
Research Article
Gastrointest Cancer Res Ther. 2016; 1(2): 1012.
Neuroendocrine Tumors of the Gastrointestinal System
Carroll KJ¹, Cortes-Santiago N², Gannon FH² and Rosen DG²*
¹School of Medicine, Baylor College of Medicine, USA
²Department of Pathology and Immunology, Baylor College of Medicine, USA
*Corresponding author: Rosen DG, Department of Pathology and Immunology, Baylor College of Medicine, Michael E. DeBakey VA Medical Center, 2002 Holcombe Blvd, Houston, TX 77030, USA
Received: November 01, 2016; Accepted: November 22, 2016; Published: November 24, 2016
Abstract
Introduction: Neuroendocrine tumors (NETs) of the gastrointestinal tract arise from the extensive serotonergic entero-endocrine system. There is very limited literature focusing on risk factors for development and progression of these tumors. Therefore, this retrospective study aims to compare large bowel, small bowel and stomach neuroendocrine tumors based on disease presentation, tumor progression and associations with certain lifestyle habits and/or the use of drugs that have the potential to modulate the neuroendocrine system.
Materials and Methods: We identified 56 patients at the Michael E. DeBakey Veterans Affairs Medical Center (Houston, TX) from 2000 to 2010. Patients were considered eligible if they were at least 21 years old with established treated or untreated primary gastrointestinal NETs. Relevant demographic factors and clinical history was obtained for all patients. Additionally, use of a broad range of medications directly or potentially affecting the serotonergic system was documented.
Results and Discussion: The majority of NETs were in the large bowel (n = 32), followed by small bowel (n = 20) and stomach (n = 4). Small bowel NETs showed a higher propensity to metastasize than large bowel or stomach tumors (p = 0.002). Conversely, there was a significant association between smoking and development of large bowel NETs (p = 0.04). No significant association with medication use was observed.
Conclusion: NETs within the small bowel have more aggressive clinical courses and may warrant more extensive initial evaluations. Furthermore, our data further supports the association between smoking and development of neuroendocrine tumors, particularly of the large bowel.
Keywords: Neuroendocrine Tumors (NET); Gastrointestinal Tract
Abbreviations
NET: Neuroendocrine Tumor; GI: Gastrointestinal; SEER: Surveillance, Epidemiology, and End Results; CPRS: Computerized Patient Record System; SSRI: Selective Serotonin Reuptake Inhibitor; TCA: Trichloroacetic Acid; PPI: Proton Pump Inhibitor; TNM: Tumor-Node-Metastasis; AJCC: American Joint Committee on Cancer; UICC: International Union Against Cancer; JNK: Jun N-terminal Kinases; ERK1/2: Extracellular Signal–regulated Kinases.
Introduction
Neuroendocrine tumors (NETs) of the gastrointestinal (GI) tract are tumors that arise from the extensive serotonergic enteroendocrine and/or bronchopulmonary neuroendocrine system. While NETs most frequently originate in the GI tract, other locations include the lungs and, less commonly, the pancreas, thyroid, ovaries and adrenal glands. NETs account for less than 0.5% of all gastrointestinal and bronchopulmonary cancers collectively [1]. These tumors were first described in 1907 as “carcinoid” since pathologist Siegfried Oberndorfer suspected these seemingly small and well-demarcated tumors to be histologically more benign than the highly aggressive adenocarcinomas [2]. However, despite its slow-growing nature, in recent years this term carcinoid has become outdated and even deemed a “misnomer” [3] due to the proclivity of many NETs of the small bowel to invade their local surroundings before metastasizing. “Endocrinocarcinoma” and NEC (neuroendocrinocarcinoma) are currently being suggested as suitable alternatives to the name “carcinoid” [2,3].
Diagnostic biochemical screens can detect secretions of peptides and vasoactive substances, including urine 5-Hydroxyindoleacetic acid (serotonin metabolite), as well as serum chromogranin A and pancreastatin levels [4,5]. Metastasis can then be monitored with cross-sectional CT or MRI imaging, somatostatin receptor scintigraphy, and/or hybrid single-photon emission CT [4]. These rare heterogeneous lesions classically present with indolent symptoms, so patients are more often diagnosed in the more advanced, incurable stages of disease [1]. Once diagnosed, treatment involves surgical resection, or alternatively, somatostatin analogues are first-line pharmacological therapies [5]. Novel peptide receptor radionuclide therapy is currently enrolled in a United States Phase III clinical trials and shows favorable outcomes in advanced, low-grade small bowel NETs resistant to somatostatin analogues [5,6].
The majority of diagnosed NETs, largely stage III or IV, have a 5-year overall survival rate of greater than 70% [7]. But despite the relatively high survival rate, the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program reports a five-fold increase in annual prevalence in NETs from 1973 to 2004 [8]. While enhanced diagnostic tools and recent advancement in detection methods could play a role in this rapid uptrend [1], further research is warranted to understand the underlying causes of neuroendocrine malignancies of gastrointestinal origin, which could in turn provide insight into early detection, prevention and predicted disease course. Thus, this study aims to compare large bowel, small bowel and stomach neuroendocrine tumors based on disease presentation, tumor progression and associations with certain lifestyle habits and/or the use of drugs that have the potential to modulate the neuroendocrine system.
Materials and Methods
Study design
This 10-year retrospective population-based study identified 56 patients with gastrointestinal NET cases diagnosed at the Michael E. DeBakey Veterans Affairs Medical Center in Houston, TX between the dates of January 1, 2000 and December 31, 2010.
Data collection
Patients were considered eligible for this study if they were at least 21 years of age with established treated or untreated primary NETs of the large bowel, small bowel or stomach of either carcinoid or adenocarcinoid histology. The neuroendocrine tumors were stratified based on primary anatomical location in the gastrointestinal tract (large bowel, small bowel and stomach). Cases of primary tumors sites outside of these three locations were excluded. Relevant demographic factors (gender and age of diagnosis) and clinical history at the time of resection or diagnosis was obtained for all patients from the Computerized Patient Record System (CPRS) electronic medical record database. Tumors were found either incidentally or after a work-up prompted by the patient’s gastrointestinal, systemic and/ or carcinoid symptoms. Additionally, documented use of a broad range of medications directly or potentially affecting the serotonergic system, including selective serotonin reuptake inhibitors (SSRIs) and other serotonin antagonists, trichloroacetic acid (TCA), and proton pump inhibitors (PPIs), as well as history of diabetes, in the last 10 years prior to biopsy or resection was recorded. Tobacco history was positive if the patient had documented evidence of smoking up to 10 years prior to biopsy or resection. Tumor-Node-Metastasis (TNM) staging was performed for NETs of the colon, small intestine and stomach in accordance with the College of American Pathologists protocol for well-differentiated neuroendocrine tumor specimens, which outlines the recommendations of the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer (UICC) [9,10,11]. Metastasis refers to presence of metastasis at time of resection or diagnosis. Follow-up was limited to documented evidence of recurrence of disease up to 15 years postdiagnosis. Follow-up data could only be collected definitively for 26 patients. The reviewers were blinded to patient demographic data.
Statistical analysis of outcomes
Descriptive statistics were calculated. Chi square tests, Fisher’s exact or ANOVA tests were used to assess the association between variables as appropriate using Microsoft Excel 2010. Only P values <0.05 were considered statistically significant. The primary endpoints of this study were NET prevalence as it relates to drug use and/or tobacco history and frequency of metastasis depending on the site of the primary tumor (small bowel, large bowel or stomach). Secondary endpoints examined the tumor’s histological characteristics, clinical stage and risk of recurrence.
Results and Discussion
During the 10-year time frame, 56 cases of NETs of the small bowel, large bowel and stomach were studied at the DeBakey Veterans Affairs Medical Center. Table 1 outlines the pertinent demographic characteristics of these patients. 92.8% of cases were male. Mean age of diagnosis of all patients was 63.1 years.
Primary tumor characteristics
Patients were diagnosed with NETs of the large intestine, small intestine and stomach, as distinguished by histopathology, respectively (Figure 1). As noted in Table 1, the majority of NETs were in the large bowel (n = 32), followed by small bowel (n = 20) and stomach (n = 4). There was no significant correlation between anatomical location of the neuroendocrine tumors and the tumor’s histological characteristics, clinical stage, nor risk of recurrence. All reported cases of small bowel tumors (n = 20) and stomach tumors (n = 4), and 87.5% of large bowel (n = 28) were of carcinoid origin histologically, as represented in Table 1. Recurrence status of the primary tumor was unknown in the majority of cases (n = 15, n = 1 and n = 4 for large bowel, small bowel and stomach, respectively). While 58% of all patients demonstrated symptoms characteristic of neuroendocrine tumors, no particular primary tumor site was more likely to present with classic symptoms (p = 0.60).
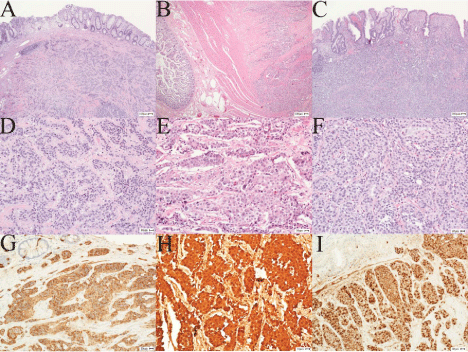
Figure 1: Images: Neuroendocrine tumors of the Gastrointestinal
system. (A-C) Hematoxylin and eosin stain, low power (4x) of large bowel,
small bowel and stomach, (D-F) Hematoxylin and eosin stain, high power
(20x) of large bowel, small bowel and stomach, (G-I) Immunostain for
synaptophysin of large bowel, small bowel and stomach, respectively.
Prevalence of metastasis
As shown in Table 1, small bowel tumors showed a higher propensity to metastasize than large bowel or stomach tumors (p = 0.002). When considering NETs of known invasive status, 63% of the small bowel tumors metastasized either at presentation or over the course of follow-up, compared to only 13% of the large bowel tumors.
Large bowel (n)
Small bowel (n)
Stomach (n)
Total (n)
Gender
Female
1
3
0
4
Male
31
17
4
52
P = 0.22
Mean age at diagnosis (range), in years
61.8 (40-89)
63.6 (38-82)
71.5 (63-79)
63.1 (40-89)
P = 0.15
Metastasis
No
21
7
2
30
Yes
3
12
1
16
Unknown
8
1
1
10
P = 0.002
Symptoms
No (incidental finding)
15
7
1
23
Yes
17
12
3
32
P = 0.60
Histology
Adenocarcinoid
1
0
0
1
Carcinoid
28
20
4
52
Neuroendocrine tumor
3
0
0
3
P = 0.52
Clinical Stage
Stage 1
15
5
1
21
Stage 2
1
1
0
2
Stage 3
0
3
0
3
Stage 4
1
1
0
2
Unstaged
15
10
3
28
P = 0.30
Recurrence
No
17
7
0
24
Yes
0
2
0
2
Unknown
15
11
4
30
P = 0.08
Table 1: Incidence of neuroendocrine tumors (number of cases, n): Stratification of patient demographics, outcomes and tumors characteristics based on tumorlocation in the gastrointestinal tract.
Lifestyle factors
A patient’s history of tobacco use was highly correlated with the development of large bowel neuroendocrine tumors when compared to small bowel and stomach tumors (p = 0.04). In fact, the number of patients with a large bowel NET who also smoked cigarettes (n = 25) more than quadrupled the number of cases without a notable smoking history (n = 7) (Table 2). Comparatively, small bowel NETs did not show the same variance dependent on tobacco use (n = 9 and n = 10, respectively). In contrast, there was no significant correlation between anatomical location of the neuroendocrine tumors and a patient’s history of chronic diabetes (p = 0.97).
Large bowel (n)
Small bowel (n)
Stomach (n)
Total (n)
Tobacco
No
7
10
2
19
Yes
25
9
1
35
Unknown
0
1
1
2
P = 0.04
Diabetes
No
19
11
1
31
Yes
13
8
3
24
Unknown
0
1
0
1
P = 0.97
Table 2: Lifestyle factors and incidence of neuroendocrine tumors (number of cases, n).
Neuro-modulatory drug use
As a potential risk factor for NET development in a specific site of the GI tract, Table 3 outlines the use of 5 drugs that could alter the neuroendocrine milieu. However, no significant correlation was found between the location of the primary NET (large bowel, small bowel and stomach) and the patient’s use of 5HT3 antagonists (p = 0.39), 5HT4 antagonists (p = 0.13), SSRIs (p = 0.95), TCAs (p = 0.46), or PPIs (p = 0.91) prior to tumor presentation.
Large bowel (n)
Small bowel (n)
Stomach (n)
Total (n)
5HT3*antagonist use
N
29
16
3
48
Yes
2
1
1
4
Unknown
1
3
0
4
P = 0.39
5HT4* antagonist use
No
29
19
3
51
Yes
2
1
3
Unknown
1
1
0
2
P = 0.13
SSRI† use
No
21
13
3
37
Yes
10
6
1
17
Unknown
1
1
0
2
P = 0.95
TCA§ use
No
27
18
3
48
Yes
4
1
1
6
Unknown
1
1
0
2
P = 0.46
PPI#use
No
18
10
1
29
Yes
13
9
3
25
Unknown
1
1
0
2
P = 0.91
Table 3: Neuroendocrine-modulatory drug use and incidence of neuroendocrine tumors (number of cases, n).
Conclusion
This 10-year retrospective study establishes that NETs of the small bowel behave more aggressively clinically than those of the large bowel or stomach. A recent neuroendocrine tumor epidemiology retrospective cohort study conducted in Ontario, Canada concluded that 60.2% of small bowel NETs metastasized, either at presentation or during the 15-year follow-up, compared to 46.9% of large bowel tumors [12]. In our study, at presentation 63% of cases of small bowel NETs had already metastasized versus 13% of large bowel tumors. While following a consistent pattern of metastatic disease, our study demonstrates a more pronounced disparity between the clinical course of small bowel and large bowel NETs. The Ontario study followed the patients for metastatic disease up to 15 years after disease presentation, which likely explains the increased percentage of large bowel malignancies. Nonetheless, synthesizing these two datasets suggests that small bowel tumors are more likely to have already metastasized at presentation, while large bowel tumors are relatively slower to progress.
Previous studies have explored smoking as a prospective risk factor for NETs of the GI tract. Two multicenter case-controlled studies have proposed a positive association between history of smoking and small bowel NET growth [13,14]. This association was not necessarily supported by the Debakey Veteran patients, as fairly equal numbers of cases of small bowel NETs were observed for patients with and without smoking exposure (n = 9 and n = 10, respectively). Conversely, more consistent with our data, the University of Texas M.D. Anderson Cancer Center found no significant correlation between smoking and any of their designated primary anatomic locations (small bowel, stomach, rectum, lung, and pancreas), but large intestine NETs were not included in their study [12]. Our study presents the association between smoking and development of large bowel neuroendocrine tumor and thereby highlights the need to redirect our attention to establishing smoking as a risk factor for NETS primarily of the large intestine. Since pack years was not consistently documented in the electronic medical record database, smoking history could not be quantified in our study. Future studies would then be necessary to determine whether longstanding heavy smokers are the patients predominately at risk, or whether even minimal smoking could predispose one to large intestine NET development.
Exogenous serotonin triggers proliferation of carcinoid cell lines via an autocrine feedback loop, facilitated primarily by the Jun N-terminal kinases (JNK) and extracellular signal–regulated kinases (ERK1/2) pathways, while serotonin inhibitors suppress this pathway [15]. Since the levels of serotonin production by NETs are known to correlate specifically with tumor site [15], it seems plausible that serotonin-modulating drugs such as SSRIs and TCAs could play a role in NET growth in varying degrees based on location. Likewise, proton pump inhibitors have been linked to gastric neuroendocrine tumor proliferation in smaller-scale population-based studies given the chronically elevated gastrin levels observed in these patients [16].
However, our study concludes that sustained serotonin-modulating drug and/or proton pump inhibitor use (Table 3) neither prompts nor impedes tumor development in a particular location along the GI tract, although our sample size is relatively small. Also, given the limited sample size, we were not able to take into account how long the patient had been on the medications and the specific dosages administered, which would be a novel focus for further research.
Our study showed no significant correlation between tumor location and whether the patient displayed symptoms at presentation or remained asymptomatic (incidental finding). Previous studies, however, have shown that individuals with a NET of the small bowel, in particular, are more likely to have metastatic disease to the liver and thus more likely to experience symptoms of Carcinoid Syndrome, including diarrhea, flushing and right heart valve disease, attributed to increased serotonin secretion [2,15]. Although perhaps a function of our limited sample size, these incongruent findings suggest that Carcinoid Syndrome would be a later finding in midgut tumors and would not necessarily be evident yet at the time of diagnosis.
Overall this study supports the clinically aggressive course of small bowel NETs, which may therefore warrant a more extensive initial evaluation and closer follow-up, and highlights the role of smoking in large intestine NET growth. Conversely, there was no significant correlation between anatomical location of the neuroendocrine tumors and the 1) likelihood of presenting symptoms, 2) the tumor’s histological characteristics, clinical stage or recurrence risk, 3) a patient’s history of chronic diabetes, or 4) medication use as referenced above. A potentially confounding factor in this study was the treatment modality for each patient, as there was no standardized means to follow-up whether the patient was compliant with his or her medications or to effectively monitor the patient’s response to surgical intervention, which could have impacted the recurrence risk. In addition, the Veterans hospital population base is male predominant and male sex is a poor prognostic factor for overall survival of patients with NETs of the gastrointestinal system [1]. Yet, considering all comparison groups were largely male, this is unlikely to significantly alter the comparisons drawn.
References
- Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015; 121: 589-597.
- Strosberg J. Neuroendocrine tumours of the small intestine. Best Pract Res Clin Gastroenterol. 2012; 26: 755-773.
- Soga J. The term "carcinoid" is a misnomer: the evidence based on local invasion. J Exp Clin Cancer Res. 2009; 28: 15.
- Wong M, Kong A, Constantine S, Pathi R, Parrish FJ, Verma R, et al. Radiopathological review of small bowel carcinoidtumours. J Med Imaging Radiat Oncol. 2009; 53: 1-12.
- Liu EH, Solorzano CC, Katznelson L, Vinik AI, Wong R, Randolph G. AACE/ACE disease state clinical review: diagnosis and management of midgut carcinoids. Endocr Pract. 2015; 21: 534-545.
- Sabet A, Dautzenberg K, Haslerud T, Aouf A, Sabet A, Simon B, et al. Specific efficacy of peptide receptor radionuclide therapy with (177)Lu-octreotate in advanced neuroendocrine tumours of the small intestine. Eur J Nucl Med Mol Imaging. 2015; 42: 1238-1246.
- Strosberg JR, Weber JM, Feldman M, Coppola D, Meredith K, Kvols LK. Prognostic validity of the American Joint Committee on Cancer staging classification for midgut neuroendocrine tumors. J Clin Oncol. 2013; 31: 420-425.
- Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008; 26: 3063-3072.
- Washington MK, Tang LH, Berlin J, Branton PA, Burgart LJ, Carter DK, et al. Members of the Cancer Committee, College of American Pathologists (2010) protocol for the examination of specimens from patients with neuroendocrine tumors (carcinoid tumors) of the small intestine and ampulla. Archives of Pathology & Laboratory Medicine. 2010; 134: 181-186.
- Washington MK, Tang LH, Berlin J, Branton PA, Burgart LJ, Carter DK, et al. Members of the Cancer Committee, College of American Pathologists (2010) protocol for the examination of specimens from patients with neuroendocrine tumors (carcinoid tumors) of the stomach. Archives of Pathology & Laboratory Medicine. 2010; 134: 187-191.
- Washington MK, Tang LH, Berlin J, Branton PA, Burgart LJ, Carter DK, et al. Members of the Cancer Committee, College of American Pathologists (2010) protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Archives of Pathology & Laboratory Medicine. 2009; 133: 1539-1551.
- Hassan MM, Phan A, Li D, Dagohoy CG, Leary C, Yao JC. Risk factors associated with neuroendocrine tumors: a U.S.- based case-control study. Int J Cancer. 2008; 123: 867–873.
- Rinzivillo M, Capurso G, Campana D, Fazio N, Panzuto F, Spada F, et al. Risk and protective factors for small intestine neuroendocrine tumors: a prospective case-control study. Neuroendocrinology. 2016; 103: 531-537.
- Kaerlev L, Teglbjaerg PS, Sabroe S, Kolstad HA, Ahrens W, Eriksson M, et al. The importance of smoking and medical history for development of small bowel carcinoid tumor: a European population-based case–control study. Cancer Causes Control. 2002; 13: 27–34.
- Sarrouilhe D, Clarhaut J, Defamie N, Mesnil M. Serotonin and cancer: what is the link? Curr Mol Med. 2015; 15: 62-77.
- Ellis L, Shale MJ, Coleman MP. Carcinoid tumors of the gastrointestinal tract: trends in incidence in England since 1971. Am J Gastroenterol. 2010; 105: 2563-2569.