
Research Article
Austin J Forensic Sci Criminol. 2015; 2(4): 1033.
Diptera Succession during Early Decomposition Stages in a Mediterranean Pinewood Umbrage
Arnaldos MI1,2, Khedre A³, Begoña I¹, Presa JJ¹, Clemente ME¹, López-Gallego E¹, Martínez AB¹, Pérez-Marcos M¹ and García MD1,2*
¹Department of Zoology and Physical Anthropology, University of Murcia, Spain
²Servicio Externo de Ciencias y Técnicas Forenses, University of Murcia, Spain
³Department of Zoology, University of Sohag, Egypt
*Corresponding author: Maria Dolores Garcia, Department of Zoology and Physical Anthropology, Campus Universitario de Espinardo, 30100 Murcia, Spain
Received: July 16, 2015; Accepted: August 09, 2015; Published: August 12, 2015
Abstract
The succession of entomosarcosaprophagous fauna depends on multiple factors, being the environment one of the most important. Thus the study of sarcosaprophagous community in different microclimatic environments is relevant, even if the different locations are close to each other. Results concerning the early sarcosaprophagous community collected during a whole year in an umbrage area located at 980 MASL in Sierra Espuña Mountain (Murcia province, SE Spain) are presented. The study was carried out using a Schoenly trap baited with 5kg piglets. A daily sample was taken during all four seasons. More than 12700 specimens, belonging to 18 orders of Arthropoda, were collected. The most abundant in all seasons was Diptera, representing 97.66% of the captures in fall. Among Diptera, Calliphoridae was the most representative family during the first stages of decomposition, representing 94.37% of all Diptera in spring, 41.05% in summer, 61.03% in fall and 80% in winter. Muscidae and Fannidae were also abundant in summer and fall. The Calliphorid species collected were: Calliphora vicina, Calliphora vomitoria, Chrysomya albiceps, Lucilia caesar, Lucilia sericata, Pollenia sp. and Stomorhina lunata. The primary species was always C. vicina. The most abundant species in the whole study was Chrysomya albiceps, being the most representative species in summer and fall. Calliphora vicina was the most representative in spring and winter. These results are compared with previous studies conducted with a piglet and chicken carcasses in a near suburban area. Differences concerning community species composition and dynamics and succession, as well as decomposition process have been detected.
Keywords: Calliphoridae; Forensic entomology; Iberian Peninsula; Sarcosaprophagous fauna
Introduction
Studies on the entomosarcosaprophagous community and the successional patterns in carcasses represent the starting point of research in forensic entomology that mainly deals with death investigations. One of the methods to estimate the delay between death and corpse finding is based on the biological principle of succession, where the colonization of the corpse occurs in a sequence that, when known, is predictable. Successional stages are represented by the variety of arthropods present in the corpse at a particular time. This diversity is, then, compared to know successional patterns for that geographic area or habitat [1]. Unfortunately, most of the studies concerning the sarcosaprophagous community and its successional patterns have been conducted in restricted areas, mainly man influenced, and few comparisons with other close and different environments have been made. In particular, wild areas have been ignored, except for some locations [1-10].
In the Iberian Peninsula, except for some data [3,4,11-15], hardly any work has been devoted to natural environments related to the sarcosaprophagous community. With exception of the information on diversity of species and relative abundance of taxa obtained from succession studies [16] no other data exists about sarcosaprophagous succession in wild conditions in the Iberian Peninsula.
As it concerns the community, the most important component for forensic purposes is the necrophagous, mainly Diptera, some of which are known to be the first arthropods to arrive at the corpse. These insects arrive immediately after death, and their dynamics depend on several factors [17], such as the corpse placement, weather conditions, and season. In addition, the corpse itself is an important factor [18-20].
Given all of above, our study attempts to provide for the first time data, for the Iberian Peninsula and also Europe, on the early sarcosaprophagous community in a mountainous wild environment.
Materials and Methods
The present study was carried out in the Murcia region of Sierra Espuña, a mountainous area located in the southeast of the Iberian Peninsula (SE Spain). The selected location was an umbrage area named Peña Apartada, at 980 MASL (UTM 30TXG6274190).
The vegetation of the area is a mixed mediterranean forest composed of Pinus halepensis, Pinus pinaster, Pinus nigra, Quercus rotundifolia, Quercus faginea, Quercus coccifera, Juniperus oxycedrus and Pistacia lentiscus.
Samples were collected daily for 15 days during the four seasons of the year defined as fall (15/09/06-29/09/06), winter (8/01/07- 22/01/07), spring (4/04/07-18/04/07) and summer (15/06/07- 29/06/07).
A modified version of the trap designed by Schoenly et al. [21] measuring 60 × 70 × 70 cm and Morril solution [22] as preservative solution were used to collect sarcosaprophagous fauna. This trap has been used previously for the study of sarcosaprophagous fauna [23-30], and has been shown to be as effective as other conventional methods by Ordóñez et al. [31].
Each season the trap was baited with a dead piglet (Sus scrofa L.) of 5kg weight. The piglet was euthanized using ketamine and saline solution, according to European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (86/609/CEE) D.O.C.E. 18.12.86. The piglets were provided by the University of Murcia Veterinary farm. As soon as the animals died, they were covered by a plastic bag to protect them during transportation to the trap.
The relative humidity and the temperature inside the trap were continuously recorded with HOBO U1O Data loggers placed in the trap.
Each sampling day, digital photographs of the bait were taken, and any other relevant data related to weather conditions, odours, etc. were also recorded.
Insect taxa were identified using different keys [32-35].
To estimate the diversity of Diptera community in our study, Margalef and Shannon indexes were calculated for each season, and for every decomposition stage. Margalef index was calculated following the equation MI=(S-1)/lnN, where S is the number of identified taxa and N is the total amount of individuals. Shannon’s index was calculated from the equation: H’=-Σ pi ln pi, where pi is the proportion of individuals found in the ith species from the total pool of species. The importance, utility and characteristics of both indexes have been extensively discussed in Magurran [36] and in Prado e Castro (pers. comm.) regarding the sarcosaprophagous community. Although the value of the Shannon index usually falls between 1.5 and 3.5 [36], the special characteristics of the sarcosaprophagous community made this index to achieve lower values, as reported in other special communities.
Results and Discussion
Climatological data
During fall, mean temperatures were quite warm (17-22oC); relative humidity varied between 43 and 89% (Figure 1). In winter, mean temperature decreased reaching a minimum of 4.5°C and a maximum of 13.5oC. Relative humidity was close to 50% reaching 70% around the middle of the sampling period. Spring was characterized by low mean temperatures, close to 10oC, and relative humidity close to 50% except for the first days. During summer, mean daily temperature was close to 23oC; relative humidity reached values in the upper 50%, increasing towards the end of the sampling period.
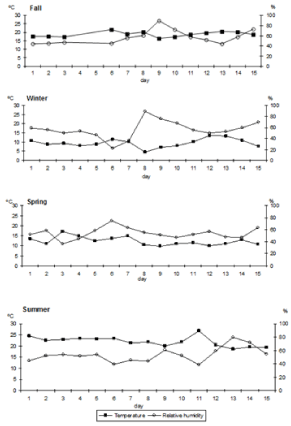
Figure 1: Mean temperature and mean relative humidity all along sampling
period.
Stages and rates of decomposition
Three decomposition stages were identified: fresh, bloated and active decay, according to the description of decomposition stages provided by Goff [37].
The rate of decomposition was quite different in each season (Table 1). The fresh stage had high variability being shorter in warm seasons than in cold seasons. The bloated stage was not visible in the winter and spring seasons, most likely as a result of cold weather. The active decomposition stage was reached at the same time during spring and summer periods. Although fall was warmer than spring and, obviously, than winter, active decomposition was reached later in this season.
season
day
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
FALL
WINTER
SPRING
SUMMER
Table 1: Decomposition stages identified in each season. Decomposition stages: fresh, bloated, active decay.
Community composition and Diptera diversity
In this study more than 12700 adult specimens have been collected, belonging to 18 arthropod orders (Table 2). The predominant collected orders were Diptera, Acarida, Coleoptera, Collembola and Hymenoptera. Diptera was the most abundant order in the whole study, as well as in every season. They represented more than 90% of the total collected fauna in summer and fall.
SPRING
SUMMER
FALL
WINTER
ALL SEASONS
ORDER
n
%
n
%
n
%
n
%
n
%
Diptera
284
75.53
5836
93.83
5474
97.66
329
55.57
11923
93.20
Acarida
107
1.72
7
0.12
182
30.74
296
2.31
Coleoptera
1
0.27
131
2.11
49
0.87
4
0.68
185
1.45
Collembola
26
6.91
56
0.90
6
0.11
58
9.80
146
1.14
Hymenoptera
8
2.13
55
0.88
37
0.66
3
0.51
103
0.81
Araneida
30
7.98
9
0.14
12
0.21
9
1.52
60
0.47
Thysanoptera
24
6.38
2
0.03
4
0.68
30
0.23
Diplopoda
13
0.23
13
0.10
Dictyoptera
12
0.19
12
0.09
Dermaptera
1
0.02
3
0.05
3
0.51
7
0.05
Psocoptera
1
0.27
2
0.03
4
0.07
7
0.05
Homoptera
1
0.27
2
0.03
3
0.02
Lepidoptera
3
0.05
3
0.02
Microcoryphia
1
0.27
1
0.01
Neuroptera
1
0.02
1
0.01
Orthoptera
1
0.02
1
0.01
Pseudoscorpionida
1
0.02
1
0.01
Siphonaptera
1
0.02
1
0.01
376
100
6220
100
5605
100
592
100
12793
100
Table 2: Frequency and capture percentage for each arthropod order in the four seasons, n: number, % percentage. The shaded cells show the most collected orders.
Although the amounts of Acarida during winter were low (only 182 specimens), they represent the 30.74% of the collected fauna during this season. In summer, similar amounts (107 specimens) were collected, although they only represented 1.72% of the total. The similar amount of specimens could be explained because of the extreme weather conditions of both seasons that could make the corpse a refuge for this fauna. Therefore, it is worth to consider that Acarida has a relative importance for this kind of studies.
Among Diptera, Calliphoridae was the most representative family during the sampling period (Figure 2). In spring it represented the 94.37% of all Diptera; in summer only 41.05%, in fall 61.03% and in winter 80.78%. Two other families were relevant in some seasons: Muscidae in summer (14.32%) and fall (32.50%), and Fannidae in summer (36.92%).
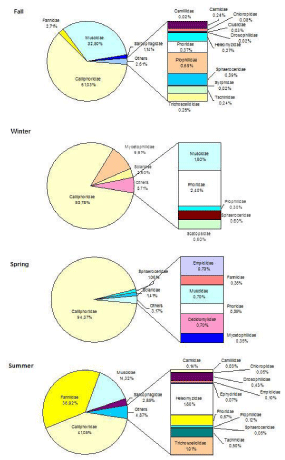
Figure 2: Capture percentage of Diptera collected in each seasons.
Comparison of our study conducted in a wild area (henceforth WA) with other studies using similar methodology with chicken carcasses (henceforth SAC) [23,24] and piglets (henceforth SAP) [38] as baits in a suburban area of the same region revealed very different results. In the wild area, 22 Diptera families have been collected while in the suburban environment only 13 families were collected. These results were independent of the bait used since piglet and chicken carcasses gave similar results.
The results obtained in the three independent studies (Table 3) indicate that Calliphoridae was the predominantly collected family in fall and winter. In spring, a different pattern appears depending on the bait and environment; thus, in WA and SAC, Calliphoridae was the most collected family while in SAP, it was Muscidae. In addition, in summer, the results appear to be different and only related to the environment; thus, in wild area, Calliphoridae was the most collected family while, in suburban area, it was Muscidae, independently of the bait used. On the other hand, during fall and summer, the wild environment was associated with more families (16) than SAC (10 and 12) or SAP (6 and 8). However, during winter and spring, SAC provided more families (10 and 12), than WA (8 and 9), and SAP (5 and 8).
WA
SA
Season
Families
Piglet
Piglet
Chicken
Fall
B
Anthomyiidae
0.45
Calliphoridae
61.03
35.14
58.36
Camillidae
0.02
Carnidae
0.24
Chloropidae
0.08
Clusiidae
0.03
Drosophilidae
0.02
Fannidae
2.71
15.60
Heleomyidae
0.27
Muscidae
32.50
16.22
15.60
Phoridae
0.37
16.22
1.51
Piophilidae
0.69
Sarcophagidae
1.40
5.06
Scatophagidae
0.06
Sphaeroceridae
0.39
Syrphidae
0.02
Tachinidae
0.24
Trichoscelididae
0.25
Cecidomyiidae
2.35
N
Chironomidae
2.70
Psychodidae
13.51
0.73
Sciaridae
16.22
0.28
Winter
B
Anthomyiidae
6.79
Calliphoridae
80.78
85.59
48.83
Fannidae
7.05
Milichiidae
0.90
Muscidae
1.80
22.72
Mycetophilidae
9.91
Phoridae
2.40
9.01
7.57
Piophilidae
0.30
Sarcophagidae
0.26
Scatopsidae
0.60
Sphaeroceridae
0.60
0.52
N
Cecidomyiidae
1.04
Psychodidae
0.90
1.04
Sciaridae
3.60
3.60
4.18
Table 3: Capture percentage of Diptera families in wild and suburban areas using different baits. WA: Wild area. SA: suburban area. B: Brachycera. N. Nematocera. The shaded cells show the most collected families in each environment and bait.
Although Sarcophagidae has traditionally been considered a compulsory part of the sarcosaprophagous Diptera community, it does not behave like that, at least in some seasons or baits, as reported by Matuszewski et al. [5]. For instance, in the three studied areas (WA, SAP and SAC), it appeared only during summer.
Diptera community (defined as families composing it) appears to be different dependent of the bait used. When using chicken, nine families were present (Anthomyiidae, Calliphoridae, Cecidomyiidae, Fannidae, Muscidae, Phoridae, Psychodidae, Sarcophagidae and Sciaridae) during all seasons, while, when using piglets, only two families (Calliphoridae and Phoridae) were present in all seasons in suburban area, and three families (Calliphoridae, Muscidae and Sphaeroceridae) in wild area. Calliphoridae is the only family present during all seasons in the three studies.
In our study, the richest and most diverse season was summer; the less rich was winter, and the less diverse spring, using the Margalef and Shannon indexes (Table 4). Nevertheless, in suburban habitats, the richest and most diverse season was spring when using chicken carcasses, but the fall when using piglets.
Margalef Index
Shannon Index
Env.
Bait
S
Fr
B
AcD
AdD
Sk
All
F
B
AcD
AdD
Sk
All
Wild
P
F
1.04
1.13
1.90
--
--
1.72
0.51
0.85
1.09
--
--
0.96
W
1.37
--
1.08
--
--
1.20
1.20
--
0.53
--
--
0.76
SP
1.24
--
1.42
--
--
1.41
0.95
--
0.30
--
--
0.32
SU
0.39
1.11
1.75
--
--
1.73
0.27
1.21
1.35
--
--
1.35
Sub
C
F
1.27
--
1.50
--
--
1.47
1.20
--
1.34
--
--
1.33
W
1.38
--
1.87
--
--
1.84
1.09
--
1.65
--
--
1.63
SP
1.68
--
2.03
1.94
--
1.85
0.94
--
2.10
1.94
--
1.87
SU
1.65
--
1.51
1.67
2.08
1.20
1.38
--
1.31
1.60
1.64
1.49
P
F
1.24
--
1.44
--
--
1.38
1.05
--
1.62
--
--
1.62
WI
0.78
--
0.87
--
--
0.84
0.69
--
0.53
--
--
0.55
SP
0
0.74
0.69
0.97
--
0.78
0
0.54
0.48
0.60
--
0.55
SU
1.24
1.00
0.79
0.60
--
0.85
0.95
0.99
0.88
0.99
--
0.96
Table 4: Diversity indexes in wild and suburban environments using different baits. AcD: Active decomposition, AdD: Advanced decomposition, All: whole season, B: Bloated stage, C: Chicken, Env.: Environment, F: Fall, Fr: Fresh stage, P: Piglet, S: Season, Sk: Skeletonization, SP: Spring, SU: Summer, Sub: Suburban, W: Winter. The shaded cells show the upper index value.
In our study, both indexes reveal the same results except for spring where, according to Margalef index, the richest stage is active decomposition, but according to Shannon index the most diverse is fresh stage.
In SAC the results of both indexes were the same in all cases, and they seem to indicate that the most advanced stages of decomposition were the most diverse and richest. On the other hand, in SAP, the diversity and taxa richness were coincidental in fall and spring, but clearly different in winter and summer. In general, SAP displayed some heterogeneity regarding the two indexes.
Calliphoridae succession
In our study, the calliphorid species collected during the whole period were: Calliphora vicina Robineau-Desvoidy, 1830, Calliphora vomitoria Linnaeus, 1758, Chrysomya albiceps (Wiedemann 1819), Lucilia caesar Linnaeus, 1758, Lucilia sericata Meigen, 1826, Pollenia sp. and Stomorhina lunata (Fabricius 1805) (Table 5). The most abundant species in the whole study was Ch. albiceps, representing more than 60%. The second most abundant species was C. vicina, in contrast to data from other Iberian mountain system [3] where the most abundant species were C. vomitoria, followed by Ch. albiceps.
SPRING
SUMMER
FALL
WINTER
ALL SEASONS
SPECIES
n
%
n
%
n
%
n
%
n
%
Chrysomya albiceps
1404
58.21
2737
75.6
4141
63.07
Calliphora vicina
163
60.82
693
28.73
792
21.90
260
96.65
1908
29.06
Calliphora vomitoria
105
39.18
243
10.07
11
0.30
9
3.35
368
5.60
Pollenia sp.
19
0.79
57
1.58
76
1.16
Lucilia sericata
52
2.16
12
0.33
64
0.97
Lucilia Caesar
8
0.22
8
0.12
Stomorhina lunata
1
0.04
1
0.02
268
100
2412
100
3617
100
269
100
6566
100
Table 5: Number and capture percentage of Calliphoridae collected in all seasons. n: number, % percentage. The shaded cells show the most collected species.
Regarding the Calliphora species, both C. vicina and C. vomitoria have been collected together in wild environment, being C. vicina the dominant species. This result agrees that reported by Prado e Castro et al. [30] for Western Iberian Peninsula confirming in Southern Europe the data reported by Greenberg [39] and Smith [40] for Northern Europe.
The number of Calliphorid species was higher in WA than in SAC and SAP. Moreover, in WA, some taxa, such as S. lunata, appears for the first time, and C. vomitoria, L. caesar and Pollenia sp. are represented, while being minimal or not appearing in SAC and SAP. On the other hand, one of the most representative species in SAP and SAC (L. sericata) was very scarce in WA. C. vicina, the second most collected species in WA (29.06%), was also represented in lower amounts in SAP and SAC (4.22 and 11.52%, respectively). Nevertheless, the most important species in all three study areas was Ch. albiceps.
Seasonally, our study (Table 5) illustrates that Ch. albiceps was only captured in summer and fall, where it was the most representative species. In SAP, Ch. albiceps was collected in spring and summer and, during all seasons in SAC. It was clearly the dominating species during fall as it occurred in Lisbon in a small patchy woodland park [30].
Calliphora vicina was collected throughout the sampling period, being the most representative species in spring and winter. It was the only Calliphorid present in SAP, and had an appreciable presence in winter. It was also the most representative during winter in SAC.
Calliphora vomitoria was collected all along the sampling period, displaying preference for spring and summer. In SAP and SAC, it was present in very low number, and it can be considered irrelevant.
Lucilia sericata and Pollenia sp. were only collected in WA in the warmer seasons of summer and fall, and the same pattern occurs in SAP and SAC. We would like to mention that L. sericata, the most representative in SAC during spring, is not present during this season in WA. This could be due, among other reasons, to the shaded character of the selected place.
In our study, the primary species was always C. vicina (Tables 6-9), although it was well represented all along the decomposition process, contrasting with SAC where it was present in all stages only in winter. C. vomitoria acted as secondary species in this environment, as well as Ch. albiceps, L. sericata and Pollenia sp. C. vomitoria was present in most of decomposition stages only in WA, and in summer. Ch. albiceps, when present, showed preference in WA for bloated and active decomposition stages but in SAP was collected in all decomposition stages registered, and in SAC in fresh and active decomposition. L. sericata although secondary in WA, is typically a primary species appearing like that in SAC in spring (Table 6), summer (Table 7) and fall (Table 8).
DAY
SPECIES
PLACE/BAIT
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
Calliphora vicina
WA
•
•
•
•
•
•
•
•
•
•
Fr
AcD
SAP
•
•
•
•
•
•
•
Fr
Bloated
AcD
AdD
SAC
•
•
•
•
•
•
Fr
AcD
AdD
Calliphora vomitoria
WA
•
•
•
Fr
AcD
SAP
•
•
Fr
Bloated
AcD
AdD
SAC
Not collected•
Fr
AcD
AdD
Chrysomya albiceps
WA
Not collected •
Fr
AcD
SAP
•
•
•
•
•
•
•
•
Fr
Bloated
AcD
AdD
SAC
•
•
•
•
•
•
Fr
AcD
AdD
Lucilia sericata
WA
Not collected •
Fr
AcD
SAP
•
•
•
•
•
•
•
•
Fr
Bloated
AcD
AdD
SAC
•
•
•
•
•
•
•
Fr
AcD
AdD
Pollenia sp.
WA
Not collected •
Fr
Active decomposition
SAP
Not collected •
Fr
Bloated
AcD
AdD
SAC
•
•
•
•
Fr
AcD
AdD
Lucilia caesar
WA
Not collected •
Fr
AcD
SAP
•
•
Fr
Bloated
AcD
AdD
SAC
Not collected •
Fr
AcD
AdD
Table 6: Calliphoridae succession in wild and suburban environment in Spring. AcD: Active decomposition, AdD: Advanced decomposition, Fr: Fresh stage, SAC: Suburban area using chicken carcasses SAP: Suburban area using chicken carcasses, WA: Wild area.
DAY
SPECIES
PLACE/BAIT
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
Calliphora vicina
WA
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
Fr
Bloated
AcD
SAP
•Not collected
Fr
Bloated
AcD
AdD
SAC
•
•
Fr
AcD
AdD
Sk
Calliphora vomitoria
WA
•
•
•
•
•
•
•
•
•
•
•
Fr
Bloated
AcD
SAP
•Not collected
Fr
Bloated
AcD
AdD
SAC
•Not collected
Fr
AcD
AdD
Sk
Chrysomya albiceps
WA
•
•
•
•
•
•
•
•
▪
▪
•
•
•
•
Fr
Bloated
AcD
SAP
•
•
▪
•
•
▪
▪
•
•
•
•
•
Fr
Bloated
AcD
AdD
SAC
•
•
•
•
•
Fr
AcD
AdD
Sk
Lucilia sericata
WA
•
•
•
•
•
•
•
•
Fr
Bloated
AcD
SAP
•
•
•
•
•
•
•
•
•
Fr
Bloated
AcD
AdD
SAC
•
•
•
•
•
Fr
AcD
AdD
Sk
Pollenia sp.
WA
•
•
•
•
•
•
•
Fr
Bloated
AcD
SAP
•Not collected
F
Bloated
AcD
AdD
SAC
•
Fr
AcD
AdD
Sk
Stomorhina lunata
WA
•
Fr
Bloated
AcD
SAP
•Not collected
F
Bloated
AcD
AdD
SAC
•Not collected
Fr
AcD
AdD
Sk
Symbols: •, 1-10, • 11-50, • 51-100, • 101-200, ▪> 201
Table 7: Calliphoridae succession in wild and suburban environment in Summer. AcD: Active decomposition, AdD: Advanced decomposition, Fr: Fresh stage, SAC: Suburban area using chicken carcasses SAP: Suburban area using chicken carcasses, WA: Wild area.
DAY
SPECIES
PLACE/BAIT
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
Calliphora vicina
WA
•
•
•
•
•
•
•
•
•
•
•
•
•
Fr
Bloated
AcD
SAP
•
•
•
•
•
Fr
AcD
SAC
•
•
•
•
•
•
•
•
•
•
Fr
AcD
Calliphora vomitoria
WA
•
•
•
Fr
Bloated
AcD
SAP
•Not collected
Fr
AcD
SAC
•Not collected
Fr
AcD
Chrysomya albiceps
WA
•
▪
▪
•
▪
▪
▪
▪
•
•
Fr
Bloated
AcD
SAP
•Not collected
Fr
AcD
SAC
•
•
▪
•
•
•
•
•
•
•
•
•
•
Fr
AcD
Lucilia caesar
WA
•
•
•
•
Fr
Bloated
AcD
SAP
•Not collected
Fr
AcD
SAC
Fr
AcD
Lucilia sericata
WA
•
•
•
•
•
•
Fr
Bloated
AcD
SAP
•
Fr
AcD
SAC
•
•
•
•
•
•
•
•
•
•
Fr
AcD
Pollenia sp.
WA
•
•
•
•
•
•
Fr
Bloated
AcD
SAP
•Not collected
Fr
AcD
SAC
•Not collected
Fr
AcD
Symbols: •, 1-10, • 11-50, • 51-100, • 101-200, ▪> 201
Table 8: Calliphoridae succession in wild and suburban environment in Fall. AcD: Active decomposition, AdD: Advanced decomposition, Fr: Fresh stage, SAC: Suburban area using chicken carcasses SAP: Suburban area using chicken carcasses, WA: Wild area.
DAY
SPECIES
PLACE/BAIT
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
Calliphora vicina
WA
•
•
•
•
•
•
•
•
•
•
•
•
•
Fr
AcD
SAP
•
•
•
•
•
•
•
•
•
•
•
•
•
Fr
AcD
SAC
•
•
•
•
•
•
•
•
•
•
•
•
•
•
Fr
AcD
Calliphora vomitoria
WA
•
•
•
•
Fr
AcD
SAP
•Not collected
Fr
AcD
SAC
•
•
•
Fr
AcD
Chrysomya albiceps
WA
•Not collected
Fr
AcD
SAP
•Not collected
Fr
AcD
SAC
•
•
Fr
AcD
Lucilia sericata
WA
•Not collected
Fr
AcD
SAP
•Not collected
Fr
AcD
SAC
•
•
•
•
•
Fr
AcD
Pollenia sp.
WA
•Not collected
Fr
AcD
SAP
•Not collected
Fr
AcD
SAC
•
•
•
•
Fr
AcD
Symbols: •, 1-10, • 11-50, • 51-100, • 101-200, ▪> 201
Table 9: Calliphoridae succession in wild and suburban environment in Winter. AcD: Active decomposition, AdD: Advanced decomposition, Fr: Fresh stage, SAC: Suburban area using chicken carcasses SAP: Suburban area using chicken carcasses, WA: Wild area.
Conclusion
The early sarcosaprophagous fauna of the wild habitat in the Murcia mountain umbrage is richer and more diverse than that of a suburban area of the same region, and these differences were not attributed to the type of animal carcass.
Some of the species present in the umbrage could be used as habitat indicators. Concerning Calliphoridae, L. caesar, S. lineata and C. vomitoria were almost absent in the suburban area, thus allowing a characterization of the wild environment community.
It is well known that detection of the length of decomposition stages and Calliphorid succession in different environments especially in wild areas provides information about the structure and dynamics of sarcosaprophagus community which is very essential for forensic application. Therefore, there is a need for such entomological studies concerning the whole decomposition process instead of only faunistic studies, and this work is one of the few attempts to gain insights, mainly in Spain.
Acknowledgement
The authors would like to thank the Director of Parque Natural de Sierra Espuña for the facilities offered to develop the study, and the staff of Servicio de Experimentación Agroforestal (Servicio de Apoyo a la Investigación) of the Murcia University for the kindness and help offered to us. This research has been supported by the project CGL2005-04668/BOS of Ministerio de Educación y Ciencia of the Spanish Government.
References
- Eberhardt TL, Elliot DA. A preliminary investigation of insect colonisation and succession on remains in New Zealand. Forensic Sci Int. 2008; 176: 217-223.
- Bourel B, Martin-Bouyer L, Hedouin V, Cailliez JC, Derout D, Gosset D. Necrophilous insect succession on rabbit carrion in sand dune habitats in northern France. J Med Entomol. 1999; 36: 420-425.
- Baz A, Cifrián B, Díaz-Aranda LM, Martín-Vega D. The distribution of adult blow-flies (Diptera: Calliphoridae) along an altitudinal gradient in Central Spain. Annales de la Société entomologique de France. 2007; 43, 289-296.
- Baz A, Cifrián B, Martín-Vega D. Patterns of diversity and abundance of carrion insect assemblages in the Natural Park "Hoces del río riaza" (central Spain). J Insect Sci. 2014; 14: 162.
- Matuszewski S, Bajerlein D, Konwerski S, Szpila K. An initial study of insect succession and carrion decomposition in various forest habitats of Central Europe. Forensic Sci Int. 2008; 108: 61-69.
- Matuszewsky S, Bajerlein D, Konwerski S, Szpila K. Insect succession and carrion decomposition in selected forests of Central Europe. Part 1: Pattern and rate of decomposition. Forensic Sci Int. 2010; 194: 85-93.
- Matuszewski S, Bajerlein D, Konwerski S, Szpila K. Insect succession and carrion decomposition in selected forests of Central Europe. Part 2: Composition and residency patterns of carrion fauna. Forensic Sci Int. 2010; 195: 42-51.
- Matuszewski S, Bajerlein D, Konwerski S, Szpila K. Insect succession and carrion decomposition in selected forests of Central Europe. Part 3: Succession of carrion fauna. Forensic Sci Int. 2011; 207: 150-163.
- Matuszewski S, Szafalowicz M, Jarmusz M. Insects colonising carcasses in open and forest habitats of Central Europe: search for indicators of corpse relocation. Forensic Sci Int. 2013; 231: 234-239.
- Moretti TC, Ribeiro OB, Thyssen PJ, Solis DR. Insects on decomposing carcasses of small rodents in a secondary forest in southeastern Brazil. European Journal of Entomology. 2008; 105: 691-696.
- Castillo Miralbés M. Estudio de la entomofauna asociada a cadáveres en el Alto Aragón (España) Monografías SEA. 2002; 6.
- Martínez-Sánchez A, Rojo S, Marcos-García MA. Sarcofágidos necrófagos y coprófagos asociados a un agroecosistema de Dehesa (Diptera: Sarcophagidae). Boletín de la Asociación española de Entomología. 2000; 24: 171-185.
- Martínez-Sánchez A, Marcos-García MA, Rojo S. Dípteros descomponedores de los pastizales de dehesas (Diptera: Calliphoridae). In: Varios Autores Biodiversidad en pastos Centro Iberoamericano de la Biodiversidad CIBIO. 2001; 281-287.
- Carles-Tolrá M, Arnaldos MI, Begoña I, García MD. Novedades faunísticas y entomosarcosaprófagas de la Región de Murcia, SE de España (Insecta: Diptera). Boletín de la Real Sociedad Española de Historia Natural (Sec. Biol.) 2014; 108: 21-35.
- Saloña Bordás MI, Moneo Pellitero J, Díaz Martín B. Estudio sobre la distribución de Califóridos (Diptera, Calliphoridae) en la Comunidad Autónoma del Pais Vasco. Boletín Asociación española de Entomología 2009; 33: 63-89.
- Keh B. Scope and applications of forensic entomology. Annu Rev Entomol. 1985; 30: 137-154.
- Anderson GS. Factors that influence insect succession on carrion. Byrd JH, Castner JL, editors. In: Forensic Entomology. The Utility of Arthropods in Legal Investigations, CRC Press, Boca Raton. 2010; 201-250.
- Nuorteva P. Studies on the significance of flies in the transmission of poliomyelitis. Annales Entomologia Fennici. 1959; 25: 137-162.
- Davies L. Species composition and larval habitats of blowfly (Calliphoridae) populations in upland areas in England and Wales. Med Vet Entomol. 1990; 4: 61-68.
- Erzinclioglu Z. Blowflies, Richmond publishing Co Ltd, Slough, England. 1996.
- Schoenly K, Griest K, Rhine S. An experimental field protocol for investigating the postmortem interval using multidisciplinary indicators. J Forensic Sci. 1991; 36: 1395-1415.
- Morril WL. Plastic pitfall trap. Environmental Entomology 1975; 4: 596.
- Arnaldos I, Romera E, García MD, Luna A. An initial study on the succession of sarcosaprophagous Diptera (Insecta) on carrion in the southeastern Iberian peninsula. Int J Legal Med. 2001; 114: 156-162.
- Arnaldos MI, Romera E, Presa JJ, Luna A, García MD. Studies on seasonal arthropod succession on carrion in the southeastern Iberian Peninsula. Int J Legal Med. 2004; 118: 197-205.
- Battán Horenstein M, Rosso B, Arnaldos MI, García MD. Estudio preliminar de la comunidad sarcosaprófaga en Córdoba (Argentina): aplicación a la entomología forense. Anales de Biología. 2005; 27: 191-201.
- Battan Horenstein M, Linhares AX, Rosso B, García MD. Species composition and seasonal succession of saprophagous calliphorids in a rural area of Córdoba: Argentina. Biol Res. 2007; 40: 163-171.
- Battán Horenstein M, Rosso B, García MD. Seasonal structure and dynamics of sarcosaprophagous fauna on pig carrion in a rural area of Cordoba (Argentina): Their importance in forensic science. Forensic Science International. 2012; 217: 146-156.
- Prado e Castro C, Arnaldos MI, García MD. Additions to the Calliphoridae (Diptera) fauna from Portugal, with description of new records. Boletín de la Asociación española de Entomología. 2010; 33: 425-437.
- Prado e Castro C, Arnaldos MI, Sousa, JP, García MD. Preliminary study on a community of sarcosaprophagous Diptera in Central Portugal. Entomologia Generalis. 2011; 33: 183-198.
- Prado e Castro C, Serrano A, Martins da Silva P, García MD. Carrion flies of forensic interest: a study of seasonal community composition and succession in Lisbon, Portugal. Medical and Veterinary Entomology. 2012; 26: 417-431.
- Ordóñez A, García MD, Fagua G. Evaluation of efficiency of Schoenly trap for collecting adult sarcosaprophagous dipterans. J Med Entomol. 2008; 45: 522-532.
- McAlpine JF, Peterson BV, Shewell GE, Teskey HJ, Vockeroth JR, Wood DM (Coord.) Manual of Neartic Diptera, Reseach Branch Agricultura Canada, Monograph nº 27, Minister of Supply and Service Canada. 1981.
- González-Mora D, Peris S. Los Calliphoridae de España: 1: Rhiniinae y Chrysomyinae. Eos. 1988; 64: 91-139.
- González Mora D. Los Calliphoridae de España, II: Calliphorini (Diptera). Eos. 1989; 65: 39-59.
- Peris SV, González Mora D. Los Calliphoridae de España, III: Luciliini (Diptera). Boletín de la Real Sociedad Española de Historia Natural (Sec. Biol.) 1991; 87: 187-207.
- Magurran AE. Measuring Biological Diversity, Blackwell Publishing, Oxford, UK. 2004.
- Goff ML. Estimation of Postmortem Interval Using Arthropods development and Successional Patterns. Forensic Science Review. 1993; 5:81-94.
- Arnaldos MI, López Gallego E, García MD. Datos preliminares sobre colonización temprana y actividad diaria de los principales dípteros sarcosaprófagos en el sureste peninsular. Ciencia Forense. 2015.
- Greenberg B. Flies and Disease, Princeton University Press, New Jersey. 1971.
- Smith KGV. A Manual of Forensic Entomology. The Trustees of the British Museum (Natural History), London. 1986.