Abstract
The estuary is one of the most variable ecosystems due to the influence of freshwater, seawater, and terrestrial inputs on its hydrodynamics, physicochemical, and biological processes. In these waters, pH affects carbonate system speciation and can be determined by potentiometry or spectrophotometry, with both methods being sensitive to common changes in temperature from ambient to in situ conditions, which are often not accounted for in some models. This study aimed to investigate the best-fit pH correction model using a Tris solution obtained at 25°C for various ambient temperatures, proposing different empirical models to correct pH for variable temperatures. The results indicated an important influence of pH correction at 25°C for in situ temperature on the carbonate system. Variations between 0.05 and 1.03 pH units caused large relative errors in different parameters of the carbonate system: CA (VC% from 6 to 188), Ω calcite (VC% from 4 to 82), Ω aragonite (VC% from 4 to 82), HCO3 - (VC% from 5 to 147), CO3 2- (VC% from 10 to 231), and DIC (VC% from 5 to 173). Our findings revealed that the newly proposed models can substantially improve the accuracy of determining carbonate system parameters in estuarine waters.
Keywords: pH measurement; Estuarine waters; Carbonate system; Empirical models; Guanabara Bay
Introduction
Estuarine waters commonly receive substantial inputs of organic matter and nutrients from urban and rural catchments [8]. These inputs can have substantial implications for the marine environment, including changes in pH levels that affect the equilibrium constants of the carbonate system [5]. The anthropogenic increase in atmospheric CO2 concentration and untreated organic effluents have led to decreases in both pH and carbonate concentrations in coastal waters over the past decades [21,28,34-39]. These growing reductions in pH are leading to ocean acidification [9], which poses a significant threat to marine organisms such as coccolithophores, corals, foraminifera, and bony fishes due to the increased concentration of H+ ions in seawater [12]. Accurate pH measurements are still needed for monitoring acidification and its impact on the speciation and quantification of the carbonate system, with errors less than 0.1 and 0.01 pH units, respectively [25].
In contrast to the stable salinity comparing field and the analysis site, seawater pH is typically determined at a standard temperature of 25°C (pH25), which often differs from in situ conditions. Due to the temperature dependence of the stoichiometric dissociation constants of the carbonate system (Ko*, K1* e K2*), pH25 measurements must be corrected to reflect the pH value at the sampling temperature (pHt). This correction requires incorporating a parameter of the carbonate system under thermodynamic conditions, such as dissolved inorganic carbon (DIC) or Total Alkalinity (TA), as established by previous studies [13,24]. However, empirical studies in the relationship between pH25 and pHt are still scarce. Based on an experimental design using Tris buffers for in-situ salinity and varying temperatures, our study aimed to propose new empirical models for converting pH25 to pHt, compare them with existing literature models, and assess their accuracy for determining carbonate system speciation in estuarine brackish waters.
Study area
Guanabara Bay is a eutrophic tropical coastal bay located on the southeastern coast of Brazil, with a surface area of 384 km², situated between latitudes 22° 41' - 22° 58' S and longitudes 43° 02' - 43° 18' W (Figure 1). The bay features a coastline spanning 131 km and an average water volume of approximately 1.87 x 109 m3. The bay's drainage basin covers approximately 4,080 km² and is intersected by 56 rivers and channels [17]. Salinity in Guanabara Bay ranges from 20 to 34 psu, with higher salinity observed in bottom waters. The temperature ranges from 14.8°C to 25.2°C in the bottom waters and from 19.6°C to 28.6°C at the surface [42].
Figure 1: Sampling sites for the pH experiment in Guanabara Bay waters:
BV1 - Boa Viagem beach 1, BV2 - Boa Viagem beach 2, BV3 - Boa Viagem
beach 3, BV4 - Boa Viagem beach 4, BV5 - Boa Viagem beach 5, CN -
Clube Naval, IF1 - Fundão Island 1, IF2 – Fundão Island 2.
Analytical Methods
pH design experimental
Water sampling
Water samples were collected in Guanabara Bay between November 27, 2017, and March 23, 2018. Sampling included five locations heavily influenced by the bay's outlet to the sea at Boa Viagem Beach (BV1, BV2, BV3, BV4, and BV5), two inner regions around Fundão Island (IF1 and IF2), and one sample from a sheltered site near the sea, named Clube Naval (CN), as shown in Figure 1. The exact sampling dates and coordinates were as follows: BV1 and BV2 on November 27 and 29, 2017, respectively, at 22°54'31.29" S, 43°7'50.52" W; IF1 on December 5, 2017 (22°50'40.29" S, 43°13'52.74" W) behind the Faculty of Physical Education building, where litter was present on the shore; IF2 on January 11, 2018 (22°51'38.77" S, 43°14'9.84" W), in the bay’s channel between the Institute CCMN/UFRJ and Vila Pinheiro. BV3, BV4, and BV5 were sampled on January 15, March 21, and March 22, 2018, respectively, at the same coordinates (22°54'31.29" S, 43°7'50.52" W), with the BV5 collection taking place during rain, accompanied by a strong leachate flow into the bay. The final sample (CN) was collected on March 23, 2018, at 22°56'5.70" S, 43°6'22.28" W. Salinity varied across the sites, ranging from 4.15 psu at IF2 to 32.95 psu at BV2. Notably, salinity dropped to 8.44 psu at BV5 during rainfall, indicating freshwater input from surface runoff. All samples were stored in 300 mL BOD bottles, preserved with HgCl2, and transported under refrigeration [34,37,39]. The experiments were conducted in the laboratory within two hours of sample collection.
Determination of salinity
Salinity was determined using the Marine Chemical Analyses Program (AQM) [36,37,38,39] based on electrical conductivity measured with a Metrohm 856 conductivimeter (5-ring probe, model 6.0915.100) connected to a Titrando 907 automatic titrator controlled by Tiamo 2.5 software. T?he dilution factor to calculate salinity (in mS/ cm) was obtained by adding deionized water to the samples by weight to fit the probe’s range (5-20 mS/cm). Measurements were taken at 25°C using a thermostatic beaker and an ultrathermostatic bath (Quimis Q214M2) to maintain constant temperature. Conductivity was validated with IAPSO Standard Seawater (Lot P156) using ultrapure water (resistivity>18 MΩ/cm) and a Shimadzu analytical balance with a precision of 0.0001 g. Standards were prepared for different salinities (3, 5, 7, 9, 10, and 11 psu) due to limitations of the conductivity cell. Replicates were converted to salinity using AQM program, yielding a mean relative error of 0.16%.
Determination of pHT (mol-kg solution) in tris buffer solution
T?he pH of the Dickson reference solution was measured at 25°C using a thermostatic beaker, an ultrathermostatic bath (Quimis, model Q214M2), and a Titrando 907 (Metrohm). A pH Iconnect 854 electrode and Tiamo 2.5 software (Metrohm) were used. The performance of the pHT (total scale) probe was predefined [35], with a Nernst constant of 59.17 mV/pH unit. The probe was calibrated with a laboratory-prepared Tris buffer solution, optimized for estuarine waters [23], and pH was measured in 10 replicates. The accuracy and precision compared to the expected pHT of 8.05 were -0.04% for RE and 0.01% for VC, respectively.
Determination of total alkalinity (μmol/Kg): Dickson solution (Batch #134)
The accuracy and precision of the total alkalinity (TA) method were determined at 25°C also using a thermostatic beaker and an ultrathermostatic bath (Quimis, model Q214M2). Ten replicates of the Dickson seawater reference solution (Batch #134) were titrated following the method by Van den Berg and Rogers (1987) [43], adapted for a Titrando 907 automatic titrator with a Metrohm IConnect 854 proble controlled by Tiamo 2.5 software. The potentials were processed using the AQM, yielding in a relative error of 0.87% (Table 1).
Replicates
TAOBS.
1
2,155
2
2,191
3
2,205
4
2,202
5
2,210
6
2,214
7
2,214
8
2,213
9
2,214
10
2,214
X
2,203.31
SD
±18.46
RE (%)
0.87
Table 1: Observed Total Alkalinity (TAOBS) measured by potentiometric titration using the Dickson reference standard (Batch #134), reported as mean ± standard deviation (X±SD) and relative error (RE). The Expected TA (TAEXP) is 2,222.61 μmol/kg.
Table 1
Determination of DIC (μmol/Kg): Dickson solution (Batch #134)
The accuracy and precision of the total alkalinity (TA) method were determined at 25°C also using a thermostatic beaker and an ultrathermostatic bath (Quimis, model Q214M2). Ten replicates of the Dickson seawater reference solution (Batch #134) were titrated following the method by Van den Berg and Rogers (1987) [43], adapted for a Titrando 907 automatic titrator with a Metrohm IConnect 854 proble controlled by Tiamo 2.5 software. The potentials were processed using the AQM, yielding in a relative error of 0.87% (Table 1).
Table 1
Determination of DIC (μmol/Kg): Dickson solution (Batch #134)
Total dissolved inorganic carbon (DIC) was calculated using AQM from pH and TA (sections 2.2 and 2.3). The results obtained were compared with DIC measurements determined using the acidified headspace method (AHS, [1]) and chromatographic analysis (Shimadzu) conducted at two laboratories: ENSP/Fiocruz (TOC-F) and Geoquímica/UFF (TOC-G) (Table 2). AQM calculations based on pH and TA exhibited the lowest relative error (RE of 0.1%).
Replicates
DICTERM
DICAHS
DICTOC-F
DICTOC-G
1
2,023.17
2,120.7
2,039.02
1,993.3
2
2,023.17
2,087.8
2,046.51
1,995.0
3
2,023.17
2,077.3
2,044.01
2,015.0
4
2,023.17
2,139.7
2,048.17
-
5
2,023.17
2,049.0
2,046.51
-
6
2,023.17
1,999.7
2,048.17
-
7
2,023.17
2,011.2
2,044.01
-
8
2,023.17
1,815.8
2,046.51
-
9
2,023.17
1,652.8
2,040.68
-
10
2,023.17
2,002.0
-
-
X
2,023.17
1,995.6
2,044.8
2,001.0
SD
± 2.4x10-13
± 150.8
± 3.2
± 12.06
RE (%)
0.1
1.2
1.2
0.9
Table 2: Comparison of Dissolved Inorganic Carbon (DIC) obtained using the Dickson reference standard from thermodynamic modeling (DICTERM), the acidified headspace method (DICAHS; Aberg & Wallin, 2014), and two Shimadzu total organic carbon analyzers (TOC-F and TOC-G). Data are reported as mean ± standard deviation (X±SD) and Relative Error (RE). The expected DIC is 2,020.1 μmol/kg.
Table 2
pH against temperature: Tris buffer solution (S = 15 psu, m = 0.03)
A Tris buffer solution was used as a reference to investigate the effect of temperature on pH. Ten replicates of this solution were measured at different temperatures: 15, 20, 25, 30, 35, and 40°C. The experiment was conducted using the same equipment and conditions outlined in section “Determination of pHT (mol-kg solution) in tris buffer solution”.
2.6. pH Model vs Temperature
To model the temperature correction of pH, water samples BV1, BV2, BV3, BV4, BV5, CN, IF1, and IF2, as described in section 2.1, were used. These samples were subjected to the same temperature conditions mentioned in section 2.5. The experiment was conducted using the same equipment and conditions outlined in section “Determination of pHT (mol-kg solution) in tris buffer solution”.
Results and Discussion
Empirical factor for converting pH25 to pHt
The empirical factor for converting pH measured at 25°C to pH at ambient temperature during water collection was determined using multi-point calibration with linear egression modeling. This approach has been proposed as a high-precision standardization procedure for measuring pH values, allowing for the standardization of pH measurements with respect to temperature [4].
Moreover, pH and temperature showed strong correlations (R² > 0.94; Figure 2) in sampling sites BV1, BV2, BV4, and IF1, unlike at BV3, BV5, CN and IF2 (0.02 < R² < 0.90). We selected only samples with linear regression R² greater than 0.93. The linear model using the Tris solution (reference) demonstrated a strong relationship between pH and temperature (R² = 0.9995), as reported previously [21,24,31,32,36,39] (Figure 3). The deviation from the ideal line observed in samples from BV3, BV5, CN, and IF2 is likely due to biogeochemical processes associated with respiration, photosynthesis, and carbonate precipitation, which disrupt the expected pattern between temperature and pH values [15,26,27].
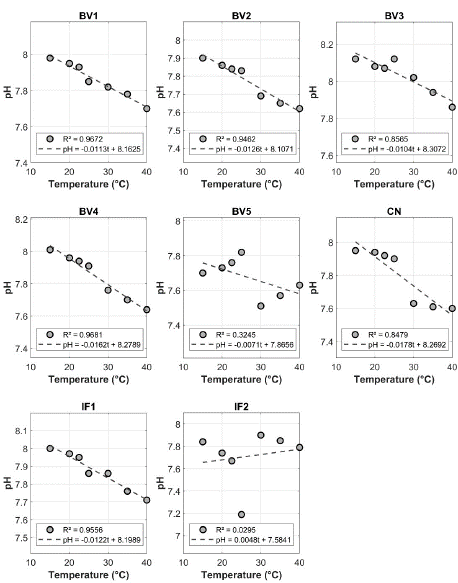
Figure 2: Relationship between temperature (°C) and pH (mol/kg-sol)
at sampling sites BV1, BV2, BV4, IF1, BV3, BV5, CN, and IF2 (linear
regression, significance level p < 0.05). Each panel reports the significant
linear equation and the coefficient of determination (R²).
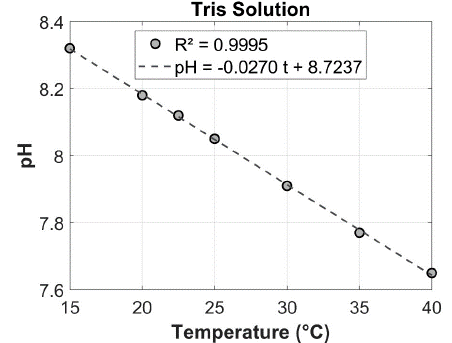
Figure 3: Relationship between temperature (oC) and pH (mol/kg-sol) of Tris
solution (linear regression, significance level p < 0.05). The linear equation
and the coefficient of determination (R²) are reported in the panel.
Figure 2
Figure 3
The non-significant relationship (p > 0.05) between pH and temperature at the BV5 site may be attributed to inputs of sewage and terrestrial organic matter as sources of organic nutrients [7], tide movements driving water renewal, algal blooms, or rainfall events. In river-dominated systems, sewage discharge typically enhances heterotrophic processes, leading to increased CO2 outgassing [45].
High components releasing protons and affecting the acid/base system of seawater, which can be estimated from DOC concentrations [20]. Concentrations of dissolved organic carbon (DOC) in water cause a systematic error in electrode response of ±0.02 pH units [14]. DOC also affects CO2 equilibrium (2CH2O + SO4 2- +2H+ = H2S + 2CO2 + 2H2O), interfering with electrode response during pH measurement and resulting in lower pH values [14]. Potentiometric measurements with a glass electrode have long been questioned for their reliability due to possible DOC interference in pH values. Humic acids (HA) are part of the DOC pool that decreases pH in waters [18]. The coastal ocean contains large amounts of HA with a wide variety of components releasing protons and affecting the acid/base system of seawater, which can be estimated from DOC concentrations [20], where KHA is the dissociation constant of HA, and [HA]T is the total concentration of HA.
In addition to natural inputs, high concentrations of DOC from anthropogenic sources can influence pH values [11]. Conversely, no evidence of DOC interference in pH measurements was found when the experiment was directed towards a homogeneous chemical form of DOC, which is not realistic for Guanabara Bay [22]. Guanabara Bay is characterized by high inputs of biomass from rivers draining densely populated urban areas, with sediment organic matter content ranging from 4% to 6% [29]. Anthropogenic humic and fulvic substances substantially reduce its water quality, such as increasing turbidity that directly affects aquatic metabolism.
The nutrient-enriched nature of anthropogenic inputs can contribute to one of the highest sea productivity rates in the world, with an average net primary production of 0.17 mol C m?² day?¹ found in Guanabara Bay [10,41]. This increased productivity is supported by high temperatures and nutrient availability throughout the year, associated with an estimated annual input of 3.2 x 10 mol P and 6.2 x 10¹ mol N, primarily from untreated sewage [44]. Thus, the regression model with the Tris solution (Figure 3) confirms the hypothesis that deviations from the ideal curve are likely due to humic and fulvic substances.
Regarding the quotient between pH and temperature obtained from water samples collected at BV1, BV2, BV4, and IF1, the observed correction coefficient for pH measured at 25°C to ambient temperature during sampling was -0.002t + 1.0493 (R² = 0.9932, p < 0.05). Testing the correction model with sample BV2 revealed a pH unit variation (UVpH) of 0.01 to -0.07 and RE of 0.08% to -0.88% (Supplementary information). The corrected pH values obtained using the model developed here, (t = in situ temperature), were better than those previously reported. The greatest variations in expected and calculated pH values were found for the model proposed by Perez & Fraga (1987) [31] for temperature ranges from 10°C to 30°C and salinity from 0 to 35 psu, showing UVpH from -0.91 to 2.92 and RE from 6.63% to 38.32%. In contrast, models generated by Gieskes (1969), Millero (1995), Ballerby et al. (1995) [6] and Lui & Chein (2017) [19] presented values more consistent with those found by Perez & Fraga (1987) [31] to Guanabara Bay, showing UVpH from 0.00 to 0.38 and RE from 0.00% to 4.99%.
Overall, the carbonate system speciation parameters of a water sample from Guanabara Bay at Boa Viagem Beach, corrected from 25°C to the ambient temperature during sampling, were highly variable compared to previous studies in other coastal waters (Table 3). This variability can be attributed to the greater sensitivity of carbonate system speciation and concentration to pH variations than to other parameters such as TA, CaT, and BT [36,39,40]. Differences in H+ concentration, which directly participate in the chemical equilibrium reactions of the carbonate system, lead to substantial differences in speciation. Proper procedures for pH measurement using glass electrodes must be employed by oceanography professionals, considering several aspects: 1- electrode verification, 2- use of the appropriate scale for seawater (i.e., total scale) defined by Tris solution, and 3- selection of the concentration unit (i.e., mol/kgsol; Marion et al., 2011). Electrodes performing within 95% to 103% of the theoretical value (100% = 59.264 mV/pH) are suitable for use [33].
CA
O calc
O arag
HCO3-
CO32-
DIC
pH (TW); pH20=7.87
1,322
2.9
1.9
1,174
74
1,261
pH (G); pH20=7.89
1,392
3.0
1.9
1,229
81
1,323
pH (P&F); pH20=8.89
28,556
11.9
7.7
12,294
8,131
20,437
pH (M); pH20=7.91
1,466
3.1
2.0
1,287
89
1,389
pH (B et al.); pH20=7.86
1,289
2.8
1.8
1,147
71
1,231
pH (L&C); pH20=7.91
1,466
3.1
2.0
1,287
89
1,389
VC
188
82
82
147
231
173
CA = Carbonate Alkalinity; pH20 = pH at ambient temperature during sampling calculated from pH measurements at 25oC; TW = This work; G = Gieskes (1969); P&F= Perez & Fraga (1987); M= Millero (1995); B= Bellerby et al (1995); L&C= Lui & Chen (2017); VC= Variation coefficient.
Table 3: Carbonate system parameters as a function of pH in a water sample from Guanabara Bay. Constant parameters: Temperature = 20°C; Salinity = 32.95 psu; TA = 2,165 μmol/kg; [Ca]T = 9,887 μmol/kg; [B]T = 411 μmol/kg; pH25 = 7.38 (mol/kg-sol).
Higher temperatures indeed result in an increased pH due to the lower solubility of CO2 under these conditions [16,30]. The pH of CO2 saturated solutions decreases with increasing NaCl concentration while maintaining constant pressure and temperature. In pH measurements using spectroscopic and electrometric methods, simple automatic temperature compensation for complex aqueous systems can lead to measurement errors. It is more appropriate to model pH as a function of the ionic species present in the sample and validate it with standards maintaining the same ionic strength and calibration temperature as the samples [2,3].
Conclusion
Our findings indicate that protocols for monitoring marine acidification across temporal and seasonal scales require more accurate pH measurement models that account for local water quality characteristics by employing cost-effective and user-friendly potentiometric methods with calomel glass electrodes. Additional experiments are needed to develop more accurate empirical models for converting pH measurements at 25°C to ambient temperatures during sampling. Therefore, this study highlights the importance of proper electrode calibration and the use of Tris buffer solution to define the pH scale and concentration unit to advance multiple fields of marine science research.
Acknowledgements
We would like to thank the Center for Study of Water, Biomass and Oil (NAB) and the Postgraduate Program in Dynamics of Oceans and Earth of Federal Fluminense University for their support and infrastructure during the development of this study. We also express our gratitude to the Coordination for the Improvement of Higher Education Personnel (CAPES) for funding the research scholarships, which were essential for the progress of this work.
References
- Aberg J, Wallin B. Evaluting a fast headspace method for measuring DIC and subsequent calculation of pCO2 in freshwater systems. Inland Waters. 2014; 4: 157-66.
- Anes B, Da Silva RJNB, Oliveira C, Camões MF. Uncertainty evaluation of alkalinity measurements on seawater samples. Measurement. 2018; 129: 395-404.
- Badocco D, Pedrini F, Pastore A, Di Marco V, Marin MG, Bogialli S, et al. Use of a simple empirical model for the accurate conversion of the seawater pH value measured with NIST calibration into seawater pH scales. Talanta. 2021; 225: 122051.
- Baucke FGK, Naumann R, Alexander-Weber C. Multiple-Point Calibration with Linear Regression as a Proposed Standardization Procedure for High- Precision pH Measurements. Anal Chem. 1993; 65: 3244-51.
- Bednaršek N, Beck MW, Pelletier G, Applebaum SL, Feely RA, Butler R, et al. Natural Analogues in pH Variability and Predictability across the Coastal Pacific Estuaries: Extrapolation of the Increased Oyster Dissolution under Increased pH Amplitude and Low Predictability Related to Ocean Acidification. Environ Sci Technol. 2022; 56: 9015-28.
- Bellerby RGJ, Turner DR, Millward GE, Worsfold PJ. Shipboard flow injection determination of sea water pH with spectrophotometric detection. Anal Chim Acta. 1995; 309: 259-70.
- Bidone ED, Lacerda LD. The use of DPSIR framework to evaluate sustainability in coastal areas. Case study: Guanabara Bay Basin, Rio de Janeiro, Brazil. Reg Environ Change. 2004; 4: 5-16.
- Cai WJ, Feely RA, Testa JM, Li M, Evans W, Alin SR, et al. Natural and Anthropogenic Drivers of Acidification in Large Estuaries. Annu Rev Mar Sci. 2021; 13: 23-55.
- Cai WJ, Hu X, Huang WJ, Murrell MC, Lehrter JC, Lohrenz SE, et al. Acidification of subsurface coastal waters enhanced by eutrophication. Nat Geosci. 2011; 4: 766-70.
- Carreira RS, Wagener ALR, Readman JW, Fileman TW, Macko SA, Veiga A. Changes in the sedimentary organic carbon pool of a fertilized tropical estuary, Guanabara Bay, Brazil: an elemental, isotopic and molecular marker approach. Mar Chem. 2002; 79: 207-27.
- Curra-Sánchez ED, Lara C, Cornejo-D’Ottone M, Nimptsch J, Aguayo M, Broitman BR, et al. Contrasting land-uses in two small river basins impact the colored dissolved organic matter concentration and carbonate system along a river-coastal ocean continuum. Sci Total Environ. 2022; 806: 150435.
- Cyronak T, Schulz K, Jokiel P. Marine Science. Encycl Environ Soc. 2016; 73: 558-62.
- Gieskes JM. Effect of temperature on seawater pH. Limnol Oceanogr. 1969; 14: 679-85.
- Herczeg AL, Hesslein RH. Determination of hydrogen ion concentration in softwater lakes using carbon dioxide equilibria. Geochim Cosmochim Acta. 1984; 48: 837-45.
- Johnson ZI, Wheeler BJ, Blinebry SK, Carlson CM, Ward CS, Hunt DE. Dramatic variability of the carbonate system at a temperate coastal ocean site (Beaufort, North Carolina, USA) is regulated by physical and biogeochemical processes on multiple timescales. PLoS One. 2013; 8: e85117.
- Kaasa B, Ostvold T. Alkalinity in oil field waters. What alkalinity is and how it is measured. In: SPE International Conference on Oilfield Chemistry. SPE. 1997: SPE-37277-MS.
- Kjerfve B, Ribeiro CHA, Dias GTM, Filippo AM, Da Silva Quaresma V. Oceanographic characteristics of an impacted coastal bay: Baía de Guanabara, Rio de Janeiro, Brazil. Cont Shelf Res. 1997; 17: 1609-43.
- Kratz TK, Cook RB, Bowser CJ, Brezonik PL. Winter and spring pH depressions in northern Wisconsin lakes caused by increases in pCO2. Can J Fish Aquat Sci. 1987; 44: 1082-8.
- Lui HK, Chen CTA. Reconciliation of pH25 and pHinsitu acidification rates of the surface oceans: A simple conversion using only in situ temperature. Limnol Oceanogr Methods. 2017; 15: 328-35.
- Lyu LN, Lu D, Sun C, Ding H, Yu LM, Yang GP. A new software of calculating the pH values of coastal seawater: Considering the effects of low molecular weight organic acids. Mar Chem. 2019; 211: 108-16.
- Marion GM, Millero FJ, Camões MF, Spitzer P, Feistel R, Chen CTA. pH of seawater. Mar Chem. 2011; 126: 89-96.
- Metcalf RC, Peck DV, Lori AJ. The influence of dissolved organic carbon on pH measurements of low solute content waters. Geochim Cosmochim Acta. 1989; 53: 773-84.
- Millero FJ. The pH of estuarine waters. Limnol Oceanogr. 1986;31(4):839-47.
- Millero FJ. Thermodynamics of the carbon dioxide system in the oceans. Science. 1995; 59: 661-77.
- Millero FJ. Chemical Oceanography. 2nd ed. CRC Press. 1996.
- Millero FJ. Chemical Oceanography. Vol. 4. CRC Press. 2006.
- Millero FJ. The marine inorganic carbon cycle. Chem Rev. 2007; 107: 308-41.
- Millero FJ. Carbonate constants for estuarine waters. Mar Freshw Res. 2010; 61: 139-142.
- Neto JAB, Gingele FX, Leipe T, Brehme I. Spatial distribution of heavy metals in surficial sediments from Guanabara Bay: Rio de Janeiro, Brazil. Environ Geol. 2006; 49: 1051-63.
- Kaasa B, Ostvold T. Prediction of pH and mineral scaling in waters with varying ionic strength containing CO2 and H2S for 0 < T (C) < 300 and 1 < P (Bar) < 500. In: CORROSION 98. OnePetro; 1998.
- Perez FF, Fraga F. The pH measurements in sea water on the NBS scale. Science. 1987; 21: 315-27.
- Pratt KW. Measurement of pHT values of Tris buffers in artificial seawater at varying mole ratios of Tris: Tris·HCl. Mar Chem. 2014; 162: 89-95.
- Ramos e Silva CA. Oceanografia Química. 2nd ed. Interciência; 2024.
- Ramos e Silva CA. Who is responsible for climate: Celestial change or human Activity? Int J Environ Clim Change. 2022; 12: 2231-4784.
- Silva CAR, Liu X, Millero FJ. Solubility of siderite (FeCO3) in NaCl solutions. J Solut Chem. 2002; 31: 97-108.
- Ramos e Silva CA, Monteiro NSC, Cavalcante LM, Tavares Junior W, Carneiro MER, De Souza FES, et al. Inventory of water masses and carbonate system from Brazilian’s northeast coast: Monitoring ocean acidification. PLoS One. 2022; 17: e0271875.
- Ramos e Silva CA, Davalos PB, Silva MP, Miranda LB, Calado L. Variability and Transport of Inorganic Carbon Dioxide in a Tropical Estuary. J Oceanogr Mar Res. 2017; 5: 1-11.
- Ramos e Silva CA, Senez-Mello, Fonseca TM, Monteiro E, Marotta H, Neto J, et al. Acidificação dos oceanos em um sopro: Prática educacional para construção de conhecimento das mudanças globais. Rev Experiências Ensino Ciências (EENCI). 2017; 12: 1-25.
- e Silva CAR, de Godoy Fernandes LV, de Souza FLS, Marotta H, da Costa Fernandes F, Senez Mello TM, et al. Carbonate system in the Cabo Frio upwelling. Scientific Reports. Scientific Reports. 2023; 13: 5292.
- Ramos e Silva CA, Dávalos PB, da Silveira Lobo Sternberg L, Soares de Souza FE, Constantino Spyrides MH, Lucio PS. The influence of shrimp farms organic waste management on chemical water quality. Estuar Coast Shelf Sci. 2010; 90: 55-60.
- Rebello ADL, Haekel W, Moreira J, Santelli R, Schroeder F. The fate of heavy metals in an estuarine tropical system. Mar Chem. 1986; 18: 215-25.
- Valentin JL, Gouvêa GV, Gomes CL. Mesozooplankton and water masses in the Guanabara Bay: Ten years monitoring. Oecol Aust. 2020; 24: 349-64.
- Van Den Berg CMG, Rogers H. Determination of alkalinities of estuarine waters by a two-point potentiometric titration. Mar Chem. 1987; 20: 219-26.
- Wagener ALR. Burial of organic carbon in estuarine zones—estimates for Guanaraba Bay, Rio de Janeiro. Quim Nova. 1995; 18: 534-5.
- Zhai W, Dai M, Guo X. Carbonate system and CO2 degassing fluxes in the inner estuary of Changjiang (Yangtze) River, China. Mar Chem. 2007; 107: 342-56.
