samples collected from urban composting sites, the same thermophilic bacterium [18]. It showed a high degrading capacity during the passaging process, indicating that the microbial agent has a good degrading effect on organic matter. Salt tolerance and the ability to degrade high concentrations of oil and grease show that the microbial agent has the potential to be used in the aerobic composting of kitchen waste. In addition, Luteimonas compost degrades proteins and belongs to the same genus of thermophilic bacteria as Luteimonas spp. isolated from food waste by Young et al [29]. Phylogenetic analysis using 16S rRNA gene sequences showed that the microbial agent is able to use organic acids to synthesize substances for its own use. Paenibacillus_dendritiformis is a cellulose-degrading strain, consistent with Bacillus arbuscularis isolated from mangrove soil samples by Sandrasekaran et al [23]. Its optimum cellulose hydrolysis temperature is 55 °C, and Mg2+ and Ca2+ increase cellulase activity.
The enzymatic activity and degradation of organic matter of most of the composite flora was better than that of any single strain of the composite flora. The inoculated piles had the fastest heating rate and the highest temperature during the high temperature period. The microorganisms in the composite were also found to be more synergistic and better adapted to the complex environment of kitchen waste.
Optimization of Culture Conditions for High-Efficiency Microbial Agents
It is common knowledge that microorganisms are the key drivers in the composting process. Therefore, it is of practical importance to optimize the culture conditions of the combined bacterial agent, improve the activity of functional bacteria and their ability to resist environmental changes, and fully utilize functional bacteria for their beneficial effects in the composting process.
Carbon sources are both essential structural materials for cells and metabolites and are also the raw material for the energy required to sustain the growth and metabolism of bacteria [14]. The best source of carbon for this experiment was glucose, whose optimum concentration is 1.5 g/L. This differs from the findings of Lai W H et al. on Lignosusrhinocerus and Hamedi A et al. on Agaricusblazei [8,12]. This is due to the varying physiological characteristics of the different bacteria involved in the composting process. Therefore, the type and concentration of carbon required are not identical.
Nitrogen sources are the basis for proteins, nucleic acids and other nitrogen-containing compounds in organisms. Nitrogen sources are the basis for proteins, nucleic acids and other nitrogen-containing compounds in organisms and play a vital role in the production of target products by microorganisms. Nitrogen sources can be divided into organic and inorganic nitrogen. The microbial agent produced in this study used organic nitrogen relatively well compared to inorganic nitrogen. This was consistent with the results of Ramesh et al. for strain R006 and Lee et al. for Paecilomyces japonica [13,24]. This may be because organic nitrogen is nutrient rich and complex and contains other growth factors in addition to nitrogen. In this experiment, the best nitrogen source was tryptone, which was most effective in promoting composting at a concentration of 12 g/L.
After optimization of the culture conditions, the number of cells in the culture solution was 34.1% higher and the organic matter degradation rate was 16.9% higher at 48 h compared to the control group. This is because glucose is a monosaccharide, tryptone is high in protein and total N, and its organic matter is more readily utilized by the microorganisms in the broth. The optimal carbon to nitrogen ratio for this experiment was lower than that of Yang and Petric et al. Higher C/N ratios result in slower composting rates and have a tendency to develop odours [19,28].
PH has a substantial effect on the growth of microorganisms because most of the enzymatic reactions that occur in microorganisms during growth require an appropriate pH range. The rate of enzymatic reactions is maximized only within the correct pH range, below or above which the growth of microorganisms is inhibited. pH affects the permeability of the cytoplasmic membrane through the permeability of the cytoplasmic membrane, the stability of the membrane structure, the solubility of substances and the redox potential of the surface charge, which affects the uptake of nutrients and the growth rate of organisms. The final optimized pH was found to be 5.5-7.5. This was consistent with the results of Zhang and Sun et al [31]. Compared to the preoptimization period, the optimized bacterial agent increased the number of cells in the culture solution by 34.1% and the organic matter degradation rate by 16.9% after 48 hours of incubation.
Therefore, optimizing the microbial medium and culture conditions for different bacteriological agents and their different applications is important to improve the concentration and activity of efficient bacteria in bacteriological agents and to promote the application and promotion of bacteriological agents.
Physicochemical Parameters of Materials in the Co-composting Process
To characterize the aerobic co-composting process, physicochemical indicators such as temperature, pH, moisture, EC and GI are commonly used.
Temperature is a key parameter in the control of the various biochemical processes that take place during composting. In terms of killing pathogens, raising the composting temperature to the thermophilic range is considered satisfactory [20]. In this study, the control group had a much lower maximum fermentation temperature and a much lower duration of fermentation throughout the co-composting process than the experimental group. The highly efficient compound bacterial agent prepared in this experiment can effectively improve the rate of aerobic composting of kitchen waste and extend the duration of high temperature in the composting stage, which is beneficial to the aerobic composting of kitchen waste.
During the composting process, the pH of the pile first dropped and then rose. This was probably because at the beginning of the temperature increase, a large amount of organic matter in the material was degraded into small molecule organic acids, resulting in a significant drop in the pH of the material. Then, as the co-composting temperature increased, the small molecule organic matter was further degraded, and ammonia was produced, causing the pH of the material to rise gradually.
In the experiment, the moisture content of the composting material was greatly reduced in the experimental group compared to the control group. This may be closely related to the temperature changes in the composting material. During the turning process, the high temperatures caused a large amount of water to evaporate from the material, and the moisture content of the experimental group decreased rapidly.
The EC value reflects the soluble salt content of the compost and gives some indication of the inhibitory and toxic effects on plant growth when applied to soil. The EC values also illustrate the quality of plant-growing compost [10]. In all treatments, EC and pH showed an inverse relationship. In this experiment, the increase in the EC may be due to the relatively high concentration of soluble salts in the raw material and the associated loss of moisture. The final EC of the samples was in accordance with the composting standards of Hong Kong and Australia. The GI is a widely used indicator of compost maturity, as it provides an immediate measure of the plant toxicity of compost products on the germination and growth of seeds [26]. As a key parameter for assessing compost maturity, GI thresholds are clearly defined in relevant standards, such as the Quality Standards for Compost and Soil Amendments. In this study, the final GI value was 67.3%. In general, a GI value above 65% indicates a high level of phytotoxicity and maturity of the compost. Taking into account the characteristics discussed above, the chosen maturity parameters indicated that the co-composting of kitchen and garden waste finally reached maturity.
And the correlation heat map was plotted by calculating the correlation coefficients (Spearman's rank correlation coefficients) between the environmental factors and the top 50 representative species, and the results are shown in Figure 7. In descending order of number of phyla affected, pH, moisture, C/N, temperature. The main environmental factors affecting the structural genus level composition of bacterial communities were pH, moisture and C/N. Microbial communities had the same correlation with pH and C/N and the opposite correlation with moisture. The pH and C/N were significantly positively correlated with 17 bacterial genera and was significantly negatively correlated with 7 bacterial genera. Moisture was significantly positively correlated with 7 bacterial genera and was significantly negatively correlated with 17 bacterial genera. Acinetobacter, Flavobacterium, Comamonas, Symbiobacterium and unclassified_f Bacillaceae were negatively correlated with temperature and were cryophilic. Norank_f A4b, norank_f norank_o SBR1031 and unclassified_f Blastocatellaceae, etc. were positively correlated with temperature and were thermophilic.
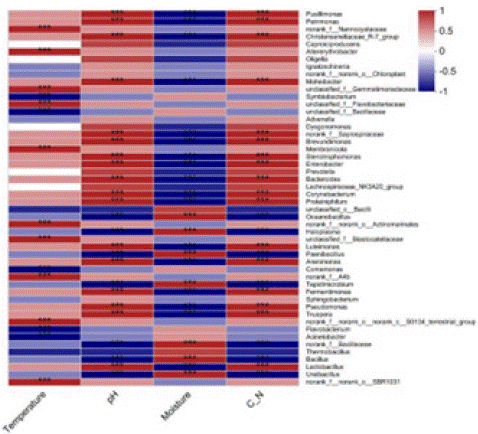
Figure 7: Correlation analysis between bacterial communities and environmental factors during co-composting of kitchen waste and garden waste.
Microbial Community in Co-composting Systems
Clustering is based on the distribution of species relative abundance and community similarity between samples, allowing high- and low- relative abundance species to be clustered separately. In addition to displaying species composition and relative abundance, the similarity and variability of community composition can be directly visualized through colour changes. The top 50 most abundant genera in the three samples (D1, D2 and D3) were selected for the heatmap, as shown in Figure 8. The dominant genera in the different periods of composting were Ureibacillus, norank-f-norank-o-SBR1031, and Lactobacillus.
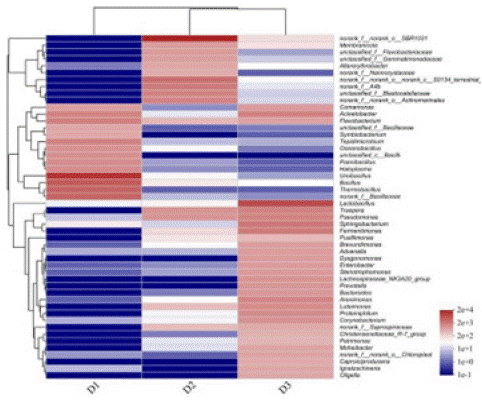
Figure 8: Heatmap of genus-level bacterial communities during co-composting of kitchen waste and garden waste.
A highly diverse community of bacteria was detected in the samples after the start of co-composting. The dominant bacterial phyla in all treatment groups were Firmicutes (the ratios of D1, D2, and D3 in Firmicutes were 88%, 1.1%, and 27%) and Proteobacteria (6.6%, 14%, and 33%), followed by Chloroflexi (0.0023%, 47%, and 0.38%) (Figure 9). These results are consistent with the data proposed by Wang et al. [25] who also found that Proteobacteria and Firmicutes were the predominant flora in the stages of sludge co-composting. The presence of this phylum reflects their capability to metabolize different substrates during composting, including lipids, sugars, cellulose, proteins, lignin and amino acids, as Firmicutes can produce various extracellular enzymes [1]. The genus is ubiquitous in lignocellulose-based composting, contributing to waste degradation due to its heat resistance [6]. Firmicutes dominance during the thermophilic phase may be due to their ability to survive thermophilic conditions [7].
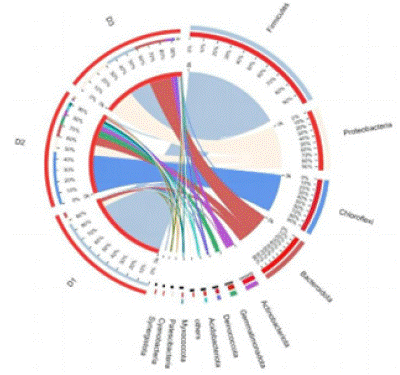
Figure 9: Composition and relative abundance at the bacterial phylum level during co-composting of kitchen waste and garden waste.
The structural composition of the bacterial community in the compost samples showed significant differences at different composting stages. The number of effective bacteria can influence the heating rate of the compost material and the duration of the high-temperature phase. In this study, the number of high-temperature bacteria in the material of the experimental group was higher than that of the control group at the end of the high-temperature phase and composting. This indicates that the dominant bacteria in the developed microbial agent could further increase the number of high-temperature bacteria in the composting material and promote the aerobic composting process of kitchen waste. The Venn diagram provides a visual indication of the similarities and differences in microbial community composition between the samples. As shown in Figure 10, there is a large overlap between the dominant bacteria in the developed bacterial agents (T1), in the high-temperature phase of co-composting (T2) and in the material at the end of co-composting (T3). The number of OTUs shared by the three samples was 15, representing 15.96% of the total number of OTUs. This shows that the prepared microbial agent can play a role in the composting process and that the microbial agent can increase the dominance of efficient bacteria in the community structure.
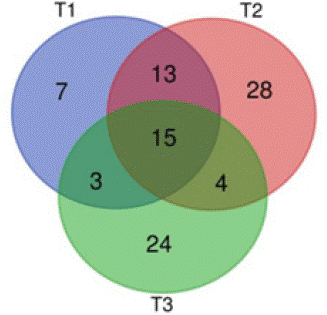
Figure 10: Overlap of dominant bacteria in the samples during co-composting of kitchen waste and garden waste.
FAPROTAX function prediction is based on a database that contains a classification of functional information of prokaryotic species to predict the function of bacterial data, and FAPROTAX functions include carbon, nitrogen, hydrogen and sulfur cycles, etc. To further explore the functional changes of the bacterial community in the endocyclic aquaculture system under different operating conditions, FAPROTAX function prediction of the bacterial community was carried out and the results are shown in Figure 11. The results showed that the bacteria had a total of 57 functions. And that the bacterial community was dominated by chemoenergetic heterotrophs with percentages of 28.7%, 30.6% and 30.2% respectively. This is due to the fact that the organic matter in the pile is the main source of energy for the biofilm bacterial community, leading to the proliferation of chemoenergetic heterotrophic bacteria as the dominant colony. Secondly, bacteria associated with the nitrogen cycle. This could be due to the predominance of Firmicutes and Proteobacteria in the microbial community, with both genera actively involved in N removal processes [22].
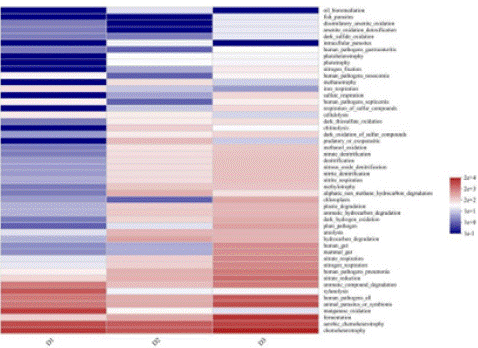
Figure 11: FAPROTAX function prediction of microorganisms in the samples during co-composting of kitchen waste and garden waste.
Conclusions
In this study, we investigated an efficient microbial preparation for co-composting kitchen and garden waste to support the application of liquid complex microbial agents in composting. In this study, the activity and number of highly efficient degrading bacteria increased significantly with the enrichment time, and the enrichment process lasted for a total of 34 days over 4 cycles. By optimizing the carbon and nitrogen sources and concentrations of the medium, the optimal medium components were determined to be tryptone 12 g/L and glucose 1.5 g/L. Compared with the preoptimized medium, the number of cells in the culture medium increased by 34.1%, and the organic matter degradation rate increased by 16.9% at 48 h. The developed high-efficiency microbial agent can shorten the heating time of the composting material and extend the duration of the high-temperature phase. In the small-scale experiment, the material temperature of the experimental group exceeded 50 on the 6th day of fermentation, and the duration of the high temperature stage was 6 d. In the medium-scale experiment, the material temperature of the experimental group exceeded 50 after 2 d of composting, which was 4 d earlier than in the small-scale experiment and 6 d earlier than in the control group. In addition, the total nutrient and organic matter contents of the compost from all treatments in this experiment were higher than the values specified in the national standard for organic fertilizers, and the components in the control group did not meet the requirements for organic fertilizers. The main dominant strains at the high-temperature stage and at the end of composting were Aneurinibacillus_sp., Ureibacillus_thermosphaericus, Paenibacillus_dendritiformis, and Trueperar adiovictrix. The percentages at the high-temperature stage were 35.4%, 11.2%, 5.6% and 5.2%, respectively, and 11.3%, 5.4%, 4.6% and 4.5% at the end of the composting period, which reflected the efficient degrading bacteria screened in this experiment. The highly efficient microbial agent prepared in this experiment promotes the aerobic composting process of kitchen waste.
Author Statements
Author Contributions
Conceptualization, Ailing Xu; methodology, Jianbo Pang; formal analysis, Lihua Qi; investigation, Qian Jiao; resources, Jianbo Pang; data curation, Xianxin Wang; writing—original draft preparation, Lihua Qi; writing—review and editing, Ailing Xu; visualization, Lihua Qi; supervision, Ailing Xu; project administration, Ailing Xu; funding acquisition, Ailing Xu. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Foundation of Shandong Natural Science Foundation (ZR2023MC193). We also thank the reviewers for their remarks and suggestions.
Data Availability Statement
The data presented in this study are available in [Rapid Co-Composting of Kitchen Waste and Garden Waste through Microbial Community Structure Modulation].
Acknowledgments
Thank you for the experimental materials provided by the Key Laboratory of Eco-environmental Engineer and Pollution Remediation in Shandong Province.
Conflicts of Interest
The authors declare no conflict of interest. The manuscript does not contain experiments using animals. The manuscript does not contain human studies. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Arab G, Razaviarani V, Sheng Z, Liu Y, McCartneyD. Benefits to decomposition rates when using digestate as compost co-feedstock: Part II – Focus on microbial community dynamics. Waste Management. 2017; 68: 85-95.
- Song C, Li M, Jia X, Wei Z, Zhao Y, Xi B, et al. Comparison of bacterial community structure and dynamics during the thermophilic composting of different types of solid wastes: anaerobic digestion residue, pig manure and chicken manure. Microbial biotechnology. 2014; 7: 424-433.
- Chen Z, Li Y, Peng Y, Mironov V, Chen J, Jin H, et al. Feasibility of sewage sludge and food waste aerobic co-composting: Physicochemical properties, microbial community structures, and contradiction between microbial metabolic activity and safety risks. The Science of the total environment. 2022; 825: 154047.
- Chenxi J, Sun S, Yang D, Sheng W, Ma Y, He W, et al. Anaerobic digestion: An alternative resource treatment option for food waste in China. Science of the Total Environment. 2021; 779: 146397.
- Feo GD, Ferrara C, Iannone V, Parente P. Improving the efficacy of municipal solid waste collection with a communicative approach based on easily understandable indicators. Science of the Total Environment. 2018; 651: 2380-2390.
- De Gannes V, Eudoxie G, Hickey WJ. Prokaryotic successions and diversity in composts as revealed by 454-pyrosequencing. Bioresource Technology. 2013; 133: 573-80.
- Guangming R, Xu X, Qu J, Zhu L, Wang T. Evaluation of microbial population dynamics in the co-composting of cow manure and rice straw using high throughput sequencing analysis. World journal of microbiology & biotechnology. 2016; 32: 101.
- Hamedi A, Ghanati F, Vahidi H. Study on the effects of different culture conditions on the morphology of Agaricus blazei and the relationship between morphology and biomass or EPS production. Annals of Microbiology. 2012; 62: 699-707.
- Julian P, Barthel M, Macnaughton S. Food waste within food supply chains: quantification and potential for change to 2050. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 365: 3065-81.
- Karak T, Bhattaharyya P, Paul RK, Das T, Saha SK. Evaluation of Composts from Agricultural Wastes with Fish Pond Sediment as Bulking Agent to Improve Compost Quality. CLEAN – Soil, Air, Water. 2013; 41: 711-723.
- Kim E, Lee J, Han G, Hwang S. Comprehensive analysis of microbial communities in full-scale mesophilic and thermophilic anaerobic digesters treating food waste-recycling wastewater. Bioresource Technology. 2018; 259: 442-450.
- Lai WH, Salleh SM, Daud F, Zainal Z, Othman AM, Saleh NM. Optimization of Submerged Culture Conditions for the Production of Mycelial Biomass and Exopolysaccharides from Lignosus rhinocerus (Pengoptimuman Kultur Tenggelam untuk Penghasilan Biojisim Miselium dan Eksopolisakarida Lignosus rhinocerus). Sains Malaysiana. 2014; 43: 73-80.
- Lee JS, Jung WC, Park SJ, Lee KE, Shin WC, et al. Culture conditions and medium components for the production of mycelial biomass and exo-polysaccharides with Paecilomyces japonica in liquid culture. Journal of Bioscience and Bioengineering. 2013; 115: 433-7.
- López MJ, Jurado MM, Lopez-Gonzalez JA, Estrella-Gonzalez MJ, Martinez-Gallardo MR, Toribio A, et al. Characterization of Thermophilic Lignocellulolytic Microorganisms in Composting. Frontiers in microbiology. 2021; 12: 697480.
- Manu MK, Wang C, Li D, Varjani S, Xu Y, Ladumor N, et al. Biodegradation kinetics of ammonium enriched food waste digestate compost with biochar amendment. Bioresource Technology. 2021; 341: 125871.
- Morales AB, Bustamante MA, Marhuenda Egea FC, Moral R. Agri-food sludge management using different co-composting strategies: study of the added value of the composts obtained. Journal of Cleaner Production. 2016: 121.
- Wang N, Huang D, Shao M, Sun R, Xu Q. Use of activated carbon to reduce ammonia emissions and accelerate humification in composting digestate from food waste. Bioresource technology. 2022; 347: 126701.
- Sedlacek P, Pernicova I, Novackova I, Kourilova X, Kalina M, Kovalcik A, et al. Introducing the Newly Isolated Bacterium Aneurinibacillus sp. H1 as an Auspicious Thermophilic Producer of Various Polyhydroxyalkanoates (PHA) Copolymers–1. Isolation and Characterization of the Bacterium. Polymers. 2020; 12: 1298.
- Petric I, Papracanin EA, Ibric N. Numerical simulation of composting process for mixture of organic fraction of municipal solid waste and poultry manure. Ecological Engineering. 2015; 75: 242-249.
- Rashad FM, Saleh WD, Moselhy MA. Bioconversion of rice straw and certain agro-industrial wastes to amendments for organic farming systems: 1. Composting, quality, stability and maturity indices. Bioresource Technology. 2010; 101: 5952-60.
- Ravindran B, Nguyen DD, Chaudhary DK, Chang SW, Kim J, Lee SR, et al. Influence of biochar on physico-chemical and microbial community during swine manure composting process. Journal of Environmental Management. 2019; 232: 592-599.
- Rìos-Montes KA, Casas-Zapata JC, Briones-Gallardo R, Penuela G. Optimal conditions for chlorothalonil and dissolved organic carbon in horizontal subsurface flow constructed wetlands. Journal of environmental science and health. Part. B, Pesticides, food contaminants, and agricultural wastes. 2017; 52: 274-281.
- Naresh S, Kunasundari B, Gunny AAN, Teoh YP, Shuit SH, Ng QH, et al. Isolation and Partial Characterisation of Thermophilic Cellulolytic Bacteria from North Malaysian Tropical Mangrove Soil. Tropical life sciences research. 2019; 30: 123-147.
- Veluchamy R. Optimization of submerged culture conditions for mycelial biomass production with enhanced antibacterial activity of the medicinal macro fungus Xylaria sp. Strain R006 against drug resistant bacterial pathogens. Current Research in Environmental & Applied Mycology. 2014; 4.
- Wang K, Mao H, Li X. Functional characteristics and influence factors of microbial community in sewage sludge composting with inorganic bulking agent. Bioresource Technology. 2018; 249: 527-535.
- Wang X, Selvam A, Wong JWC. Influence of lime on struvite formation and nitrogen conservation during food waste composting. Bioresource Technology. 2016; 217: 227-32.
- Wei H, Wang L, Hassan M, Xie B. Succession of the functional microbial communities and the metabolic functions in maize straw composting process. Bioresource Technology. 2018; 256: 333-341.
- Yang F, Li G, Shi H, Wang Y. Effects of phosphogypsum and superphosphate on compost maturity and gaseous emissions during kitchen waste composting. Waste Management. 2015; 36: 70-6.
- Young CC, Kampfer P, Chen WM, Yen WS, Arun AB, Lai WA, et al. Luteimonas composti sp. nov., a moderately thermophilic bacterium isolated from food waste. International journal of systematic and evolutionary microbiology. 2007; 57: 741-744.
- Yu Q, Chen L, Wang W, Wang Q, Bai R, Zhuang X, et al. Impact of blending on hydrolysis and ethanol fermentation of garden wastes. Journal of Cleaner Production. 2018; 190: 36-43.
- Zhang L, Sun X. Influence of bulking agents on physical, chemical, and microbiological properties during the two-stage composting of green waste. Waste Management. 2016; 48: 115-126.
- Zhicheng X, Ma Y, Zhang L, Han Y, Yuan J, Li G, et al. Relating bacterial dynamics and functions to gaseous emissions during composting of kitchen and garden wastes. Science of The Total Environment. 2021; 767: 144210.
