
Research Article
Austin J Forensic Sci Criminol. 2015;2(3): 1026.
Towards a Working Methodology for Using Total Hip and Knee Joint Replacements to Support Identification
Bryson D*
Department of Forensic Science, College of Life and Natural Sciences, University of Derby, Kedleston Road, Derby, DE22 1GB, United Kingdom
*Corresponding author: Department of Forensic Science, College of Life and Natural Sciences, University of Derby, Derby, United Kingdom
Received: May 12, 2015; Accepted: June 12, 2015; Published: June 29, 2015
Abstract
Hip and knee prostheses have occasionally been used to support identification of unknown persons along with other medical devices and implants. This paper looks at the specific issues around using hip and knee implants, suggesting a working methodology for their use in supporting identification during and after a post-mortem.
The value of Total Knee Replacements (TKR) and Total Hip Replacements (THR) as a means of identification along with other implants is a very recent area of interest in Forensic Science considering the long history of implants. This together with the recent introduction of Joint Replacement Registries means that using hip and knee implants to support identification is likely to become automatic in the future but is not currently automatic.
The paper looks at the accumulative collection of evidence as well as the range of issues including; the types and changes in early prostheses, examination of the body for external indications of implants, radiological recording prior to autopsy for confirmation of identification using matching of features with ante-mortem images, actual harvesting and collection of all parts of the joint replacement including cement and any other components, specific differences between TKR and THR.
In developing an approach to the problems associated with identifications using TKRs and THRs a stepwise process and the full recording of all of the features associated with the implant as well as manufacturers details and identification numbers is suggested so that the cumulative nature of these features will help to narrow down possibilities towards a more certain identification and confirmation of that identification.
Keywords: Hip; Knee; Identification; Joint; Replacement; Autopsy
Abbreviations
AJRR: American Joint Replacement Registry; NHS: National Health Service; NJR: National Joint Registry for England and Wales; THR: Total Hip Replacements; TKR: Total Knee Replacements; UK: United Kingdom
Introduction
The use of implants to support identification has been described in the literature in general for example [1,2], and as individual cases reporting on the positive identification of burnt remains from an automobile accident based on a bone healing stimulator [3], using a tibial plate [4], using a femoral plate [5], using hip replacement radiographs after a car accident [6] and a partially mummified woman [7] and from implants and hip prostheses in the Tri-State Crematorium incident [8] the use of metal screws in heavily disrupted human remains [9].
This paper outlines a working methodology for using hip and knee joint implants which can;
- in some circumstances, reliably be used as the sole form of identification
- are another item that can support identification
- help to narrow down the pool of likely matches for identification
In 2007 the National Joint Registry for England and Wales (NJR) recorded 68,950 hip procedures and 72,480 knee replacement procedures, these figures include revisions or re-operations (2008). These are the most useful of the implants as Clarkson comments ‘the usefulness of an implanted device in determining identity depends on the ability to associate that item to an individual’ (2007) and these two procedures have been compulsorily part of a data collection procedure in the United Kingdom (UK) since April 2003.
Historically hip joint replacements have been around for longer than knee replacements, see Table 1 developed from Scales paper on the history of hip replacements (1966) summarizing some of the changes in types of hip replacement. The number of people having joint replacements has steadily increased with the availability of modern surgical techniques but even reports on early operations, prospective studies, are looking at patient numbers in the high hundreds. Judet reported on 400 cases in 1952 and in 1955 Aufranc reported on 1,000 cases of hip arthroplasty over a 15 year period [10].
Year
History
1890
Thomas Gluck experimented with materials and suggested possibility of an ivory ball and socket joint.
1922
Ivory femoral head replacement, Hey Groves.
1923
Floating cup covering end of femoral head, Smith Peterson
1934
Rehn may have been originator of the metallic fixed acetabular cup.
1938
Wiles originator of metallic (stainless-steel) total hip replacement
1945
Judet acrylic femoral head
1950
Austin Moore femoral head prosthesis Thompson femoral head prosthesis using cobalt-chromium alloy ‘Vitallium’
1951
McKee total hip replacement Leventhal, titanium femoral prosthesis
1952
Self-curing acrylic cement
1956
Stanmore cobalt-chromium alloy total hip replacement
1959
Charnley cemented polytetrafluoroethylene (PTFE) and steel total hip replacement
1963
Polyacetyl and steel total hip replacement
Table 1: Dates in the early development of hip arthroplasty (after Scales 1967).
Early developments were looking at metal-on-metal until low friction combinations of metal-on-polymer were developed like the Charnley total hip replacement. More recent developments in joint replacement technology have been covered by [11] looking at hip replacement but whilst there are papers looking at modern developments of knee replacements there is no overview of their history and origin.
Working Methodology
Examination of the body for surgical scarring
This is looking for the presence of healed surgical scars or scarring around a joint that can indicate that the subject has undergone a procedure whether elective or emergency. The type of scarring will be indicative of the possible nature of the operation. Surgery involving the whole joint may or may not include joint replacement so scarring is only indicates that an operation has taken place not the type of operation, see Figure 1.
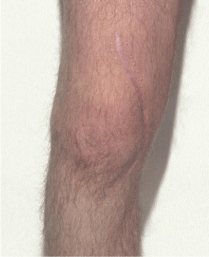
Figure 1: Close-up of healed scar following knee surgery note the curved
nature of the incision.
Most joint replacement operations are designed to fully correct the patients problems e.g. osteoarthritis. However, other features may be visible for example; difference in leg length comparing right and left, this may have led to a change in gait, deficiency in the lateral or medial collateral ligaments can lead to a varus or valgus deformity in the knee joint or excessive joint mobility, the presence or absence of this would obviously depend on the post-mortem interval.
The specific type of incision and their placement can also be indicative of surgeon preferences for operative procedures. So photographs of the scar when circulated could help to narrow down the hospital and surgical team responsible and so support identification.
Radiography of joints
Standard radiographic procedures have been described as below;
Primary survey: Initial triage and assessment
Secondary survey: Standard examination of specific body parts (e.g. dentition).
Tertiary examination: Specific examinations performed in response to findings during primary or secondary surveys or during pathology, odontology, or anthropology assessment [12].
Standard radiographs are taken to confirm the presence of any unique identifying features including joint replacements [12,13]. The views taken should be the same as those used in post-operative care of patients so that post-mortem radiographs can be directly compared with ante-mortem records.
This technique has been used for foot deformities by Sudimack [14] and a study by Rich [15] showed that radiographs of ankle surgery pre and post surgery could be relied on to support identification “Results indicate that surgical intervention with subsequent healing does not preclude positive identification in foot and ankle radiographic comparisons” Brogdon [15] shows an example of identification using ante and postmortem radiographs of an air crash victim who had undergone hip replacement surgery [6].
Radiographs of the prosthesis in-situ should be taken prior to removing the implant in skeletal remains so that the relationship can be seen between bony features and the implant itself. Where the subject is known or there are possible matches the radiographs can be used to provide a positive identification through assessment of the morphological characteristics of the prosthesis and bone. If further detail is needed the next stages can be undertaken and the prosthesis details compared directly with the subjects clinical records.
Examination in situ and collection of parts of joint prostheses
The joint including replacement should be examined and photographed as signs may be found of immediate response of the tissues to the prosthesis, remodeling of bone around implants or bone overgrowth [16]. The longer the prosthesis has been in place the more likely remodeling and bone overgrowth will have occurred, photography is useful as unlike radiography it can record the cartilage growth (See Figure 2).
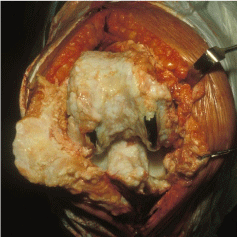
Figure 2: Overgrowth of bone and cartilage in the knee a natural response
from the body to the presence of a joint implant.
The number of parts and type of evidence that needs to be collected will vary depending on the type of joint replacement. All pieces should be recorded as they are collected including samples of the cement from each area, as chemical analysis may be needed to establish the type of cement used and there may be variations in cement used for different components (Acetabular compared to femoral). As some prostheses are non-cemented the presence/ absence of cement should be recorded.
Samples of the synovial fluid may be useful as the presence of fine or larger particles of polymers in the synovial fluid around the joint may be indicative of wear, similarly floating pieces of bone, cartilage or other tissues and an effusion and/or presence of blood may be indicative of joint trauma ante-mortem.
Hip and knee joints should be collected and collated with subject information including separating materials from right and left leg e.g. in a situation where there are bilateral implants or more than one joint has been replaced. This will provide extra information as the same prosthesis may or may not have been used on opposite sides, helping to narrow down the identity.
Hip joint: This is normally considered as having 2 components acetabular and femoral.
Acetabular component - This consists of 1 or more parts; the acetabular component is usually one piece but may be two pieces and fitted with or without cement though some types require screws into the hip bone.
Femoral component – This is usually seen as one piece but some modern replacements have two or more parts to them the long femoral piece to go into the medulla and a ball which fits onto the end of the femur and articulates with the acetabular component.
Knee joint: The knee joint is viewed as three joints in one, a tri compartmental unit, the lateral and medial compartments of the femur and tibia together and the patellofemoral joint. The medial tibial plateau is less circular than the lateral with the semi-lunar cartilages or menisci attached. The lateral meniscus can shift out of the way, being more mobile, with the medial bearing the brunt of injuries and often splitting or tearing, see Figure 3 for parts of the knee joint.
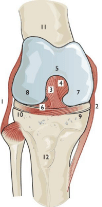
Figure 3: Anatomy of the knee joint. 1 – lateral collateral ligament, 2 – medial
collateral ligament, 3 – anterior cruciate ligament, 4 – posterior cruciate
ligament, 5 – intercondylar notch, 6 – tibial plateau, 7 – medial femoral
condyle, 8 – lateral femoral condyle, 9 – medial meniscus, 10 – lateral
meniscus, 11 – femur, 12 – tibial tuberosity, taken from Bryson 1999.
The standard joint replacement would be replacing all three parts of the joint so you would have a tibial, femoral and patellar components together with a meniscal component or components (Lateral and medial). A full knee joint replacement, referred to as a total knee replacement (TKR) replaces the whole joint as can be seen in Figure 4a. However, when the joint problem is uni-compartmental it may be only the medial or lateral compartments that are replaced, see Figure 4b. The uni compartmental knee replacement consists of a tibial plate, femoral component and meniscus which slides on the tibial plate the patella may or may not be replaced depending on its condition. The position medial or lateral should be recorded. As with the THR cement is often used with knee joint replacements but some types of replacement are cement free or may have screws.
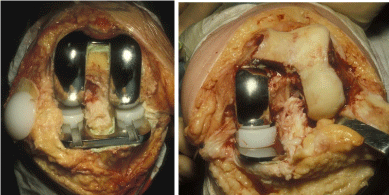
Figure 4: Knee replacements seen post-operatively a) Total knee
replacement b) Unicondylar knee replacement (Oxford knee).
In some cases for both a hip replacement and knee replacement bone grafting may also have been required.
This may be visible with removal of the joint or on the radiographs. If osteonecrosis has occurred parts of bone may be loose or clearly deficient in blood supply. At each stage evidence should be looked for as to whether the implant is a primary procedure or a revision.
Implants may be difficult to extract either due to strength of the cement or the overgrowth of cartilage and bone over the implant, see Figure 2. If the prosthesis type and manufacturer can be determined without it removal from the bony matrix this should be done.
Examination and identification of the joint prostheses
The aim of this stage is to collect data to support identification of the subject from the prosthesis. This data can then be compared against data held by a Joint Replacement Registry. The information needed is for the main components to match the data recorded by the National Joint Registry (NJR), see Table 2, further details that have been collected may be useful to narrow down or confirm identification once a possible match or matches have been found.
Areas Information is Recorded
Areas Information is not Recorded
Hip/knee components
Wire / Mesh
Bone cement (if used)
Cables / Plates
Accessories
Screws
Surgical tools, eg blades
Endoprosthesis
Bipolar heads
Table 2: Types of components that the National Joint Registry for England and Wales records information on.
The identification can be supported by distinctive features added by a manufacturer including, if they have added it to the implant, their company logo, product or individual serial number. Ubelaker suggests in his paper that “Like dentures, surgically implanted orthopedic devices may contain markings and information that likely will facilitate identification” [17]. The plethora of companies supplying implants and the specialist applications mean that their logos are not as identifiable compared to those used for common household objects. There are websites that support logo identification e.g. https://www.brandsoftheworld.com/ and https://www.seeklogo. com/.
One of the difficulties is not just identifying the manufacturer but the type of prosthesis, its size and other features that will help to narrow down the number of likely matches. Components should be accurately photographed so visual records can be sent to the manufacturer to identify the precise model and combination of components. After determining the manufacturer it is the characteristics of the design that can help determine the exact model. Until a reference database of old and new implants is available manufacturers or local orthopaedic surgeons would be the best experts to classify the type of implant.
Indications of wear on prostheses have been examined using a range of techniques including the use of ultraviolet fluorescent powder on femoral surfaces [18] and its extent should be noted as it may be indicative along with cartilaginous and bony overgrowth of the length of time the implant has been in place. Removal of bony growth may be needed to view an implants manufacturers logo or serial number.
Problems associated with using hip and knee implants for identification purposes
Recent establishment of registries when hip implants have been in use for almost 100 years: The recent establishment of the NJR, first data collected in 2003, and if you look at individual regions or hospitals statistics even that date may not be when hospitals actually started to upload data. The total number of operations recorded in 2003/04 was 62,191 climbing to 107, 172 in 2005/06 and 132, 578 in 2006/7 with a slight decrease in 2007/08 so full records are only really available from 2005.
Coverage is national not international with some countries having no registry in place: The National Joint Registry for England and Wales (Web address https://www.njrcentre.org.uk), established in 2002, is managed by the Healthcare Quality Improvement Partnership and is funded by levies on joint components acetabular for hip, femoral component for knee. The aim of this registry is to support and monitor the outcome of surgery; however data requests can be made. Other countries have a joint registry including Sweden, Norway, Australia and Canada.
Device tracking in the United States, by the Food and Drug Administration [19], is limited to those devices considered a serious risk to health e.g. pacemakers and other electrical devices [20]. The Kaiser Permanente National Total Joint Replacement Registry is reported as having collected data on total hip replacements, for clinical analysis from 2001-2008 but coverage is restricted to a number of regions recording 16,945 total hip replacements compared to the estimated annual 600,000/year undertaken in the United States [21].
The American Academy of Orthopaedic Surgeons discussed these issues in 2007 [22] and has since developed the American Joint Replacement Registry (AJRR) https://teamwork.aaos.org/ajrr/ default.aspx following a pilot study in 2010 with the goal in their 2013 report of becoming ‘the first comprehensive national hip and knee orthopaedic implant registry in the United States’ [23] and to enroll 90% of all institutions conducting hip and knee replacements [24].
Lack of complete linkage between a record and NHS patient number
There is currently a lack of complete linkage between a record and NHS patient number, see Figure 5, with a linkage rate of only 69% in 2006/07 with a target of 90% for 2008 [25].
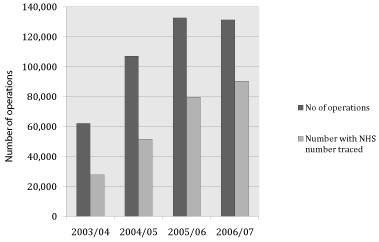
Figure 5: Graph showing linkage between Number of operations and number
with NHS number traced (National Joint Registry for England and Wales
2008b).
Lack of compliance to ensure records are complete
The lack of compliance to ensure records is complete and accurate from every hospital [25].
The number of manufacturers and number of implants they each produce
“In 2007, 129 different brands of acetabular cups and 144 different brands of femoral stems were used and recorded in the NJR. The total number of acetabular Cups listed is 165 and 187 different brands of femoral stem: an increase of 7% over 2006 for both stems and cups. This was a result of new suppliers entering the market, the introduction of new brands by existing suppliers and/or improved reporting” [25].
For femoral stem brands entered onto the database in 2007 the choice is between cemented or non-cemented, primary or revision hip procedure, then manufacturer, and brand (Really model or type e.g. name like Exeter V40 or Stanmore modular). For cemented stems there are 20 manufacturers and 65 different brands, with Biomet for example having 11 different brands/stems in use.
The high frequency with which some components are used
The most popular cemented brand during 2007 for primary and revision procedures was the Exeter V40 made by Stryker Howmedica Osteonics used for 18,524 primary operations with 54% of the market and 1,118 of revision procedures 47.7% of the market [26]. Similarly for non-cemented the top primary hip replacement is the Corail by DePuy with 44.5% (9,477) of the market. Other factors like size of implant and corresponding acetabular cup may help but this does means that an exact match for either of these implants if found at post-mortem would be unlikely without other supporting evidence i.e. serial numbers. However, the opposite is also true as in 2007 some 213 component combinations, femoral stem and acetabular cup were unique to individual patients for primary procedures.
In knee implants similar high frequency occurs for some brands with the DePuy PFC Sigma Bicondylar knee having a 34.8% market for primary (20,859) and revision (640) replacements.
Conclusion
This paper proposes through this stepwise approach that it is more likely to be the accumulation of evidence about the prostheses which will be valuable evidentially rather than a direct hit on a registry database and that the evidence collected will also be available to provide confirmation if a direct hit on the database does occur with those individuals that have had a joint replacement over the past 5-10 years.
For identification we need to know what has been used not just now but in the past to fully cover the possibilities when a post-mortem examination reveals an implant. Someone who was born 70-100 years ago will have had very different treatment during their life than we have or children being born now will have in the future. Research should include testing of the proposed working methodology, collection and analysis of hip and knee implants including data and photographs both of new and used, looking at the effect of the body and wear processes on the implant, of burial/post-mortem conditions on implants and wear or wear patterns.
References
- Clarkson J Schaefer M. Surgical intervention. In: Thompson T, Black S, editors. Forensic human identification: An introduction. London: CRC Press. 2007.
- Simpson EK, James RA, Eitzen DA, Byard RW. Role of orthopedic implants and bone morphology in the identification of human remains. J Forensic Sci. 2007; 52: 442-448.
- Bennett JL, Benedix DC. Positive identification of cremains recovered from an automobile based on presence of an internal fixation device. J Forensic Sci. 1999; 44: 1296-1298.
- Dedouit F, Telmon N, Guilbeau-Frugier C, Gainza D, Otal P, Joffre F, et al. Virtual autopsy and forensic identification-practical application: a report of one case. J Forensic Sci. 2007; 52: 960-964.
- Pushparani C, Ravichandran CP, Sivakumari K. Radiography Superimposition in Personal Identification - A Case Study Involving Surgical Implants. Journal of Forensic Research. 2012; 3: 1-4.
- Brogdon, BG. Forensic Radiology. London: CRC Press. 1998.
- Dolinak D, Matshes E. Identification. In: Dolinak D, Matshes EW, Lew EO, editors. Forensic pathology: Principles and practice. 2nd ed. 2005: 557.
- Steadman DW, Sperry K, Snow F, Fulginiti L, Craig E. Anthroplogical investigations of the Tri-State Crematorium incident. In: Adams B, Byrd J, editors. Recovery, analysis, and identification of commingled remains. Humana Press: Totowa, NJ. 2008.
- Blau S, Robertson S, Johnstone M. Disaster victim identification: new applications for postmortem computed tomography. J Forensic Sci. 2008; 53: 956-961.
- Scales J. Arthroplasty of the hip using foreign materials: A history. Proc Inst Mech Engrs. 1966-67; 181: 63-84.
- Dowson D. Hip replacement: tribological principles, materials and engineering. In: Revell, P.A. ed. Joint replacement technology. Cambridge: Woodhead Publishing Limited. 2008.
- Viner MD. The use of radiology in mass fatality events. In: Adams BJ, Byrd JE, editors. Recovery, analysis, and the identification of commingled human remains. New York: Humana Press. 2008: 167-168.
- Gould P. X-ray detectives turn images into evidence. 2003.
- Sudimack JR, Lewis BJ, Rich J, Dean DE, Fardal PM. Identification of decomposed human remains from radiographic comparisons of an unusual foot deformity. J Forensic Sci. 2002; 47: 218-220.
- Rich J, Tatarek NE, Powers RH, Brogdon BG, Lewis BJ, Dean DE. Using pre- and post-surgical foot and ankle radiographs for identification. J Forensic Sci. 2002; 47: 1319-1322.
- Revell, P.A. The healing response to implants used in joint replacement. In: Revell PA, editor. Joint replacement technology. Cambridge: Wood head Publishing Limited. 2008.
- Ubelaker D, Jacobs C. Identification of orthopedic device manufacturer. Journal of Forensic Sciences. 1995; 40: 168-170.
- Ward DM. The use of fluorescence markers to record prosthetic wear patterns. J Audiov Media Med. 1996; 19: 123-129.
- Food and Drug Administration. Medical device tracking.
- Weitzman JB. Electronic medical devices: a primer for pathologists. Arch Pathol Lab Med. 2003; 127: 814-825.
- Paxton EW, Inacio M, Slipchenko T, Fithian DC. The kaiser permanente national total joint replacement registry. Perm J. 2008; 12: 12-16.
- Hayashi A. Building a national joint replacement registry. AAOS Now. 2009; 7.
- American Joint Replacement Registry. Fall 2103 Update. 2013.
- Smith MA, Smith WT. The American Joint Replacement Registry. Orthop Nurs. 2012; 31: 296-299.
- National Joint Registry for England and Wales. Supporting data for figures for part 1, 5th Annual Report. 2008b.
- Sibanda N, Copley LP, Lewsey JD, Borroff M, Gregg P, MacGregor AJ, et al. Revision rates after primary hip and knee replacement in England between 2003 and 2006. PLoS Med. 2008; 5: e179.