
Research Article
Austin J Forensic Sci Criminol. 2015;2(3): 1029.
Predictive Models as Screening Tools for DNA Recovery from Baked and Burned Porcine Bones
Velzen IV², Shaw M¹, Raveendran M² and J. Gonzalez-Rodriguez²*
¹University of Lincoln, School of Life Sciences, Brayford Pool, United Kingdom
²University of Lincoln, School of Chemistry, Brayford Pool, United Kingdom
*Corresponding author: J. Gonzalez-Rodriguez, University of Lincoln, School of Chemistry, Brayford Pool, Lincoln, United Kingdom
Received: June 01, 2015; Accepted: June 25, 2015; Published: June 30, 2015
Abstract
Burnt bones and skeletal remnants continue to challenge the proficiency of forensic investigations in human individualization and identification. The various natural disasters and human inflicted crimes involving fire leave the forensic investigators with very little to work on. Thus, demand for practical studies to obtain useful facts for improvisation of current techniques and to overcome the short comings is a prerequisite. In this study Design of Experiments (DOE) as an investigative and screening tool to relate the different variables (burning temperature, time, thickness of flesh, presence of accelerants) involved in the burning process and to detect the probability of obtaining successful DNA identification from burnt bones is proposed. We show that high temperature and large base pair PCR primer have a significant effect on DNA retrieval and amplification. The baking study provides reproducible DNA identification with maximum retrieval temperature of 320°C for the smallest (106bp) amplicon. The study involving accelerants demonstrates that those with high specific heat capacity decrease DNA recovery, hence suggesting probable damage to DNA. Through this study the positive effect of presence of flesh for DNA recovery was also verified with a maximum DNA recovery temperature of 500°C. Utilizing all these information through DOE, predictive models were also created with regression equations to calculate positive DNA amplification and to predict the different variables respective to the burning process. These models created using porcine bones could be related for real scenarios and with more data procurement it could be used effectively in forensic investigations.
Keywords: Design of Experiments; DNA recovery; Forensic investigation; Burnt bones
Introduction
Forensic investigations involving identification and individualization of burnt human bones remain a challenge. Human bodies subjected to extreme temperatures as a result of accidents, mass disasters [1, 2, 3, 4, 5], death by fire [6, 7, 8, 9] and in some cases as an act to conceal crime [10, 11, 12] often leave behind only burnt bones or fragments of bones and dental remnants at the crime scene. In these cases, with loss of skin and tissue, visual identification and fingerprinting methodologies are not possible. Occasionally, even dental identification becomes impossible or difficult due to lack of records. Histological studies may shed light on the origin of skeletal remains but DNA analysis is needed for singular identification. Thus, under these circumstances genetic finger typing becomes a much-expected choice, but the absence of flesh or proper substrate for DNA makes extraction problematic. In severely burnt/charred bones there is a high likelihood of degraded DNA template, which makes successful amplification difficult, reducing the probability of identification.
Studies in this area have led to the development of modified and improved DNA isolation procedures [13] to overcome problems and achieve effective DNA extraction. Yet identifying severely burnt bones through DNA methods remains problematic as the process of burning changes the bone both physically and chemically [14] degrading the DNA. To understand the different complications, research concerning the histology of burnt bones, elemental analysis of burnt bones, and relation between burning temperature and changes in bone has been conducted [15, 16, 17]. The above studies provide valuable information regarding the burning temperature, indication about the tests to be performed and even estimations on how the burnt bones had been treated. Reports investigating the probability of successful DNA retrieval from bones subjected to high temperatures seem to show that the positive DNA results are a factor of both temperature and duration of burning.
While some studies show a positive DNA extraction and amplification even at high burning temperatures [18], most studies were only able to yield positive results at lower temperatures [19, 20]. Studies also indicate mitochondrial DNA (mtDNA) to be a better choice than nuclear DNA [18] as mtDNA is available in greater numbers and thus there is a higher chance to achieve positive extraction and amplification. Contradicting results reporting the lack of amplifiable DNA (even mtDNA) at all temperatures [21] could be due to the different DNA extraction procedures used or burning temperatures and procedures. Nevertheless it is of evidence through these studies that the probability of successful DNA extraction and amplification is higher in bones burnt at a lower temperature for a short period, and the difficulty increases with increasing burning period and temperature.
From the above practical conclusions, the possibility of various factors affecting the probability of DNA extraction, amplification and individual identification from burnt bones should be recognized. While studies on burning temperature and burning time as variables are available and acknowledged, as stated above, and since the already available experimental studies involve burning bones in an electrical or industrial furnace, a study concentrating on several variables like effect of ignitable liquids, presence of flesh, effect of baking including burning temperature and time is required. As the majority of crimes related to burning victims (accidental fire, arson cases, etc.) contains use of accelerants or fuels [22], the present study providing information linking ignitable liquids-burning temperature/time and DNA retrieval is needed to accept or reject any theories concerning inhibitory effect of ignitable liquids on DNA extraction and amplification (if any).
Previous works [23, 19] list histological studies and analytical techniques as approaches to evaluate the potential of DNA recovery. These procedures provide us an indication if the bone samples are capable of a subsequent positive result, thus acting as a diagnostic tool. These tests not only save money and energy wasted on timeconsuming procedures but also suggest useful information regarding temperature and time the bones were burnt, which is relevant in a forensic investigation. With regard to this fact, the use of statistics to build a model, by experimental design (DOE), to be used as a diagnostic tool was attempted in this study.
The major aim of this study is to design a predictive model that could be used for calculating burning time, burning temperature and also work as a diagnostic tool to ascertain a probability of recovering DNA from burnt bones. Analysis of burnt and baked bones with ignitable liquids, temperature, time and flesh as variables was performed to study the associations between them. The data collected from this study and the designed model would serve as an indicative tool to assess burnt bones for various forensic purposes. The recurrent encounters of burnt bones in forensics and the constant attempts by the suspect to mislead and conceal evidence [24, 25, 26] necessitates more experimental work to be done in this field. This will bring about reliable methods and useful data to diminish limitations and overcome drawbacks that are encountered with present procedures. Thus this project proposes the use of statistics to study the association between different variables involved in burning bones and depicts a basic model as a screening tool, alike which further models could be designed for various other requirements.
Methods and Materials
Bone samples preparation
Porcine bones selected for this experiment were acquired from a butchers shop, Lincoln, UK. Similar sized phalangeal and rib bones (Figure 1) were used in this study. The bones were divided into three groups and labeled BRF (burned with flesh), BR (burned with ignitable liquids) and Baked without flesh (BK) for ease of reference and separate temperature studies were generated for each group. Group BR and BK bones were de-fleshed and cleaned before further analysis. Group BKF consisted of bones with flesh of different thickness (5mm, 10mm, and 15mm). The bones were burnt with and without using ignitable fluids in a crucible inside a chemical hood/ gas chamber, and the oven set at different temperatures was used for baking.
Experimental Conditions
Burning with ignitable liquids (BR): De-fleshed phalangeal and rib bones were burnt with white spirit, petrol and ethanol at different temperatures and then tested for retrievable DNA. The bones were weighed and 0.5ml/g to 2ml/g accelerant with an increase of 0.5ml was added for the burning procedure. Maximum burning time and temperature association of different ignitable liquids used were also studied by burning the accelerants on their own in a crucible. The maximum time taken to self extinguish and the related maximum temperatures reached were recorded using a stop clock and an infrared thermometer. Temperature studies relating to the particular accelerant used, accelerant volume, burning time and the probability of obtaining a positive DNA amplification were also implemented.
Baking without flesh (BK): Bones were baked at temperatures between 50ºC to 400ºC for 10 to 40 minutes and DNA was extracted using either Chelex or Phenol chloroform method. Two separate DOE models were built for both the extraction procedures. The baking studies in the oven were done to maintain a constant and nonfluctuating temperature, thus establishing an accurate temperature model. The main aim of the study was to chart the effect of baking on DNA recovery. The use of two different extraction procedures would shed light on the limits of the method in relation to the maximum burning temperature and time till which DNA extraction is possible. This study could also postulate evidence on the more capable DNA extraction procedure of the two.
Burning with flesh (BRF): The phalangeal and rib bones with flesh of various thicknesses (5 to 15 mm) were first burnt without any temperature or time constraint to gain an overview of temperatures and time. The uncontrolled experimental conditions would also provide information about how fleshed bones burn and how the other variables have an effect on it. The fleshed bones were then subjected to different sets of temperature and time period ranging between 100ºC to 500ºC and 5 to 30 minutes. The effect of thickness of flesh on extracting DNA and its relationship with burning time and temperature was established. All the temperature models built were designed using the statistical Design of experiments (DOE) technique as explained below, using the stat graphics software.
Design of experiments
Design of Experiments (DOE) is a systemic statistical way of designing a model that delivers the most information out of a fewest number of experiments by creating an equal data. It has three main phases, creating an experiment, analyzing the results and further experimentation.
- The first phase was to select the experimental factors and limits for the program to predict an optimum set of conditions that would produce a perfect dataset. This way one would not be wasting time and resources performing numerous experiments to obtain enough data that has relevant information. The program produces an optimal design for the given parameters.
- The next phase was to practically test the conditions created by the program and input the resultant data back to the program, to analyze the results by fitting various statistical simulations and ultimately come up with a model that could be used to define the parameters. In this phase the design allows us to perform the following four functions.
- Comparison – To assess if a change in any one experimental factor leads to a change or improvement to the whole process.
- Characterization – To screen and rank the important factors that have a significant effect on the process.
- Model – to design a model of the process with predictive function.
- Optimization – to determine optimal settings for the experimental factors in order to optimize the process response.
- The final phase is to augment the design by specifying different parameter settings or experimental factor limits and optimizing the resultant data to an even better value if needed.
The parameters considered for this study were burning temperature, burning time, effect of ignitable liquids (IL) and volume of IL and the presence of flesh. Two major designs one for studying the relationship between baking temperature, time and its effect of obtaining DNA and one for studying the influence of flesh in obtaining DNA at different burning temperatures and time was performed. The effect of different type of ignitable liquids on DNA extraction was also tested as a univariate function. The bones were heat-treated (burned or baked) to a set of temperatures for appropriate time periods as predicted by statistical Design of Experiments (DOE) and different models were developed to test the parameters considered in this study.
DNA analysis – chelex and phenol chloroform method
Bone samples taken from each group were grounded finely using pestle and mortar and DNA was extracted from 0.25 grams of ground bones using Chelex and Phenol Chloroform [Phenol: Chloroform: Isoamyl Alcohol 25:24:1, pH 8] methods as stated elsewhere [27, 28]. The two methods were compared to figure out the most effective and plausible way of extracting usable DNA from burnt bones. The DNA pellets were suspended in 50μl TE buffer and stored at -20 degree Celsius. The concentration and purity of the extracted DNA was analyzed using a thermo scientific Nanodrop UV spectrophotometer.
DNA amplification and detection
Three forward and reverse primer pairs (Table 1) targeting different sized fragments (106bp, 199 bp, 411 bp) of 12s rRNA gene of Sus scrofa were designed and purchased from Sigma Oligo. Polymerase chain reaction (PCR) was performed to amplify the indicated fragments in 0.2ml PCR tubes. The primers were optimised to a concentration of 10 pmol to obtain clear and reproducible bands. The different annealing temperatures for each primer set were also optimised. Refer supplementary data, Table 10, for cycling parameters- temperatures and times, for each primer set.
Targeted fragment length
Forward primer
Reverse primer
106 bp
5-CGACTCATTAATAACCCACAA-3
G5-TTAGTGATCAGGTTTGGCCTTT-3
199 bp
5-TATCCGCGCCCCGGTGAGAA-3
5-GCGGTGGCTGGCACGAGATT-3
411 bp
5-AAAGGACTTGGCGGTGCT-3
5-GTTACGACTTGTCTCTTCGTGCA-3
Table 1: Primers used in the study.
Amplified DNA was analysed using gel electrophoresis procedure on a 2% agarose gel in TAE and visualized under UV light (Figure 2). Positive DNA amplification was documented with presence of bands for 106bp, 199bp, and 411bp fragments, absence of visual bands was indicative of lack of amplification and hence noted as negative.
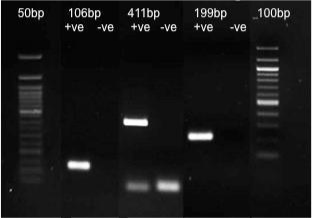
Figure 2: Optimal primer concentration (10 pmol) producing bands for 106bp,
411bp and 199 bp fragments of Sus scrofa 12s rRNA gene. (Please note that
the lanes are stitched together from different gels ran separately).
Results
Comparing DNA extraction methods for DNA recovery
Two DNA extraction techniques, Phenol chloroform and Chelex method were compared for their effectiveness to retrieve usable DNA from heat subjected (baked using oven) bones. The entire statistical design that resulted in a predictive model was done using stat graphics. The different temperature and time periods the bones were baked and the respective DNA retrieval ability of the Chelex extraction procedure is listed (Table 2).
Temperature (°C)
Time
(Minutes)
Bone Sample
106bp
199bp
411bp
50
25
Rib
Yes
Yes
Yes
Phalange
Yes
Yes
Yes
50
10
Rib
Yes
Yes
Yes
Phalange
Yes
Yes
Yes
50
40
Rib
Yes
Yes
Yes
Phalange
Yes
Yes
Yes
200
10
Rib
Yes
Yes
Yes
Phalange
Yes
Yes
Yes
200
25
Rib
Yes
Yes
Yes
Phalange
Yes
Yes
Yes
200
25
Rib
No
No
No
Phalange
No
No
No
200
40
Rib
Yes
Yes
Yes
Phalange
Yes
Yes
Yes
350
10
Rib
No
No
No
Phalange
No
No
No
350
25
Rib
No
No
No
Phalange
No
No
No
350
40
Rib
No
No
No
Phalange
No
No
No
Table 2: Chelex extraction method - DOE variables design. The different temperature and time ranges and their respective PCR amplification results for the three target fragments.
It could be seen from the results that high temperature had a significant effect on DNA retrieval, whereas burning time affected DNA recovery with respect to the temperature. Bones baked at temperatures of 350°C showed no visible bands post amplification irrespective of the time. But temperatures 200°C and 50°C showed fluctuations in successful amplifications when subjected to a longer time period of above 25 minutes. The length of the targeted fragment also had an effect on PCR, while shorter fragments (106 and 199 bp) exhibited positive amplifications; the large 411 bp was not amplified sometimes, even at lower temperatures and shorter time frames. Similar results were obtained for both Chelex and phenol chloroform extraction.
Two separate analysis for Chelex and phenol chloroform extraction designed with 20 runs to be run in 2 blocks (1 replicate) studied the effects of two factors (temperature and time) on the response variable (DNA amplification). With a fully randomized experimental setup estimated effects for the response variable and the interactions of the factors were obtained.
The ANOVA test (for Chelex method) indicating statistical significance of the factors at a 95.0% confidence level showed temperature had a significant effect on the response variable (amplification of 106 and 199 bp target fragments) whereas both temperature and time had a significant effect on amplifying the longer 411 bp fragment. Irrespective of individual effects there was no significant interaction between the factors for all three fragments. These results were also supported by the Pareto chart and normal probability chart (refer Figure 7- supplementary material).
These charts furthermore provide a visual representation of the important and real factors hence allowing us to remove background noise or the factors without a significant effect. Even though R squared value of 73.21%, 73.21% and 70.73% for 106, 199 and 411 bp respectively indicates a low fit of the created model to the response data (i.e. only the indicated % of the variability is accounted for), the low average error (0.18 for the shorter and 0.2 for larger fragment) in predicting the response using this fitted model makes it a good enough tool.
Similar experimental designing (Table 3) for the model tested with phenol chloroform extraction method showed a high R squared value of 84.87, 73.21 and 100 % for 106, 199 and 411 bp fragments respectively; which indicated a very well fitted model compared to the one tested with Chelex extraction procedure. The factors also showed different effects in that only temperature had a significant effect on all fragments irrespective of burning time.
The fitted models were tested for predictive values and again various plots were used to understand the results. While the main effects plot for the Chelex model showed temperature has the most impact on the response for 106 and 199 bp, both time and temperature played a role in amplifying the 411bp fragment. The interaction plot (refer figure 1 supplementary material) supported the Pareto chart in indicating no significant interaction between the factors. It also suggests that the effect of one factor (temperature) affects the other (time) for 411 bp but not 106 and 199 bp fragments.
Figure 1: Phalangeal and rib bone used in the study.
Temperature (°C)
Time
(Minutes)
Bone Sample
106bp
199bp
411bp
50
10
Phalange
Yes
Yes
Yes
Rib
Yes
Yes
Yes
50
20
Phalange
Yes
Yes
Yes
Rib
Yes
Yes
Yes
50
30
Phalange
Yes
Yes
Yes
Rib
Yes
Yes
Yes
225
20
Phalange
Yes
Yes
No
Rib
Yes
Yes
No
225
10
Phalange
Yes
Yes
No
Rib
Yes
Yes
No
225
30
Phalange
Yes
Yes
No
Rib
No
Yes
No
225
20
Phalange
Yes
Yes
No
Rib
Yes
Yes
No
400
10
Phalange
No
No
No
Rib
No
No
No
400
20
Phalange
No
No
No
Rib
No
No
No
400
30
Phalange
No
No
No
Rib
No
No
No
Table 3: Phenol Chloroform method - DOE variables design. The different temperature and time ranges and their respective PCR amplification results for the three target fragments.
Similarly there were no interactive effects observed amidst the factors in Pareto chart and ANOVA for phenol chloroform model. However the interaction plot showed a slight change in the factors (with respect to the other) for 106 bp, whereas none was observed for the larger fragments (refer Figure 8-supplementary material for charts).
The optimum values of temperature and time predicted to produce a positive PCR amplification of the fragments predicted through the surface and contour response curves are listed in Table 4 below along with the respective regression equation for the fitted model.
DOE
Model
Target
Fragment
Optimum Temperature
(°C)
Optimum Time period (minutes)
Regression Equation
(Temperature=T, time – t)
Chelex
106 bp
68.75
10
106bp=1.39683-0.047619*t+ 0.00174603*T+ 0.000952381*t^2 + 0.0*t*T 0.0000126984*T^2
199 bp
68.75
10
199 bp = 1.39683 + 0.00174603*T - 0.047619*t - 0.0000126984*T^2 + 0.0*T*t + 0.000952381*t^2
411 bp
50
40
411 bp = -0.746032 + 0.00309524*T + 0.0761905*t - 0.00000634921*T^2 - 0.000111111*T*t - 0.000634921*t^2
Phenol Chloroform
106 bp
102.49
16.10
106 bp = 0.719145 + 0.00239067*T + 0.0345238*t - 0.0000116618*T^2 + 0.0*T*t - 0.00107143*t^2
199 bp
137.52
24.31
199 bp = 1.39683 + 0.00174603*T - 0.047619*t - 0.0000126984*T^2 + 0.0*T*t + 0.000952381*t^2
411 bp
50
20
411 bp = 1.46939 - 0.0102041*T + 0.0*t + 0.0000163265*T^2 + 0.0*T*t + 0.0*t^2
Table 4: Optimal (Best) variable values and regression equation for both DNA extraction models.
The predicted response values plotted as a response surface plot show that the highest recovery of DNA from the burned bones predicted by the model can be obtained at temperatures less than 100°C for the Chelex model. The phenol chloroform model showed a highest response for 411 bpat temperatures less than 50°C and near 200°C for 106 and 199 bp fragments, burning time had a uniform response all through the temperature range for both models (Figure 3).
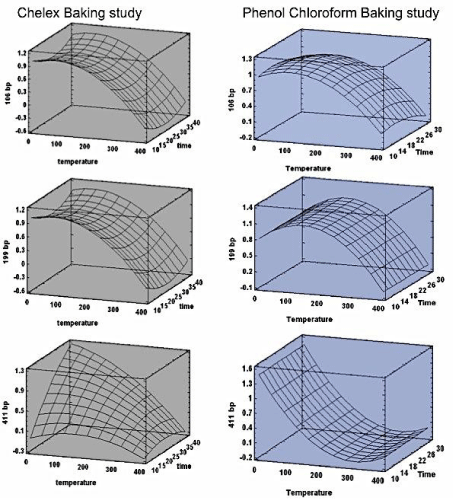
Figure 3: Surface response charts - predicting the values of temperature and time at which the highest response could be achieved. Column one (gray) contains
the response surface charts for Chelex method and column two (blue) has the response charts for PC.
The temperature study with DOE provided an optimum temperature and burning time with which positive DNA amplification is possible; it also provided a range of temperatures at which DNA recovery is reproducible. To further confirm the maximum temperature at which positive DNA amplification is possible, and to further test the range of positive DNA amplification temperatures, a second temperature study (Table 5) from 220°C to 340°C with a 20°C increase was carried out with a constant optimal burning time of 20 minutes. Phenol chloroform extraction procedure was opted for this study as the DOE model for PC provided a better fit than Chelex model. The temperature and time range was applied to the previously fitted model to generate the predictive values indicated as DOE fitted value in Table 5; the outcomes were double checked by practically carrying out the experiments also.
Bone sample
Temperature (°C)
Conc. of DNA obtained
(ng/μl)
Post PCR Band visibility on agarose Gel
(bp)
DOE Fitted value
106
199
411
106bp
199bp
411bp
1
220
132
Yes
Yes
Yes
0.957447
1.01388
0.0146939
2
240
318
Yes
Yes
No
0.897971
0.953469
-0.0391837
3
260
195
Yes
Yes
No
0.829167
0.88
-0.08
4
280
216
Yes
Yes
No
0.751033
0.793469
-0.107755
5
300
93
Yes
Yes
No
0.663569
0.693878
-0.122449
6
320
-
Yes
No
No
0.566776
0.581224
-0.124082
7
340
-
No
No
No
0.460654
0.45551
-0.112653
Table 5: Baking temperature study model generating predictive values for DNA amplification.
From this study the new maximum temperature for DNA recovery was identified as 300°C at the shorter fragments, temperatures any higher up to 320°C show successful amplification only for the smallest fragment of 106 bp lengths and for temperatures further higher no visible bands were seen. The larger fragment only produced vaguely visible bands for 220°C and didn’t produce any for higher temperatures. The Predictive values from the PC model supported this. Values closer to 1.0 show a better probability in obtaining reproducible visible bands after PCR amplification whereas decreasing values closer to 0.0 and negative values point towards a negative PCR amplification result. As observed from the table, the DOE fitted values are decreasing with respect to increase in burning temperature, which further confirms the model that temperature as a factor has an hard influence on DNA retrieval from severely burned bones.
Effect of ignitable liquids
To understand the properties of ignitable liquids used in the study, they were initially individually burned on their own. The highest burning temperature reached and the time taken for self-extinguish ion was noted to chart the temperature and time relationship. Of the three, white spirit burned the longest and reached the highest temperature of 300°C. Petrol burnt the quickest (10 minutes) and reached a temperature of 260°C, whereas ethanol burned for 11 minutes and reached 230°C. The effect of different ignitable liquids in varying quantities on positive DNA recovery with reference to the burning time and temperature reached was studied. Table 6 shows positive DNA amplification recorded for all the different trials.
Ignitable liquid
Sample
Volume 0.5ml/g
Time
DNA Conc.
DNA Amplification
Temperature
Minutes
(ng/μl)
106,199,411bp
Average recorded
Highest reached
White spirit
R1
3.2
3.10
1314
Yes
210
312
R2
4.75
1.40
2193
Yes
140.9
296
P1
1.1
3.11
285
Yes
167.1
262
P2
1.2
1.35
132
Yes
100.9
203
Petrol
R1
4.9
0.46
1293
Yes
189.2
254
R2
3.6
0.50
1118
Yes
180.7
266
P1
1.15
0.29
606
Yes
125.3
162.9
P2
1.2
0.24
957
Yes
103.3
150.4
Ethanol
R1
3.8
0.50
1857
Yes
180.3
261
R2
3.8
1.07
2208
Yes
171.6
254
P1
1.3
0.34
435
Yes
153
223
P2
1.45
0.41
273
Yes
186.7
259
Positive control
P
-
-
-
Yes
-
-
Negative control
N
-
-
-
No
-
-
R= Rib bone, P= phalange bone.
Table 6: Results of Burning bones with different ignitable liquids.
Volumes up to 2ml/g of accelerants and the high temperatures reached as a result of accelerant-induced heat had no negative effect on DNA extraction and amplification for this particular study. It was also noted that the increasing temperature was directly proportional to the volume of accelerant used.
It can be observed that White spirit produces the lowest DNA concentration, which could be related to its high burning temperature and time. It could be speculated that ignitable with high specific heat capacity burned the longest and reached highest temperatures resulting in low quantities of DNA. But given that petrol has more impurities than the rest of the ignitable used, its interference with DNA extraction could be a hindrance and thus despite its lower heat capacity than ethanol, it results in lower DNA concentration. Nevertheless the DNA amplification process was successful irrespective of these differences.
The increasing volume of accelerants used to burn the bones did not have any significant effect on DNA extraction and amplification of shorter base pair targets. Quantities up to 2ml/g was tested (Table 7), while increasing volumes resulted in blurred faint bands post amplification for short fragments, the longer fragments showed absence of amplified products even at lower volumes (Figure 4). This shows that the combined effect of large accelerant volume and a large fragment selected for PCR would hinder successful DNA amplification.
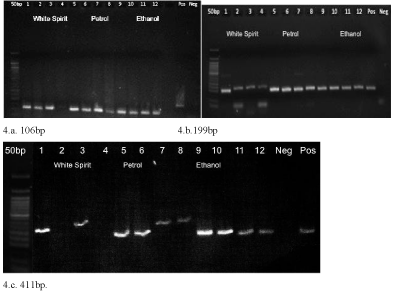
Figure 4: Gel electrophoresis images for DNA amplified from bones burned
with ignitable liquids. In pictures 4.a, 4.b, and 4.c lane 1 to 4 – white spirit
(0.5,1.0,1.5,2.0 ml), lane 5 to 8 – petrol (0.5,1.0,1.5,2.0 ml), lane 9 to 12 –
ethanol (0.5,1.0,1.5,2.0 ml).
Ignitable liquid
Volume
ml/g
106 band
199 band
411 band
White spirit
0.5
Yes
Yes
Yes
1.0
No
Yes
No
1.5
Yes
Yes
Yes
2.0
No
Yes
No
Petrol
0.5
Yes
Yes
Yes
1.0
Yes
Yes
Yes
1.5
Yes
Yes
Yes
2.0
Yes
Yes
Yes
Ethanol 100%
0.5
Yes
Yes
Yes
1.0
Yes
Yes
Yes
1.5
Yes
Yes
Yes
2.0
Yes
Yes
Yes
Positive control
-
Yes
Yes
Yes
Negaitive control
-
No
No
No
Table 7: Bones burnt with increasing volumes of accelerants and their PCR amplification results.
We suggest that accelerants with high heat capacity that burn longer are capable of reaching higher temperatures, thus are more likely to damage DNA. In this case, bones burned with white spirit could be relatively problematic for DNA retrieval than petrol and ethanol. Large quantities of accelerants also have a negative effect on successful amplification. It should also be noted that the burning temperatures in this study did not reach any higher than 300°C, as the amount of samples burned were small. Even though theoretically from this study one would need a very high volume of accelerant to completely destroy a full skeleton to deprive it of usable DNA, the real crime scenes like fire accidents, self-incineration, etc. would produce a very high degree of heat, seriously impacting DNA extraction and amplification. Nevertheless this small-scale study could be helpful to relate to real case crime scenes.
Effect of flesh
To test the theory that presence of flesh preserves DNA after a fire, bones with 5, 10 and 15 mm thickness flesh were burned for different time intervals within a recorded temperature range of 350- 500. To avoid contamination, the DNA extractions were made from ground bones thoroughly cleaned after removal of burnt flesh. It was observed that bones with thicker flesh produced positive DNA amplification even if it was burned for longer time periods (Table 8). The concentration of DNA retrieved had a positive relation with increasing thickness of flesh. Figure 5 shows the gel electrophoresis image for some of the DNA extractions from bones burnt at temperature and time ranges shown in Table 8.
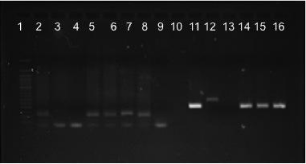
Figure 5: Gel electrophoresis image of DNA amplification from bones burned
with flesh. Lane 2-4min, 3-7min, 4-11min (106 bp, 5 mm flesh) ; lane 5-4min,
6-7min, 7-11min (106bp, 10 mm flesh) ; lane 8 +ve control ; lane 9 –ve
control ; lane 11-4min, 12-7min, 13-11min (199bp, 5mm flesh) ; lane 14-4min,
115-7min, 16-11min (199bp, 10mm flesh).
Flesh
Time
Concentration
ng/μl
106 band
199 band
411 band
5 mm
4 min
526
Yes
Yes
Yes
5 mm
5.5 min
1074
Yes
Yes
Yes
5 mm
7 min
Not detectable
No
No
No
5 mm
11 min
Not detectable
No
No
No
10
4min
593
Yes
Yes
Yes
10
7 min
590
Yes
Yes
Yes
10
11 min
240
Yes
Yes
Yes
10
15 min
898
Yes
Yes
Yes
10
20min
756
No
No
No
15
15 min
812
Yes
Yes
Yes
15
20min
1182
Yes
Yes
Yes
Table 8: DNA amplification results for bones burnt in-flesh for the respective variable ranges.
Thus it was obvious from this experiment that bones burned with flesh have a positive effect on DNA retrieval. To further analyse this theory and create a model to test for DNA retrieval for bones burned with flesh, a DOE was designed to examine bones with various flesh thickness (0 to 15mm) within a temperature range of 100°C to 500°C and time period of 10 to 30 minutes (Table 9).
Bone samples
Temperature
(Degree Celsius)
Time
(Minutes)
Flesh
(mm
DNA Conc.
ng/μl
PCR amplification
106bp
199bp
411bp
1
100
5
5
2241
Yes
Yes
Yes
2
100
5
10
1995
Yes
Yes
Yes
3
100
5
7.5
2118
Yes
Yes
Yes
4
100
17.5
15
1479
Yes
Yes
Yes
5
100
17.5
0
2427
Yes
Yes
Yes
6
100
30
5
1995
Yes
Yes
Yes
7
100
30
10
3270
Yes
Yes
Yes
8
100
30
7.5
2632
Yes
Yes
Yes
9
300
5
15
3018
Yes
Yes
Yes
10
300
5
0
570
Yes
Yes
Yes
11
300
17.5
5
1452
Yes
Yes
Yes
12
300
17.5
5
2013
Yes
Yes
Yes
13
300
17.5
7.5
2820
Yes
Yes
Yes
14
300
17.5
10
2574
Yes
Yes
Yes
15
300
30
0
-
No
No
No
16
300
30
15
-
No
No
No
17
500
5
5
843
Yes
Yes
No
18
500
5
7.5
1891
Yes
Yes
No
19
500
5
10
2940
Yes
Yes
No
20
500
17.5
15
-
No
No
No
21
500
17.5
0
-
No
No
No
22
500
30
5
-
No
No
No
23
500
30
10
-
No
No
No
Table 9: DOE for bones burnt in-flesh. The experimental design with the variable ranges and their PCR amplification results along with extracted DNA concentration.
The experimental design outlined through the ANOVA test showed that there is a positive significant effect of flesh upon DNA extraction. The interactive effect of flesh with other variables (temperature and time) portrayed in the Pareto chart and interaction plot (supplementary material Figure 9) was also clearly significant. The interactive plot between time and flesh shows that increasing thickness of flesh has a positive effect on DNA extraction even with a longer burning period. Thus the model clearly ascertains the afore mentioned theory. The equation of the fitted model as given below allows us to predict the DNA concentration that could be extracted from bones burned at certain temperatures and time periods with regard to presence of flesh.
DNA Conc. = 352.95 + 9.92444 *temperature + 247.594 *time + 76.254 *flesh -0.0286439 *temperature^2 - 0.782627 *temperature *time +1.41445 *temperature *flesh - 5.97924 *time^2 + 8.06873 *time *flesh - 26.0433 *flesh^2
This model with 87.36% R squared value indicates the optimum burning temperature as 475°C (Figure 6), which seems high compared to the previous models constructed in this study, but the presence of 15 mm thick flesh and a minimal exposure time of 5 minutes (optimal values provided by the model) could be the reason for this unexpected positive retrieval of DNA at these high temperatures. The presence of flesh elevates the possibility of retrieving DNA from bones burnt at higher temperatures and thus the experiment and model strongly suggest the ability of flesh to preserve bone DNA.
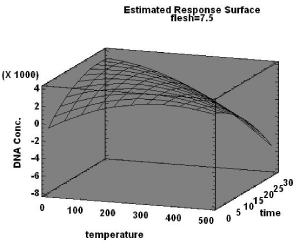
Figure 6: Response surface chart for bones burnt in-flesh.
Discussion
This report consists of multiple studies analyzing various variables for their effect on DNA retrieval. All the different variable sets were designed using DOE of statgraphics to arrive at statistical models that could be used as a diagnostic or screening tool to test the possibility to extract and amplify usable DNA from burnt bones. The first study involves analyzing the effects of baking bones on DNA amplification at various temperature and time frames. The study within itself consists of a comparative analysis for DNA extraction between phenol chloroform and Chelex techniques. Presence or absence of clear bands for the three intended target regions of the DNA reflected the positive and negative amplification of the DNA and hence is a visual measure of efficacy of the extraction technique itself and the amplification process.
The report by Imaizumi et al. [29] studied microwave irradiation effects on DNA, and its application as to whether microwave irradiation can be used in DNA extraction procedures. Our study concentrates at the actual recovery of DNA from oven treated baked bones and its reproducibility. Comparing the effects of baking to other methods of heat treatment like burning and use of ignitable liquids for burning would also provide an insight at the temperature studies of burned and baked bones and resolve whether they exhibit different effects on DNA recovery.
Use of baking as a heating method produced negative effects on DNA amplification as reported by Imaizumi et al. [29], and the reduced amount of DNA from baked bone samples in comparison to positive (unbaked) bone samples indicate DNA degradation. Nevertheless the reproducibility was high enough to provide proper DNA identification and surprisingly the baking temperature study is in general agreement with studies involving other heating methods like accidental fires [18], and electric muffle furnace [20].
Our positive DNA retrieval temperature range matches the conclusions of Schwark et al. [18] that reliable and reproducible DNA profiles could be obtained from semi burned (low temperature) bones. The use of similar sized target fragments between our two studies also compels the fact that shorter fragments are easier and more probable for positive DNA amplifications. The designed model showed that burning temperature affects DNA recovery substantially and burning time was insignificant, but this changed as we increased the fragment length targeted for PCR amplification. Imaizumi et al. [20] also noted this difference in amplification between different sized amplicons.
Amplification of larger fragments was affected by both time and temperature and was predicted to show more negative amplification. This was evident through clear reproducible bands for shorter fragments and inconsistent and negative amplification results for larger. While Imaizumi et al. [20] was unable to retrieve successful amplifications above 200°C, our study produced a higher, maximum DNA retrieval temperature. We found that the length of the target fragment affected the maximum temperature of positive DNA amplification. Whilst short fragments were successfully amplified from bones burnt at high temperatures of 300°C, the larger fragment became irretrievable at a much lower temperature of 240°C. Hence we propose short fragments are best suited for DNA recovery from severely burnt bones. Between the two designed models a better fit and consistency was established for Phenol chloroform than Chelex extraction technique and so was selected as the extraction procedure for all further designs in this study.
The second study with ignitable liquids was done as a univariate analysis since there were no available resources to control the burning time and temperature. This specific experiment showed that within the accelerants compared those with a high specific heat capacity (with purity as a variable) have the longest burn time and reaches the highest temperatures. Thus these accelerants were speculated also to cause the most damage to DNA resulting in lower extraction concentrations and correspondingly more negative amplifications. However, again the length of the target fragment had a significant influence on the effect of accelerants. While the largest fragment (411 bp) was unrecoverable at increasing volumes of accelerants, the smaller fragments were all amplifiable.
The unexpected all-positive DNA amplification could be because the temperatures never reached any higher than 300°C in all the cases, adding on to this the very short burning time, made DNA retrieval rather very likely and effective. Given that this is a small-scale experiment utilizing singular bones with low amounts of accelerants, the whole scenario in real life would be a lot more drastic. The large amounts of accelerant that would be used to burn a whole body (or skeleton) would cause more damage, enough to intervene with DNA retrieval, which is the case with many crime scenes.
For example, the study by Cattaneo et al. [21] showed negative amplification of DNA from all their burnt bone samples, as the temperature range was 800°C to 1200°C which according to the authors is similar to those in real life scenarios. One could reason that the many different factors and variables like the burning method, fluctuating burning temperatures, burning time, presence and absence of accelerants etc., could also have lead on to these different results. But the real crime scene samples (charred bones) used in the same study from two car fires also showed negative results which leads us to speculate that bone DNA survival at very high temperatures are rare except in cases like Sajantila et al. [4] and Schwark et al. [18] where charred tissues were still present.
On the other hand analysis of real crime scene samples by Schwark et al. [18] where, burnt bone samples collected from 13 burnt bodies showed variable grades of burning. Their conclusions of successful DNA identification from few bones suggests that not all fire deaths result in complete degradation of DNA. Difference in heat exposure through the body results in different grades of burning within a skeleton itself, this slightly increases the chance of DNA recovery from at least few bones in the skeleton.
Presence of tissues on a burnt body increases the possibility of positive DNA profiling. Although DNA extraction from the burnt muscles, tissues itself could result in positive PCR amplification and identification, the probability of contamination and presence of PCR inhibitors due to the extreme conditions of burning makes us think about the reproducibility of these methods and limitations. However the protective nature of flesh could be useful for bone DNA retrieval rather than DNA recovery from flesh itself.
Thus the third and final part of this report comprises of the temperature study involving presence of flesh as a variable. Previous reports by Baby [30] and Bindford [31] show that there is a considerable difference in the way fleshed and dry bones burn. The process they undergo upon heat treatment and the end results have also been cited [32]. Here we report that there is a difference in DNA recovering capacity amidst bones burnt with flesh and dry bones. The statistical model designed from the practical trials strongly suggests that bones burned with flesh enhance DNA retrieval over those burned without flesh (green bones and dry bones). Positive amplification of DNA was possible from fleshed bones burned at 500°C whereas bones burned without flesh had no usable DNA after 300°C. The higher maximum-DNA-retrieval temperature of 475°C and the greater DNA concentrations also proves presence of flesh allows better quality and quantity DNA.
While analytical tools like FTIR [23] and isotope analysis [19] proposed, as a diagnostic tool to detect DNA viability is an interesting option, we propose a statistical methodology for the same. The regression equations developed from our study serves as a diagnostic technique to test the probability of arriving at a positive DNA extraction and amplification. The exciting feature of our model is that they could also be used as a predictive tool to test any different data within the range of the respective variables for their effect on DNA recovery. The models from baking experiment predict the likelihood of DNA amplification of three different sized target regions. With a known approximate burning temperature and time we could use this model to test if we can get a positive DNA amplification. The model from the third study predicts the quantity of DNA that could be extracted from bones burnt in-flesh and without flesh. These models could also be used to deduct the different temperature and time the bones were burned at, if DNA extraction is possible. It should be noted that these models are reliable prediction tools for variables within the same range as used to design the model, if used for other ranges the results should be interpreted cautiously as the values may not always come under the curve of the fitted model. Thus using design of experiments to create the dataset that results in most relevant information and optimal results is less time consuming and simpler by comparison to the above mentioned tools.7. Conclusion
This small-scale attempt to produce a statistical model capable of deducing DNA retrieval from burnt bones under various circumstances was successful with this study. The different individual and interactive effects of variables like burning temperature, burning time, presence of flesh and ignitable liquids were also extensively analysed. Our results facilitate the prediction of probable DNA retrieval and amplification and estimation of the above said variables of burnt bones.
In summary our results support the theory of other previous reports that temperature has a significant effect on DNA recovery and amplification. We also propose that burning time affects DNA with respect to burning temperature. The results from our statistical model is in agreement with other studies which makes us to speculate that effects of baking are not significantly different from other types of heat treatment. Our experiment with ignitable liquids show no high substantial effect on DNA, but the decreasing DNA concentration with increasing volumes of accelerants and temperature suggest that DNA degradation would be more drastic in actual life fire events. The experiment and model for burning bones in-flesh strongly suggest the ability of flesh to preserve bone DNA.
Given that this report exploiting the effects of baking, fleshed bones and ignitable liquid is first of its kind, more experimentation and data acquirement is needed. However the information gained through this modest study, conducted in laboratory conditions with porcine bones, will be beneficial for interpreting data obtained from burnt human remains for forensic purposes.
References
- Budimlija ZM, Prinz MK, Zelson-Mundorff A, Wiersema J, Bartelink E, MacKinnon G, et al. World Trade Center human identification project: experiences with individual body identification cases. Croatian Medical Journal.2003; 44: 259–263.
- Holland MM, Cave CA, Holland CA, Bille TW. Development of a quality, high throughput DNA analysis procedure for skeletal samples to assist with the identification of victims from the World Trade Center attacks. Croat Med J. 2003; 44: 264-272.
- Meyer HJ. The Kaprun cable car fire disaster--aspects of forensic organisation following a mass fatality with 155 victims. Forensic Sci Int. 2003; 138: 1-7.
- Sajantila A, Ström M, Budowle B, Karhunen PJ, Peltonen L. The polymerase chain reaction and post-mortem forensic identity testing: application of amplified D1S80 and HLA-DQ alpha loci to the identification of fire victims. Forensic Sci Int. 1991; 51: 23-34.
- Whitaker JP, Clayton TM, Urquhart AJ, Millican ES, Downes TJ, Kimpton CP, et al. Short tandem repeat typing of bodies from a mass disaster: high success rate and characteristic amplification patterns in highly degraded samples. Biotechniques. 1995; 18: 670-677.
- Calacal GC, Delfin FC, Tan MMM, Roewer L, Magtanong DL, Lara MC, et al. Identification of exhumed remains of fire tragedy victims using conventional methods and autosomal/Y-chromosomal short tandem repeat DNA profiling. The American Journal of Forensic Medicine and Pathology. 2005; 26: 285–291.
- Copeland AR . Suicidal fire deaths revisited. Z Rechtsmed. 1985; 95: 51-57.
- D.J. DiMaio, V.J.M. DiMaio, Deaths due to fire. D.J. DiMaio, V.J.M. DiMaio, Editors. In: Forensic Pathology, CRC Press, Boca Raton, Ann Arbor, London, Tokyo. 1933; 327–345.
- Lutz S, Weisser HJ, Heizmann J, Pollak S. mtDNA as a tool for identification of human remains. Identification using mtDNA. Int J Legal Med. 1996; 109: 205-209.
- Fanton L, Jdeed K, Tilhet-Coartet S, Malicier D. Criminal burning. Forensic Sci Int. 2006; 158: 87-93.
- Nelson CL, Winston DC. Detection of medical examiner cases from review of cremation requests. Am J Forensic Med Pathol. 2006; 27: 103-105.
- Sivolap Y, Krivda G, Kozhuhova N, Chebotar S, & Benecke M. A homicide in the Ukraine: DNA-based identification of a boiled, skeletonized, and varnished human skull, and of bone fragments found in a fireplace. The American Journal of Forensic Medicine and Pathology. 2001; 22: 412–414.
- Ye J, Ji A, Parra EJ, Zheng X, Jiang C, Zhao X, et al. A simple and efficient method for extracting DNA from old and burned bone. J Forensic Sci. 2004; 49: 754-759.
- Hiller JC, Thompson TJ, Evison MP, Chamberlain AT, Wess TJ. Bone mineral change during experimental heating: an X-ray scattering investigation. Biomaterials. 2003; 24: 5091-5097.
- Grupe G & Hummel S. Trace element studies on experimentally cremated bone. I. Alteration of the chemical composition at high temperatures. Journal of Archaeological Science. 1991; 18: 177–186.
- Kalsbeek N & Richter J. Preservation of Burned Bones: An Investigation of the Effects of Temperature and pH on Hardness. Studies in Conservation. 2006; 51: 123–138.
- Ubelaker DH. The forensic evaluation of burned skeletal remains: a synthesis. Forensic Sci Int. 2009; 183: 1-5.
- Schwark T, Heinrich A, Preusse-Prange A, von Wurmb-Schwark N. Reliable genetic identification of burnt human remains. Forensic Sci Int Genet. 2011; 5: 393-399.
- Harbeck M, Schleuder R, Schneider J, Wiechmann I, Schmahl WW, & Grupe G. Research potential and limitations of trace analyses of cremated remains. Forensic Science International. 2011; 204: 191–200.
- Imaizumi K, Taniguchi K, Ogawa Y. DNA survival and physical and histological properties of heat-induced alterations in burnt bones. Int J Legal Med. 2014; 128: 439-446.
- Cattaneo C, DiMartino S, Scali S, Craig OE, Grandi M, Sokol RJ. Determining the human origin of fragments of burnt bone: a comparative study of histological, immunological and DNA techniques. Forensic Sci Int. 1999; 102: 181-191.
- Hess, K & Orthmann CH. Criminal Investigation. 9th edition. Cengage Learning. 2009.
- Fredericks JD, Bennett P, Williams A, Rogers KD. FTIR spectroscopy: A new diagnostic tool to aid DNA analysis from heated bone. Forensic Sci Int Genet. 2012; 6: 375-380.
- Eckert WG. The medicolegal and forensic aspects of fires. Am J Forensic Med Pathol. 1981; 2: 347-357.
- Owsley DW. Identification of the fragmentary, burned remains of two U.S. journalists seven years after their disappearance in Guatemala. J Forensic Sci. 1993; 38: 1372-1382.
- Pope EJ1, Smith OC. Identification of traumatic injury in burned cranial bone: an experimental approach. J Forensic Sci. 2004; 49: 431-440.
- Hoff-Olsen P, Mevåg B, Staalstrøm E, Hovde B, Egeland T, Olaisen B. Extraction of DNA from decomposed human tissue. An evaluation of five extraction methods for short tandem repeat typing. Forensic Sci Int. 1999; 105: 171-183.
- Maloy SR. Experimental Techniques in Bacterial Genetics. Jones and Bartlett.1990.
- Imaizumi K, Taniguchi K, Ogawa Y. An evaluation of the effect of microwave irradiation on bone decalcification aimed to DNA extraction. Leg Med (Tokyo). 2013; 15: 272-277.
- Baby RS. Hopewell cremation practices, Ohio Hist. Soc., Papers Archaeol.1954; 1: 1–17.
- Binford LR. An analysis of cremations from three Michigan sites, Wisconsin Archaeol. 1963; 44: 98–110.
- Thurman M & Willmore L. A Replicative Cremation Experiment. North American Archaeologist.1981; 2: 275–283.