
Research Article
Austin J Forensic Sci Criminol. 2015;2(1): 1011.
Post-Implant Morphological Changes of Mammary Tissue: Polyvinyl Sponge (Ivalon) vs. Silicone Breast Prosthesis
Audrey Ekhaguere, Mohammed Al-Tikriti*,Gerald Bertetta, Elizabeth Rega
Department of Medical Anatomical Sciences, Western University of Health Sciences, USA
*Corresponding author: Mohammed Al-Tikriti, Department of Anatomy, College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, 309 E 2nd St, Pomona, CA 91766, USA
Received: November 03, 2014; Accepted: January 12, 2015; Published: January 14, 2015
Abstract
We report histomorphological changes of the capsules associated with Polyvinyl (Ivalon) sponge and silicone breast prosthesis implants, were removed from two Caucasian female cadavers between the ages of 50 and 80, and histologically analyze the reactions of the surrounding breast tissue to each type of breast implant.
Measurements and the volumes of the implants were taken and recorded. 1 cm x 1 cm tissue samples were cut and prepared through the tissue preparation for light microscopy method. The polyethylene bag did not completely prevent infiltration of living cells into the sponge. Many factors may have played a part in the extent of calcium deposition seen in the capsule and subcutaneous mammary tissue of the Ivalon breast as compared to the subcutaneous mammary tissue silicone breast implants.
Histological stains revealed living cell infiltration into the polyvinyl sponge, calcification of the Ivalon capsule, and calcium deposition in the subcutaneous mammary tissues of both the Ivalon and silicone breast implants.
Keywords: Cadaver; Breast implant; PVA; Ivalon; Silicone
Introduction
Breast implants have been used for nearly half a century for both cosmetic and reconstructive purposes. Many commercially available synthetic polymers, such as polyvinyl alcohol (PVA), show physicochemical and mechanical properties comparable to those biological tissues to be substituted [1,2]. One of the earliest types of breast prosthesis was the Ivalon sponge. It is composed of polyvinyl alcohol, where formaldehyde, in the presence of sulfuric acid, reacts with 80% of its hydroxyl groups [3,4]. It showed no evidence of local systemic toxicity associated with PVA. In 1949, Grindlay and Clagett [5] established use of the polyvinyl alcohol sponge as a prosthetic implant. The polyvinyl sponge’s porous composition allowed for penetration by blood and other connective tissue of the body, in turn deeming it a living mass [6]. The polyvinyl sponge (commercially known as Ivalon) breast implants, composed of an oval piece of polyvinyl alcohol sponge enclosed in a polyethylene bag, were first used during the 1950s [4]. Organic fibrous tissue formation surrounding polyethylene implants had previously been documented, although there was a lack of long-term follow-ups beyond three years [7].
It was later evident that complications, including increased weight and hardening (possibly calcification), were associated with the implants [4]. In the early 1960s, breast implant prosthesis was revolutionized by the establishment of silicone gel breast implants. Due to rupture and many reported claims of connective tissue disease related to them, the FDA placed a ban on silicone gel breast implants for use in cosmetic purposes. The purpose of this report was to look at the histomorphological changes induced by these breast implants and hopefully draw a conclusion as to which type has the least reaction to the surrounding connective tissue of the breast.
Materials and Methods
A pair of polyvinyl sponge breast implants was found in a 78 year old Caucasian female cadaver. The implants with the capsules were removed and weighed. Another pair of silicone breast implant that was found in Caucasian female cadaver between the ages of 50 and 65, was also removed, and their volumes was calculated by using the displacement method: a beaker was filled to the 675 ml line with tap water. The length and volume of the implants were measured and recorded (Table 1). Pictures of the Ivalon sponge implant at each of the aforementioned distances were all taken. 1 cm x 1 cm samples were cut from the polyvinyl sponge, its hardened capsule, the subcutaneous tissue directly underlying the capsule, the tissue directly surrounding the silicone breast implant (considered the capsule), and the subcutaneous tissue underlying the silicone capsule. As the tissue samples have already been formalin preserved, the samples were then dehydrated, cleared, molded into a paraffin block, sectioned at five μm (micrometers), and placed on a slide, as per standard histological procedures. The slides were then stained with hematoxylin and eosin (H&E), Alizarin Red, and Masson’s Trichrome. The slides were mounted and analyzed.
Sponge
Silicone
Measurements
W- capsule
WO-capsule
W-capsule
WO-capsule
length
10.3cm
10.1cm
15.1cm
17.1cm
Volume
209.8cc
201.7cc
450cc
509.6cc
Table 1: Parametric measurement of the sponge and silicone breast implant. (W- and WO-) with or without capsule.
Result and Discussions
The Ivalon prosthesis with its capsule, and the silicone capsule, measured 10.3 cm (Figure 1) and 17.1 cm (Figure 2), respectively. A hematoxylin & eosin stain of the sponge shows infiltration of living cells into the polyvinyl sponge (Figure 3), suggesting that the body recognizes the sponge as a living mass [6]. A Polyethylene bag was probably used to envelope the polyvinyl sponge because it is more difficult for cells to attach to a smooth surface [8] and it will limit cell growth into the sponge, which will lead to hardening of the implants if infiltration occurs. The polyethylene bag does not completely seal the polyvinyl sponge, as the bag was merely wrapped around the sponge, therefore allowing infiltration of the tissue fluid into the sponge. The mechanism that the body uses to take up the polyvinyl sponge is unknown but it is clear that the body does not recognize it as foreign material and therefore allows tissue fluids, with cells following, to enter [9]. Also, as the sponge was not hardened when cut into, this suggests that no severe calcification occurred inside the sponge. This conclusion was confirmed upon an Alizarin Red stain of the sponge showing a lack of calcium deposits inside the sponge (Figure 4). Figure 5 shows collagen fibers and plenty of adipose cells in the subcutaneous tissue near the silicone implant. This is evidence that the subcutaneous tissue is not completely calcified. An Alizarin Red stain of the silicone subcutaneous tissue verifies early and incomplete calcification, due to the amount of irregular collagen fiber deposition seen in the tissue (Figure 6).
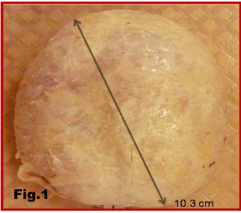
Figure 1: External surface of the fibrous capsule of Ivalon sponge implant
enclosed in the fibrous capsule.
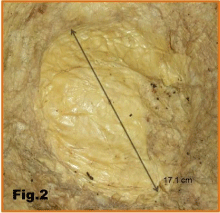
Figure 2: Capsule of the silicone implant.
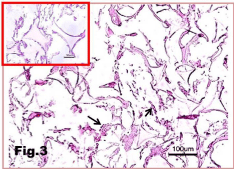
Figure 3: Sponge-10X: insert 20X: Sponge is either rectangular or triangular
cells, formed by concave sided and meet at their angles, insert (20X) shows
that are invaded by blood leukocytes (arrow).
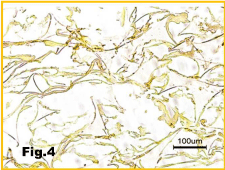
Figure 4: Sponges implant showing no calcification. Stained by Alizarin Red.
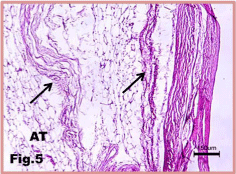
Figure 5: Silicon implant: Subcutaneous tissue of the implant showing
connective tissue collagen bundles (arrows) intermingled with adipose tissue
(AT). H&E stain.
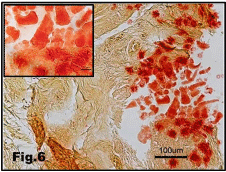
Figure 6: Calcium deposition (red particles) in the subcutaneous tissue of the
silicon implanted breast. Insert: high magnification of Ca++ deposit. Alizarin
Red stain.
The irregularly patterned collagen fibers seen in the capsule of the silicone implant (Figure 8) are indicative of possible calcium deposition, as seen in the subcutaneous tissue. Since no calcium deposits are observed in the silicone capsule (Figure 7), it is verified that the body’s immune reaction to foreign objects begins in subcutaneous tissue surrounding the implants and works its way toward the implants. The immune response mechanism to foreign body begins with the recruitment of neutrophils, the body’s first line of defense. If the neutrophils are unable to defeat the foreign body, macrophages and B and T lymphocytes, which all produce cytokines, will be recruited. If that line of defense fails as well, the cytokines will then stimulate fibroblast formation to build a protective wall around the foreign body [10]. The fibroblasts will randomly lay down elastic and collagen fibers in a disorganized manner (Figure 9), in order to create a matrix. The matrix will then mineralize via various mineralization processes and finally become calcified.
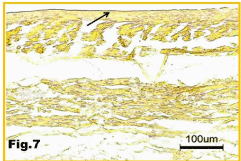
Figure 7: No calcium deposition in the capsule (arrow) of the silicone implant.
Alizarin stain.
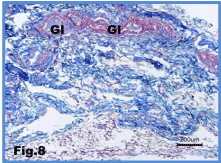
Figure 8: Subcutaneous tissue of the sponge implant breast, showing
Irregular blue stained collagen fibers of the connective tissue between the
ducts of the gland (Gl). Mason’s trichrome stain.
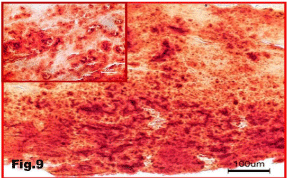
Figure 9: Calcium deposit in the subcutaneous tissue of sponge implant
breast. Alizarin Red stains.
Samples of the subcutaneous mammary tissue from the Ivalon breast implant show severe calcium deposits. The significant amount of calcification observed (Figure 9), as compared to the subcutaneous tissue of the silicone implants (Figure 6), can be due to either or a combination of three factors: irritation, the composition of the polyethylene bag, and time.
Extreme irritation of the surrounding tissue can result from the implants being inserted aggressively. When polyethylene is necessary for inertness, a pure Polyethylene, which allows for minimal tissue response in humans, is used. In the case of breast implants, inertness is not sought. Therefore, the other alternative was the du Pont polyethylene, which contained added chemicals that promoted fibroblast proliferation [7]. The body’s response then eventually leads to rapid calcification of tissue surrounding the bag. Although there is no available information regarding the length of time of which each breast implant has been in each recipient, there is plenty of dark red reaction seen in the Alizarin red stain of the calcium subcutaneous tissue of sponge implant (Figure 9). This suggests that the calcification has been there for a longer period than that seen in the subcutaneous tissue of the silicone implants (Figure 6).
Conclusion
A histological section of stained polyvinyl sponge shows evidence of tissue cellular infiltration into the sponge. Although histological examination proved calcium deposition in mammary tissue of both types of implants, it revealed that the body has a more severe reaction to the Ivalon breast implants than to those of silicone. This severity seen in the polyvinyl sponge implants can be due to a combination of factors: how aggressive the implantation procedure was, the chemical composition of the polyethylene bag, and time. A special stain of mammary tissue surrounding both types of implants suggests that the foreign body reaction begins in the subcutaneous tissue in close proximity to the implants, and then moves toward the implants. Essentially, the body reacts to both the Ivalon and silicone breast implants.
References
- DeMerlis CC, Schoneker DR. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem. Toxicol. 2003; 41: 319–326.
- Seabra AB, De Oliveira MG. Poly(vinyl alcohol) and poly(vinyl pyrrolidone) blended films for local nitric oxide release. Biomaterials. 2004; 25: 3773-3782.
- Patel AR, Vavia PR. Evaluation of synthesized cross linked polyvinyl alcohol as potential disintegrant. J Pharm Pharm Sci. 2010; 13: 114-127.
- Liu LW, Truong LD. Morphologic characterization of polyvinyl sponge (Ivalon) breast prosthesis. Arch Pathol Lab Med. 1996; 120: 876-878.
- Grindlay JH, Clagett OT. A plastic sponge prosthesis for use after pneumonectomy; preliminary report of an experimental study. Proc Staff Meet Mayo Clin. 1949; 24: 538.
- Peters WJ, Smith DC. Ivalon breast prostheses: evaluation 19 years after implantation. Plast Reconstr Surg. 1981; 67: 514-518.
- Rubin LR. Polyethylene--a three year study. Plast Reconstr Surg (1946). 1951; 7: 131-142.
- Edwards BF. TEFLON-SILICONE BREAST IMPLANTS. Plast Reconstr Surg. 1963; 32: 519-526.
- Dukes CE, Mitchley BC. Polyvinyl sponge implants: experimental and clinical observations. Br J Plast Surg. 1962; 15: 225-235.
- Spargo BJ, Rudolph AS, Rollwagen FM. Recruitment of tissue resident cells to hydrogel composites: in vivo response to implant materials. Biomaterials. 1994; 15: 853-858.