
Research Article
Austin J Forensic Sci Criminol. 2015; 2(4): 1034.
Implication of Meconium-aspirated Lungs in Stillborns and Neonatal Deaths
Satoh F, Kakimoto Y, Miyashita K, Tsuboi A, Seto Y and Osawa M*
Department of Forensic Medicine, Tokai University School of Medicine, Japan
*Corresponding author: Motoki Osawa, Department of Forensic Medicine, Tokai University School of Medicine, Shimokasuya 143, Isehara, Kanagawa 259- 1193, Japan
Received: July 16, 2015; Accepted: August 08, 2015; Published: August 11, 2015
Abstract
Intrauterine passage of meconium into amniotic fluid is thought to occur in response to fetal distress, in which meconium aspiration syndrome is a serious complication in delivery. This retrospective study describes postmortem histological examination to detect amniotic elements in the lungs of stillborns and neonatal deaths. Alcian blue stain and immunohistochemistry using antibodies to sialyl Tn and cytokeratin were performed for formalin-fixed tissues obtained from nine subjects: four stillborns and five neonates. Meconium masses occluded the bronchiolar and alveolar spaces in the lungs of five infants to varying extents. One case of clinically diagnosed meconium aspiration syndrome and another of an abandoned neonate exhibited a high degree of meconium occlusion. In the latter case, fetal distress was presumed to have occurred, as inferred from the histopathological features. The other three stillborns demonstrated a mild degree of small meconium, distributed mainly to alveolar spaces, in which it remained unknown whether this amount of meconium affected stillbirth. As another perspective, amniotic squames were evaluated by immunohistochemistry. Even fewer squames were present in alveoli of the lungs of all live-born subjects, indicating that complete exhalation or degeneration of amniotic elements occurs after several days following birth. Forensic autopsy cases of deceased infants often lack clinical management and detailed information. Meconium contamination in the lung sections is a finding that can indicate antepartum or intrapartum compromised status of infants.
Keywords: Meconium aspiration syndrome; Fetal distress; Stillbirth; Forensic autopsy; Immunohistochemistry
Introduction
It is meaningful to confirm the presence of amniotic fluid contents in forensic autopsy and subsequent histological examination. For instance, patients with amniotic fluid embolism exhibit dyspnea and cyanosis after broken waters, which is sometimes fatal. After invasion of amniotic fluid into the maternal circulation, it can occlude the pulmonary microcirculation. In postmortem diagnosis, various fetal elements, such as epithelial squames from the fatal skin, lanugo hairs, vernix caseosa, and mucin from respiratory and gastrointestinal tracts, are evident within the pulmonary vasculature. Histopathological procedures to assist in detection of the elements have been established [1-3], in which special stains, such as Alcian blue for acid muco-polysaccharide in mucin, Attwood for squamous cells and mucin, and Sudan Black or Oil Red O for lipid droplets, are effective. Immunohistochemistry using antibody to cytokeratin is also employed to demonstrate squames [4,5]. For another instance related to infants, microscopic examination often reveals amniotic fluid in the lung sections of stillborns and neonates [6]. Innocuous amniotic fluid is filled from the bronchi to alveoli because of spontaneous intrauterine respiratory movements [7,8]. Moreover, Meconium Aspiration Syndrome (MAS) is a severe complication in delivery [9]. Infants who have inhaled meconium develop respiratory distress, including airway obstruction, surfactant dysfunction, and pneumonitis. Meconium is normally stored in the intestines until after birth, but it is expelled into the amniotic fluid prior to birth in response to fetal compromise. Hypoxia in utero has been considered the cause of intestinal contraction and relaxation of the anal sphincter [10]. Amniotic fluid is normally clear, but becomes greenish because of the admixture of meconium. Therefore, the presence of meconiumstained amniotic fluid may be a serious sign of fetal distress. The passage of meconium occurs in a median of 14% (range, 6–25%) of all births [11,12]. In histology, Alcian blue staining of the lung sections of infants is effective to detect a plug of meconium, in contrast to less specific detection by staining with hematoxylin and eosin. Moreover, sialyl Tn of NeuAc α2-6GalNAc, which is produced by goblet cells of the intestine, is well known as an antigen that reacts with mucintype glycoprotein in meconium. The sensitive monoclonal antibody directed to sialyl Tn is TKH-2 [13], which specifically confirms meconium in immunohistochemistry. However, to our knowledge, the morphological appearance of amniotic fluid and meconium in the infant lung sections has not been fully evaluated from the perspective of forensic pathology [14-16]. In this retrospective study, to evaluate the amniotic fluid contents in the lung sections using histology and immunohistochemisry, we examine acute deaths of neonates and infants at or near full term from our forensic autopsy file. Furthermore, we compare observations related to stillbirths and live-births.
Materials and Methods
In all, nine cases were retrieved from the autopsy file of our department for a four-year period of 2008 to 2012, including four stillbirths and five live-births. Lung tissues were fixed in 10% buffered formalin for one to two weeks; then paraffin-embedded tissues were prepared. In each case, multiple sections along the parallel planes from four blocks including all lung lobes were examined. For histological evaluation using microscopy, 4-μm-thick sections were stained with Mayer’s hematoxylin and eosin, and Alcian blue. The immunohistochemical evaluation was performed as described previously [17]. Briefly, mouse monoclonal antibody to sialyl Tn (TKH-2; Novocastra Laboratories Ltd., Newcastle, UK), was incubated with autoclave-treated sections. Prior to application of anti-cytokeratin antibody AE1/AE3 (Dako Cytomation, Carpinteria, CA), sections were treated with 0.1% trypsin at 37oC for 30 min. No pretreatment was performed for anti-surfactant apoprotein antibody, SP-A (Dako). The antibodies were incubated at a dilution of 1:50 or 1:100 for 1 h at room temperature, followed by introduction of the biotinylated second antibody and streptavidin-conjugated peroxidase (LSAB System; Dako). After washing, enzyme activity was visualized with 0.02% 3, 3’-diaminobenzidine containing 0.002% hydrogen peroxide. Sections using TKH-2 and AE1/AE3 were counter-stained respectively with hematoxylin and methylgreen. The immunohistochemical profile of TKH-2 for meconium was evaluated semi quantitatively as eight fields per section with 200× magnification using the following scores: negative stained, –; positive in less than 50% fields, +; 50%–75%, 2+; and more than 75%, 3+. That of cytokeratin for squames was evaluated as the mean number of positive stains in four fields per section with 400× magnification using the following scores: negative stained, –; in less than 10 stains per field, +; 10–20 stains, 2+; and more than 20 stains, 3+.
Results
Case summary
(Table 1) summarizes the causes of death, results of the hydrostatic tests, and findings at forensic autopsy and histological examinations, with numbering of the nine cases. The subjects’ age of gestation was presumed to be from 31-42 weeks. Four infant deaths were judged as stillbirths because no portion of the lungs, even the stomach, floated in water during hydrostatic testing, except for those of one subject #3, who had received clinical management. Perinatal deaths during delivery or fresh stillbirths attributable to intrapartum death were evident in all cases because of little or no maceration. Hypoxia and bleeding caused by antepartum placental abruption was suspected as the cause of death in two subjects, #1 and #4; the mother of subject #4 was also found death at the scene. Subject #3 died of umbilical cord looping around the neck during delivery. Among the other five neonates, subject #5 had been diagnosed with MAS at a hospital, in which greenish meconium debris was present in the external ear and nasal cavities. Suctioning of the trachea was done after birth. In subject #6, with congenital diaphragm herniation, cyanosis occurred after normal delivery. For #9, asphyxia by smothering after normal birth was suspected. In the other two cases, the cause of death was unknown, but neither malformation nor inflammation was evident. One subject #8 had been abandoned. For that reason, the precise alive time after birth was unknown, but it appeared to have been for a short period. Overall, of the nine cases, conditions of fetal distress were presumed to pertain in four cases, #2-#5, based on their clinical courses and death-scene investigations.
No.
Gender, height, weight, alive time after birth
Cause of infant death
Hydrostatic test
Meconium
Squames
Surfactant stain
Interstitial emphysema
Chorioamnionitis
#1
Male, 39 cm, 1800g, stillborn
Placental abruption
-
-
+
Granular
-
NA*
#2
Female, 49.5 cm, 2972 g, stillborn
Unknown
-
+
3+
Granular
-
-
#3
Male, 55 cm, 3333 g, stillborn
Umbilical cord looping
Partially +
+
3+
Granular
-
NA
#4
Female, 50.5 cm, 3075 g, stillborn
Placental abruption
-
+
2+
Granular
-
+
#5
Male, 52 cm, 3737 g, 1 day
MAS
+
2+
3+
Stretched
-
-
#6
Male, 51.5 cm, 2930 g, 12 h
Congenital diaphragm hernia
+
-
+
Stretched
-
-
#7
Female, 50 cm, 3293 g, 4 days
Unknown
+
-
+
Stretched
+
NA
#8
Female, 45 cm, 2195 g, unknown
Unknown
+
3+
2+
Stretched
+
+
#9
Male, 53 cm, 3300 g, 5 days
Suspected asphyxia
+
-
2+
Stretched
+
NA
*NA; the placenta was not available at autopsy.
Table 1: Summary of findings at autopsy and histopathological examinations of the lung and placenta sections in nine examined cases.
Appearance of amniotic fluid contents in the lungs
In order to demonstrate the presence of amniotic fluid in histology, consecutive sections of formalin-fixed lung tissues were examined in this series of study. In sections from all stillborn infants, diffuse faint blue staining by Alcian blue were observed to varying degree in intra-bronchial and intra-alveolar spaces. In five among the nine examined cases, masses of meconium were focally evident in the bronchiole to terminal alveoli with intensive stains by Alcian blue. Immunohistochemical co-localization using TKH-2 confirmed the intensive blue stains as meconium. Two neonates of subject #5 diagnosed with MAS and subject #8 found after abandonment exhibited meconium stains in bronchiole histologically. In MAS case #5, moderate formation of mecoinum plugs was observed, accompanied by mild focal hemorrhage and interstitial fibrosis. However, the pulmonary artery wall thickness and formation of hyaline membrane were not observed. In subject #8, the plugs were extensive; the terminal bronchiole and alveoli were fully expanded. (Figure 1) presents a meconium plug in a bronchiole of subject #8. However, pulmonary hemorrhage, artery thickness, and hyaline formation did not accompany. In contrast, the three other stillborns, #2-#4, exhibited only a mild degree of small meconium, distributed mainly to alveolar spaces (Figure 2). No apparent pneumonitis with infiltration of inflammatory cells was observed in any examined subject. Sections were incubated with the antibody mixture to low-molecular-weight cytokeratin (AE1) and high-molecular-weight cytokeratin (AE3) to detect amniotic squames by immunohistochemistry. The basal cells of the bronchial epithelium were stained positively, though with much less intensity than amniotic squames; they were therefore readily distinguishable from background staining. (Figure 3A) portrays the positive brown staining, in which numerous cells were evident in the alveolar space of full-term stillborn cases of #2-#4. In addition, the placenta was accompanying at autopsy in five cases, as indicated in (Table 1). Histological examination revealed chorioamnionitis in two cases of #4 and #8.
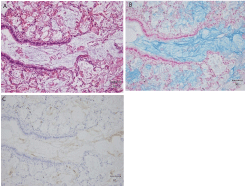
Figure 1: Obstacle of meconium mass in a fully expanded terminal bronchiole
in consecutive lung sections of neonate subject #8: (A) hematoxylin and
eosin stain; (B) Alcian blue stain; and (C) immunohistochemistry using TKH-2
counterstained with hematoxylin (× 400).
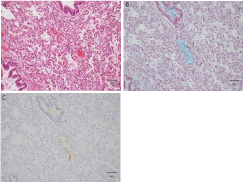
Figure 2: Small mass of meconium in an atelectatic alveolus in consecutive
lung sections of stillborn subject #3: (A) hematoxylin and eosin stain; (B)
Alcian blue stain; and (C) immunohistochemistry using TKH-2 (× 200).
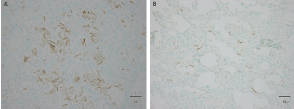
Figure 3: Epithelial squames in the terminal airway, as detected by
immunohistochemistry using cytokeratin antibody AE1/AE3: (A) lung section
of stillborn case #2; and (B) lung section of neonate case #7 (× 400).
Differences between stillbirth and live-birth
The judgment of stillbirth and live-birth is an important issue in forensic autopsy. Microscopic features of amniotic fluid were compared from this standpoint. The alveolar wall was distended in live-born neonates in immnuohistochemistry using surfactant antibody (data not shown) [18,19]. Apparent interstitial emphysema in histology was also evident in three live-born subjects [20]. These examinations clearly supported the judgment. In contrast, Alcian blue stain remained in the alveolar space, except for aerated portions. In particular, fewer squames that were positive by immunohistochemistry were evident in alveoli of the lungs of all liveborn subjects (Figure 3B).
Discussion
Meconium expelled by fetal distress typically adheres to the perianal region and external ear at birth. However, greenish or yellowish, for the old one [9], meconium-stained amniotic fluid is usually not confirmed by external observation at autopsy because the newborn’s body has been washed postnatally through clinical management; the newborn has also undergone tracheal suctioning. In some cases, careful gross observation of the airway potentially reveals meconium plugs. Otherwise, it is indispensable that the meconium contamination is confirmed by histological examination of the lung sections. In fact, in the subject #5, who had been clinically diagnosed with MAS, the postmortem confirmation of meconium depended upon histological examinations. To prove the presence of amniotic fluid elements, routine hematoxylin and eosin stain was insufficient. Multiple lung sections should be submitted for special staining and immunohistochemical analyses using TKH-2, in which the staining methods and microscopic features are compatible with those in amniotic fluid embolism, except for cases with distinct distribution of obstacles in the airway or circulation [2,3]. Actually, MAS occurs in approximately 14% of cases with meconium passage in utero. The mortality rate is approximately 10% of MAS, which occurs in cases of severe MSA [11]. Cleary and Wiswell [11] define MAS as respiratory distress in an infant born through meconium-stained amniotic fluid whose symptoms cannot be explained otherwise. It is controversial which of meconium aspiration or chronic asphyxia in utero is primarily critical [12]. The combination of meconium-stained amniotic fluid and fetal asphyxia enhances the potential for a poor neonatal outcome because the incidence of moconium contamination increases concomitantly with a lower Apgar score [21]. The role of the forensic pathologist in infant deaths is primarily to determine whether the subject was stillborn or live-born; then whether the cause of death resulted from a natural or an unnatural cause. Sims and Collins [22] described that a substantial fraction of fetal deaths are classified as having unexplained causes in any event involving intrauterine compromise. Moreover, some forensic cases of deceased infants lack clinical management and detailed information. We think, therefore, that the presence of meconium in histopathology should be considered a positive finding, indicating the occurrence of fetal distress in utero or in delivery. For instance, neonaticide was suspected in the live-birth case of the #8 abandoned infant. The expanded airway in histopathology appeared to indicate that respiratory efforts occurred, but the movement failed to exhale amniotic fluid due to extensive meconium obstructions. In addition to the absence of injuries on the subject’s body, chorioamnionitis of the placenta was evident. Therefore, it was possible that the general condition of the neonate was very poor after birth. The relatedness of the degree of meconium contamination to persistent pulmonary hypertension and further mortality in neonates was obscure. Perlman et al. [23]. Demonstrated that the degree of meconium contamination did not correlate with the complication and survival time, although sticky meconium induces severe general conditions in infants [11,24]. In contrast, in the three stillbirth cases, smaller amounts of small meconium masses were identified mainly in alveoli. It remains unknown whether this degree of meconium contamination is a physiological phenomenon or a pathogenic one that affected stillbirth. As another perspective, microscopic findings of amniotic fluid in the lungs of stillborns at or near full-term might serve as a distinction from those born alive. After delivery, live-born infants drain the amniotic fluid and breathe in extra-uterine. Except for putrid cases, a hydrostatic test on the lung tissues is definitive for the judgment: aeration of the alveoli during extra-uterine respiration induces flotation in water. In contrast, histological interpretation sufficient to discriminate stillbirth from live-birth is sometimes difficult because the features are nearly indistinguishable [14]. Aeration induces distension of alveoli, which can be demonstrated immnohistochemically using anti-surfactant antibody [18,19] Lavezzi et al. [2]. Demonstrated that pulmonary interstitial emphysema provides morphological evidence of live-birth. However, the aerated appearance is often patchy and uneven in microscopy in cases of infants who died after a short time [14]. Moreover, the lungs removed at autopsy result in collapse, tortuous alveolar wall with atelectasis, caused by the loss of negative pleural pressure and handling at autopsy [25]. In the present study, the bronchi and terminal airways of stillborn infants are filled with amniotic fluid, but considerable amounts of the elements remained in the lungs of neonates, particularly amniotic squames. This result indicates that the complete exhalation or degeneration of the squames occurs only after several days following live-birth [8]. Based on this retention of amniotic fluid, microscopic features of the contents were not attributable to the distinction between stillbirth and live-birth. In conclusion, infant deaths in near-term and term deliveries are rare in forensic pathology. However, their death investigation is unique in several aspects: gestational age, distinction of stillbirth or live-birth, abandoned infants, unknown mother, and so on. To confirm the presence of meconium in the lungs might be important knowledge to determine the physical status of infants during or before delivery. Because the number of examined subjects was limited in the present study, further evaluation of these findings is necessary.
References
- Lau G. Amniotic fluid embolism as a cause of sudden maternal death. Med Sci Law. 1994; 34: 213-220.
- Marcus BJ, Collins KA, Harley RA. Ancillary studies in amniotic fluid embolism: a case report and review of the literature. Am J Forensic Med Pathol. 2005; 26: 92-95.
- Christiansen LR, Collins KA. Pregnancy-associated deaths: a 15-year retrospective study and overall review of maternal pathophysiology. Am J Forensic Med Pathol. 2006; 27: 11-19.
- Garland W, Thompson WD. Diagnosis of amniotic fluid embolism using an antiserum to human keratin. J Clin Pathol. 1983; 36: 625-627.
- Lunetta P, Penttilä A. Immunohistochemical identification of syncytiotrophoblastic cells and megakaryocytes in pulmonary vessels in a fatal case of amniotic fluid embolism. Int J Legal Med. 1996; 108: 210-214.
- Kalousek DK, Gilbert-Barness E. Causes of stillbirth and neonatal death. In: Gilbert-Barness E, editor. Potter’s pathology of the fetus and infant, Mosby, St. Louis. 1997; 128-161.
- Seppälä M, Aho I. Physiological role of meconium during delivery. Acta Obstet Gynecol Scand. 1975; 54: 209-211.
- Cox JN. Respiratory System. In: Berry CL, editor. Paediatric Pathology. Springer, New York. 1995; 313-448.
- Wiswell TE, Bent RC. Meconium staining and the meconium aspiration syndrome. Unresolved issues. Pediatr Clin North Am. 1993; 40: 955-981.
- Manning FA, Martin CB Jr, Murata Y, Miyaki K, Danzler G. Breathing movements before death in the primate fetus (Macaca mulatta). Am J Obstet Gynecol. 1979; 135: 71-76.
- Cleary GM, Wiswell TE. Meconium-stained amniotic fluid and the meconium aspiration syndrome. An update. Pediatr Clin North Am. 1998; 45: 511-529.
- Ghidini A, Spong CY. Severe meconium aspiration syndrome is not caused by aspiration of meconium. Am J Obstet Gynecol. 2001; 185: 931-938.
- Kobayashi H, Ooi H, Hayakawa H, Arai T, Matsuda Y, Gotoh K, et al. Histological diagnosis of amniotic fluid embolism by monoclonal antibody TKH-2 that recognizes NeuAc alpha 2-6GalNAc epitope. Hum Pathol. 1997; 28: 428-433.
- Shapiro HA. Microscopy of human fetal lung and the diagnosis of postnatal respiration. Leg Med Annu. 1977; 1976: 39-52.
- Althoff H, Cremer U. Forensic medicine and morphologic aspects of fatal amniotic fluid aspiration. Z Rechtsmed. 1989; 102: 11-23.
- Ikeda N, Yamakawa M, Imai Y, Suzuki T. Sudden infant death from atelectasis due to amniotic fluid aspiration. Am J Forensic Med Pathol. 1989; 10: 340-343.
- Osawa M, Satoh F, Horiuchi H, Tian W, Kugota N, Hasegawa I. Postmortem diagnosis of fatal anaphylaxis during intravenous administration of therapeutic and diagnostic agents: evaluation of clinical laboratory parameters and immunohistochemistry in three cases. Leg Med. 2008; 10: 143-147.
- Zhu BL, Ishida K, Quan L, Fujita MQ, Maeda H. Immunohistochemistry of pulmonary surfactant apoprotein A in forensic autopsy: reassessment in relation to the causes of death. Forensic Sci Int. 2000; 113: 193-197.
- Shimada I, Matsui K, Kominato Y, Hata Y, Takizawa H, Nishida N. Immunohistochemical study of thyroid transcription factor-1 and surfactant-associated protein A for investigation of peripheral airway structure in perinatal fatality. Leg Med. 2008; 10: 96-100.
- Lavezzi WA, McKenna BJ, Wolf BC. The significance of pulmonary interstitial emphysema in live birth determination. J Forensic Sci. 2004; 49: 546-552.
- Rao S, Pavlova Z, Incerpi MH, Ramanathan R. Meconium-stained amniotic fluid and neonatal morbidity in near-term and term deliveries with acute histologic chorioamnionitis and/or funisitis. J Perinatol. 2001; 21: 537-540.
- Sims MA, Collins KA. Fetal death. A 10-year retrospective study. Am J Forensic Med Pathol. 2001; 22: 261-265.
- Perlman EJ, Moore GW, Hutchins GM. The pulmonary vasculature in meconium aspiration. Hum Pathol. 1989; 20: 701-706.
- Rossi EM, Philipson EH, Williams TG, Kalhan SC. Meconium aspiration syndrome: intrapartum and neonatal attributes. Am J Obstet Gynecol. 1989; 161: 1106-1110.
- Hausmann R, Bock H, Biermann T, Betz P. Influence of lung fixation technique on the state of alveolar expansion-a histomorphometrical study. Leg Med (Tokyo). 2004; 6: 61-65.