
Special Article - Esophageal Cancer
Gastrointest Cancer Res Ther. 2017; 2(1): 1014.
Exercise Prehabilitation during Neoadjuvant Cancer Treatment in Patients with Gastrointestinal and Thoracic Cancer: A Systematic Review
Bott RK1#*, Zylstra J1,2#, Whyte G2,3 and Davies AR1,4
¹Department of Upper GI Surgery, St Thomas’ Hospital, London, UK
²Faculty of Science, Liverpool John Moore’s University, Liverpool, UK
³Centre for Health and Human Performance, Harley Street, London, UK
4Gastrointestinal Cancer, Division of Cancer Studies, King’s College London, UK
#Two authors contributed equally to the completion of the final manuscript
*Corresponding author: Bott RK, Department of Upper GI Surgery, St Thomas’ Hospital, Westminster Bridge Road, London, SE1 7EH, United Kingdom
Received: December 23, 2016; Accepted: January 19, 2017; Published: January 23, 2017
Abstract
Aim: To evaluate the current evidence assessing the use of exercise prehabilitation interventions during neoadjuvant cancer treatment in those patients with gastrointestinal or thoracic cancer.
Methods: A comprehensive and systematic database search was performed to identify all published clinical studies involving exercise prehabilitation during neoadjuvant cancer treatment for patients diagnosed with gastrointestinal or thoracic cancer. Pre-defined criteria were used to identify relevant articles and the Modified Downs and Black checklist was used for quality assessment purposes.
Results: The search identified 508 relevant abstracts. After screening, 18 full-text articles were assessed for eligibility and inclusion. Three full-text articles met all the search criteria and were included in the review. Physical fitness was the main outcome measure and an improvement in physical fitness was observed in all three included studies. There was good adherence to the exercise programmes with a lack of associated adverse events, suggesting safety and feasibility of such interventions in this setting. A differentiation between ‘responders’ and ‘non-responders’ to exercise training was identified for the first time.
Conclusions: This is the first systematic review assessing the use of exercise prehabilitation during neoadjuvant cancer treatment in patients with gastrointestinal and thoracic cancer. Although this review demonstrates the safe use of exercise prehabilitation during neoadjuvant cancer treatment in patients with gastrointestinal and thoracic cancer, there is still insufficient evidence to support any robust conclusions regarding the ideal characteristics of an exercise prehabilitation intervention and the impact it may have on clinical and post-operative outcomes.
Keywords: Exercise prehabilitation; Oncology; Surgery; Neoadjuvant cancer treatment; Gastrointestinal; Thoracic
Introduction
‘Prehabilitation’ is the process of preparing and improving functional capacity ahead of a planned physiological stressor [1]. Surgical prehabilitation is being used in a variety of settings but is novel in the field of neoadjuvant therapy. In patients with upper and lower gastrointestinal cancer there is good evidence demonstrating a decrease in physical fitness when patients have multi-modality therapy (chemotherapy in combination with radiotherapy and surgery) in the neoadjuvant setting [2,3]. In addition, those patients who receive chemotherapy in combination with radiotherapy and surgery have decreased levels of physical fitness when compared to those patients who receive radiotherapy or surgery alone [4]. These affects have been shown to decrease objectively measured physical fitness, assessed using cardiopulmonary exercise testing (CPET), which in turn has been associated with worse surgical outcomes, e.g. post-operative morbidity and mortality [2,3]. There is therefore a current interest in assessing the use of exercise prehabilitation in conjunction with neoadjuvant cancer treatment to maintain and improve the levels of physical fitness for cancer patients prior to surgery.
Regular physical activity during and after cancer treatment has also been shown to reduce the risk of recurrence and improve overall survival [5]. However, following a diagnosis of cancer, physical activity levels tend to decrease [6]. Exercise prehabilitation during neoadjuvant cancer treatment may therefore have an important role in preparing patients for the physiological stress of both neoadjuvant treatment and the surgical resection that follows. Prehabilitation with exercise can lead to skeletal muscle adaptations which improves oxygen uptake and increases mitochondrial content, both contributing to improved aerobic capacity, commonly accepted as the gold standard measure of physical fitness [7]. In colorectal cancer patients, an increase in physical activity levels by 50% following diagnosis, has been shown to decrease the risk of colorectal cancerspecific as well as all-cause mortality [8]. Additionally, for women with breast cancer, it has been suggested that those who exercise for thirty minutes or more, on five or more days a week, at moderate intensity, will have a lower risk of death [9].
There has been increasing interest in recent years in exerciseoncology and the use of pre-operative prehabilitation. A systematic review in 2011, demonstrated that it was feasible and safe for patients with non-small cell lung cancer (NSCLC) to take part in exercise programmes both during and after their cancer treatment [10]. Currently, ongoing studies are predominantly focussed on prehabilitation before surgery as a single modality treatment, or rehabilitation after surgery prior to adjuvant treatment. Most studies are in patients with breast, prostate and rectal cancer and the applicability of these results to other tumour groups is uncertain. To date, no published systematic reviews have examined exercise prehabilitation in patients with gastrointestinal and thoracic cancer. Accordingly, the objective of this review was to systematically evaluate the methods and outcomes of all studies assessing exercise prehabilitation and neoadjuvant cancer treatment in patients with gastrointestinal and thoracic cancer.
Methods
Search strategy
A comprehensive, systematic search was performed on 3rd November 2016 and updated on 20th November 2016 and 28th November 2016 to identify all clinical studies that involved any form of exercise prehabilitation in addition to receiving neoadjuvant cancer treatment for patients with gastrointestinal and thoracic cancer. The following keywords and MeSH search terms were used: ‘exercise prehabilitation’, ‘cancer’, ‘surgery’, ‘treatment’, ‘neoadjuvant’, ‘oesophageal’, ‘gastrointestinal’ and ‘thoracic’. Following an initial search for ‘exercise prehabilitation’ all further searches were carried out using Boolean terms and the above listed keywords. The following databases were used to obtain relevant studies for this review;
- Pubmed
- Ovid Medline (Journals@Ovid Full Text)
- Web of Science
- SCOPUS
- Cochrane Central Register of Controlled Trials (CENTRAL)
- ClinicalTrials.gov
The reference lists of all identified full-text articles were used to manually assess for any additional studies. References from previous review studies on exercise prehabilitation and cancer treatment were also assessed for inclusion.
Study selection
For this new and emerging area of research, the inclusion criteria were broad and included randomised controlled trials (RCTs) and non-RCTs, pilot studies and all adult human trials. The studies needed to include an exercise prehabilitation intervention and any form of neoadjuvant cancer treatment in patients with gastrointestinal or thoracic cancer. All types of exercise prehabilitation interventions were included and this could take place before, during or after the neoadjuvant cancer treatment. The exclusion criteria for this review were trials that were not yet recruiting or where data was unpublished, studies where patients received adjuvant cancer treatment, studies with surgical intervention only and any studies with other cancers (non-gastrointestinal and non-thoracic). Case reports, published abstracts and previous reviews were also excluded.
Participants were therefore adults (>18years) with a gastrointestinal or thoracic cancer who were enrolled in any type of exercise prehabilitation intervention, where physical fitness was an outcome measure, who also had some form of neoadjuvant cancer treatment (chemotherapy or chemoradiotherapy).
Data extraction & quality assessment
Data extraction and the assessment of methodological quality were performed independently by two reviewers (RB and JZ) and the results were collated. The studies which met all the inclusion criteria were assessed for different study characteristics such as study design and length, participant’s characteristics, specific types of cancer, and the timing of the exercise prehabilitation intervention. The type of exercise prehabilitation intervention was also assessed including mode, duration, frequency, location and drop-out rates. The outcome variables that were assessed included cardiopulmonary exercise testing (CPET) derived variables, leg and arm muscle strength and functional exercise capacity, measured by maximum walking distance. Additional outcomes were safety and feasibility, health related quality of life measures and post-operative complications.
The Modified Downs and Black checklist [11] was used for the assessment of methodological quality of the included studies. This checklist can be used for assessing both randomised and nonrandomised studies [12] and it consists of 27 questions, with scores between 0 and 2, and a total score out of 28. The checklist assesses different aspects of quality including reporting, external validity, internal validity and bias, as well as assessing power. Higher quality studies are those with a higher score from the checklist. All scoring was done independently (RB and JZ) and then compared. Any inconsistencies were discussed and agreed.
Results
A PRISMA style flow chart [13] is used to demonstrate the results of the initial systematic database search (Figure 1). A total of 502 relevant abstracts were identified through database searching with an additional 6 abstracts being identified using reference lists from other relevant papers and reviews. Following the removal of any duplicates, all the relevant abstracts were screened by two independent reviewers (RB and JZ) and 323 abstracts were excluded. 18 full-text articles were then assessed for eligibility based on the inclusion and exclusion criteria outlined in the methods section. A further 15 articles were excluded as they did not meet all of these criteria. As demonstrated in the flow chart, following full screening and assessment for eligibility by two independent reviewers, three full text articles were identified for inclusion in this review. A full qualitative synthesis of these papers is provided however due to the small number of studies and the heterogeneity of outcome data being used, a meta-analysis has not been performed.
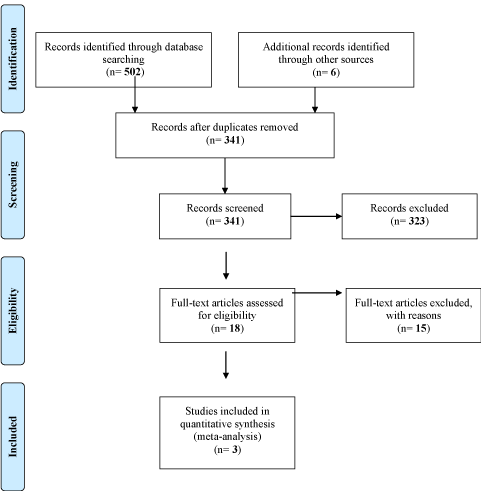
Figure 1: PRISMA-style flow chart demonstrating the different phases of the systematic review [13].
Study characteristics
The main characteristics of the three included studies are outlined in Table 1. There were no randomised controlled trials included in this review. The main aim of the three studies was to identify improvements in the physical fitness of cancer patients using exercise prehabilitation interventions in relation to neoadjuvant cancer treatments for those patients with gastrointestinal and/or thoracic cancers. Two of the included studies were prospective pilot studies [14,15], and one study was blinded at the point of data collection [15]. Heldens at el. carried out a single group study and West et al. used a parallel group study with an intervention (exercise prehabilitation) group and a control group [15]. The third study, by Huang et al. was a retrospective cohort study [16].
First Author & Year of Publication
Study Design
No. of Subjects
Cancer Type
Neoadjuvant Treatment
Exercise Prehabilitation Intervention
Outcomes
Measurements
Heldens et al, 2016
Single group prospective pilot study
13 (20)
Locally advanced resectable rectal cancer
NACRT (chemoradiotherap)
5.5 weeks
45 Gy in 25 fractions + boost dose
Oxaliplatin and Capecitabine
Individual supervised outpatient physical exercise training programme- 2 sessions a week
1. Feasibility of physical exercise programme during NACRT
2. Physical Fitness (functional exercise capacity and muscle strength)
3. Quality of life and perception of fatigue
- Maximum walking distance- 6-minute walk test (6 MWT)
-Muscle strength- submaximal multiple repetition test (X-RM)- leg press and chest press
West et al,
2015
Non-randomised, parallel group, interventional prospective pilot study (blinded)
35 (39)
Locally advanced resectable rectal cancer
NACRT(chemoradiotherapy)
5 weeks
45 Gy in 25 fractions + boost dose
Capecitabine
6 week supervised in-hospital exercise training programme- 3 sessions a week
1. Safety and feasibility of exercise intervention
2. Physical Fitness (oxygen uptake at lactate threshold)
3. Physical Activity (average number of steps)
- Changes in oxygen uptake at estimated lactate threshold (VO2LT)
-Changes in oxygen uptake at peak exercise (VO2peak)
-Changes in the number average number of steps
Huang et al,
2015
Retrospective cohort study
26 (32)
Oesophageal, gastric, colorectal and thoracic cancer
NACRT (chemoradiotherapy)
-No further details given
Individual exercise training programme with progressive aerobic and strength training exercises- 3 to 5 sessions a week
1. Responders vs Non-responders
2. Physical Fitness (oxygen uptake at anaerobic threshold)
3. Post-operative Complications
- Oxygen uptake (VO2) at anaerobic threshold (AT)
- Peak oxygen uptake (VO2peak)
- Oxygen uptake (VO2peak)/body surface area (BSA)
- Peak Work (Watts)
Table 1: Summary of Study Characteristics and Outcome Measures.
The number of study participants in the included studies ranged from 13 patients to 39 patients. Heldens et al. recruited 20 participants, however five patients (25%) refused to take part and two more were excluded, leaving 13 (65%) patients enrolled in the exercise prehabilitation programme. Only nine of these patients (69.2%) completed the whole exercise prehabilitation programme [14]. West et al. recruited 39 subjects who were allocated into an exercise group (22 out of 22 subjects) and a control group (13 out of 17 subjects), with four subjects (10.3%) dropping out prior to starting the exercise prehabilitation programme. Huang et al. referred 32 consecutive patients to their study and of those who were eligible; six participants (18.7%) were excluded, leaving a total of 26 patients whose results were analysed. All three studies included both male and female patients with a greater percentage of male patients reported throughout. The mean age of participants was over 60 years in all three studies.
Two of the studies assessed exercise prehabilitation in the context of neoadjuvant treatment for locally advanced resectable rectal cancer [14,15]. Huang et al. included patients having three different types of surgery; oesophago-gastrectomy, complex colorectal surgery and thoracic cancer surgery. The two prospective studies included patients who underwent neoadjuvant treatment in the form of chemoradiotherapy prior to surgical resection. Of these two studies, one assessed exercise prehabilitation during this treatment [14] and the other assessed exercise prehabilitation following completion of the neoadjuvant treatment but prior to surgery [15]. Both rectal studies used a similar chemoradiotherapy regimen which lasted 5 to 6 weeks with radiotherapy consisting of 45Gy in 25 fractions of 1.8Gy for 5 weeks with a boost dose given in the sixth week. Chemotherapy was given as oral capecitabine twice daily on radiotherapy days by West et al. whilst Heldens et al. administered oxaliplatin intravenously on day one in combination with oral capecitabine twice daily. The retrospective study also included patients undergoing chemoradiotherapy as a neoadjuvant treatment, with exercise prehabilitation being used following completion of treatment, however no further treatment details were provided [16].
Additionally, two of the studies assessed the safety and feasibility of implementing an exercise prehabilitation intervention in these patient groups [14,15]. Other outcomes that were assessed included physical activity measurements; health related quality of life, perception of fatigue and post-operative complications.
Exercise prehabilitation characteristics
All three studies included an exercise prehabilitation intervention alongside a form of neoadjuvant cancer treatment prior to plan surgical resection. The main characteristics of the exercise prehabilitation interventions used in the studies are summarised in Table 2. Heldens et al. was the only study which assessed the use of an exercise prehabilitation intervention during neoadjuvant treatment. Both of the other studies assessed the use of an exercise prehabilitation intervention following completion of the neoadjuvant treatment but prior to planned surgical resection [15,16].
First Author & Year of Publication
Study Design
Timing of Exercise Prehabilitation Intervention
Supervision and Location of Exercise Session
Exercise Session Duration & Frequency
Exercise Session Intensity
Type of Exercise
Duration of Exercise Prehabilitation
Attendance Rate
Heldens et al,
2016
Single group prospective pilot study
During NACRT- exercise prehabilitation commenced during first week of neoadjuvant treatment
Individual training sessions, supervised, in-hospital
45-60 minutes
(2 x week)
Moderate Intensity
Endurance (treadmill and ergometer) and resistance exercises
9-17 weeks
95.7%
West et al, 2015
Non-randomised, parallel group, interventional prospective pilot study (blinded)
Post-NACRT- exercise prehabilitation commenced immediately following completion of neoadjuvant treatment
Paired training sessions, supervised, in-hospital
40 minutes
(3 x week)
Moderate to Severe Intensity
Interval training (ergometer)
6 weeks
96%
Huang et al, 2015
Retrospective cohort study
Post- NACRT- exercise prehabilitation commenced following completion of neoadjuvant treatment
Individual training sessions, supervised and unsupervised, at home (50%), in-hospital (23%) and in the community (27%)
20-45 minutes
(3-5 x week)
Severe Intensity
Progressive interval training (aerobic) and strength training exercises
26- 461 days (median 74 days)
77% (completed > 80% of the exercise programme)
Table 2: Summary of Exercise Prehabilitation Intervention Characteristics.
The location and supervision of the exercise prehabilitation intervention was varied across the studies. Although all three studies used an individualised training programme, West et al. allowed subjects to exercise in pairs to encourage camaraderie and improve adherence [15]. In the other studies, participants exercised alone, however they were supervised in hospital by trained physical therapists in the single group study by Heldens et al. [14]. Huang et al. allowed subjects to exercise individually at home (50%), in the community (27%) or supervised in the hospital (23%).
The exercise prehabilitation intervention sessions lasted between 20 and 60 minutes across the three studies and were performed between two and five times a week [14-16]. The intensity of the exercise intervention session was also varied across the three studies. Huang et al. used a severe intensity exercise training programme that was progressive over its duration and was based on subjects working to 60-80% of their maximum heart rate measured at peak oxygen consumption (VO2peak). West et al. used an exercise training programme which alternated between moderate and severe intensity, also defined using maximum heart rate (50-80%) and peak oxygen consumption (VO2peak). A moderate intensity exercise training programme (50-60% maximum heart rate; estimated using the formula: 220-age) was used by Heldens et al. [14].
Different types of exercise were used for the exercise prehabilitation interventions. Interval and endurance training using an ergometer was used in all three studies [14-16]. In addition, Heldens et al. used three resistance exercises (leg press, chest press and lateral pull down) as part of their exercise prehabilitation programme. Huang et al. also included strength training exercises but no further information regarding these exercises was provided [16].
The duration of the exercise prehabilitation programmes was diverse (26-461 days). In two of the studies it was partly dependent on the length of time between completing neoadjuvant treatment and the planned surgical resection [14,16]. West et al. used a structured six-week exercise training programme following completion of neoadjuvant treatment. Adherence to the exercise prehabilitation programme was similar in the two prospective studies (>95% attendance rate) [14,15]. In contrast, adherence was much lower in the retrospective study where 77% in the ‘responder’ group and as low as 39% in the ‘non-responder’ group was noted [16].
Exercise prehabilitation measurements and outcomes
Measurements: All three of the included studies used physical fitness as a primary outcome measure. The measurements for physical fitness were performed differently in each study. Heldens et al. used functional exercise capacity, measured by a six-minute walk test (6MWT), with maximum walking distance as a primary outcome measure. Muscle strength was also measured using the sub maximal multiple-repetition (X-RM) test procedure for two different resistance exercises: leg press and chest press (14). All measurements were taken at baseline (B), after five weeks of training (T1), after ten weeks of training (T2) and eight weeks post-operatively (T3). West et al. measured physical fitness using cardiopulmonary exercise testing (CPET) derived variables and also assessed physical activity using a biaxial accelerometer measuring average step count over a 72-hour period. The CPET-derived variables assessed were: changes in oxygen uptake at estimated lactate threshold (VO2LT), changes in oxygen uptake at peak exercise (VO2peak) and changes in the number average number of steps [15]. Measurements were taken two weeks before NACRT (baseline), immediately post-NACRT (week 0) and then at weeks 3, 6, 9, and 14 before their surgery during week 15. Both these studies additionally assessed the safety and feasibility of using the exercise prehabilitation programme. Huang et al. also measured physical fitness using CPET-derived gas exchange variables. They included oxygen uptake (VO2) at anaerobic threshold (AT), peak oxygen uptake (VO2peak), and oxygen uptake (VO2peak)/body surface area (BSA) [16]. In this study subjects were also defined as ‘responders’ to the exercise prehabilitation programme if they had >10% improvement in AT, based on 10% being reported as the coefficient of variation for repeated CPET assessment [17]. ‘Non-responders’ were those patients who had less than 10% increase in AT following the exercise prehabilitation programme [16]. CPET measurements were performed at baseline, prior to the exercise prehabilitation programme (CPX1) and following the exercise prehabilitation programme, prior to surgery (CPX2). For those patients who had neoadjuvant chemotherapy (11 of 26 subjects), a third CPET test was performed following the completion of treatment.
Additional secondary outcomes included the assessment of health-related quality of life and patient perception of fatigue using structured self-reported questionnaires [14]. Post-operative complications were also assessed to see if the exercise prehabilitation programme was associated with improved post-operative outcomes. This was performed using the Clavien-Dindo Classification, where a major post-operative complication was defined by a score of >3 which the authors felt was more likely to represent impaired cardiopulmonary reserve rather than a surgical or technical complication [16].
Outcomes: All the included studies demonstrated some improvement in the main primary outcome, physical fitness, following the use of an exercise prehabilitation programme. In the Heldens et al. study functional exercise capacity as measured by walking distance improved across the duration of the exercise prehabilitation programme but none of the observed differences were statistically significant [14]. The biggest change was observed when walking distance at T2 was compared to walking distance at baseline, where seven of eight (87.5%) patients walked a longer distance (mean +/- SD distance 555.6 +/- 101.7m vs 509.6 +/- 124.5m, respectively; P=0.075). However, some observed changes in leg and arm muscle strength were shown to be statistically significant [14]. Although both arm and leg muscle strength improved, no statistically significant results were observed between measurements at baseline and T1 or between T1 and T2. At T2 compared to baseline, eight out of eight (100%) patients had improved their leg muscle strength (mean +/- SD strength of 144.8 +/- 45.6kg vs 104.0 +/- 32.3kg, respectively; P <0.001). This represented a mean improvement in leg strength of 39.2%(14).Also at T2 compared to baseline, eight out of eight patients (100%) had improved their arm muscle strength (mean +/- SD strength of 48.7 +/- 13.8kg vs 36.1 +/- 11.0kg respectively; P= 0.002, representing a mean improvement in arm muscle strength of 34.9%) [14].
West et al. used a CPET measurement immediately following completion of neoadjuvant chemoradiotherapy and demonstrated a significant reduction in VO2LT (-1.91ml kg-1 min-1; 95% CI -1.27 to -2.55; P< 0.0001) and VO2peak (-2.52ml kg-1 min-1; 95% CI -1.33 to -3.71; P<0.0001) [15]. During the intervention period (weeks 0-6), the exercise group showed a significant improvement in both primary endpoints. VO2LT improved in the exercise group by +2.12mlkg-1min-1 (95% CI +1.34-2.90; P< 0.0001), whilst in the control group it did not (-0.65ml kg-1min-1, 95% CI -1.66 to +0.37; P=0.204). VO2peak results were similar with improvement in the exercise group by +2.65 ml kg-1min-1 (95% CI +1.19-4.10; P= 0.0005), whereas the control group reduced by -1.25 ml kg-1min-1 (95% CI -3.14 to +0.64; P=0.19). All these analyses were adjusted for potential confounders which had a negligible effect [15]. A significant difference was also observed in the average number of steps for the exercise and control groups between week 0 and week 6 (P< 0.0001 and P=0.003) as well as between baseline and week 0 for all subjects (P=0.0004) [15].
In the retrospective cohort study of Huang et el., a significant overall improvement in functional capacity was observed across three of the measured CPET-derived domains: AT increased 9% from 10.4 to 11.6 ml kg-1min-1; P=0.046; VO2peak increased 9% from 16.0 to 17.7 ml kg-1min-1; P=0.002; and VO2peak/BSA increased 10% from 658 to 726 ml min-1m-2; P=0.004 (16). Half of the study subjects (50%) were defined as ‘responders’ to their individual exercise prehabilitation programme. For all patients, there was a median improvement between CPX1 and CPX2 in peak work performed of 14% from 100 to 113 W; P=0.0018. ‘Responders’ showed an increase in peak work of 19% (94-115 W; P=0.024) but this improvement was not observed in the ‘non-responder’ group(16). In a comparison of baseline characteristics between the ‘responder’ and ‘non-responder’ groups, no statistically significant differences were observed. For those patients who underwent neoadjuvant treatment, functional capacity demonstrated an insignificant decline following the completion of treatment (AT decreased 5.1%, IQR -5.9% to 16.1%, from 10.4 +/- 2.8 to 9.7 +/- 1.3mlkg-1min-1; P=0.23 and VO2peak decreased 6.8%, IQR -3.6% to 17.2%, from 15.7 +/- 4.6 to 14.7 +/- 3.0mlkg-1min-1; P=0.24). However, a significant improvement was observed between the postneoadjuvant treatment CPET test and the final pre-surgical CPET (CPX2) test (AT increased 13.6%, IQR 4.6% to 22.7%, from 9.7 +/- 1.3 to 11.4 +/- 1.6mlkg-1min-1; P<0.001 and VO2peak increased 11.2 %, IQR 2.7% to 19.8%, from 14.7 +/- 3.0 to 16.5 +/- 3.6mlkg-1min-1; P=0.004) [16].
Additionally, ‘non-responders’ were observed to trend towards suffering more major post-operative complications (Clavien-Dindo classification >3= 30.8%, ‘non-responders’ vs 0% in ‘responders’; P=0.096). ‘Non-responders’ were also more likely to have completed their exercise prehabilitation programme unsupervised, at home (69% vs. 31%, ‘responder’ vs. ‘non-responder’ respectively, P=0.03) [16]. Heldens et al. reported the exercise prehabilitation programme to be both safe and feasible based on adherence rates (95.7%) and a lack of adverse events occurring during the intervention [14]. West et al. also concluded that the exercise prehabilitation used was both safe and feasible, with good overall adherence (96%) and no adverse events reported [15]. No significant differences were observed using the multi-dimensional fatigue index (MFI) or the short form 36 health survey (36) used to assess perception of fatigue and health related quality of life respectively [14].
Quality assessment: The methodological quality of the three included studies was assessed using the Modified Downs and Black Checklist [11]. Each study was independently reviewed (RB and JZ). The highest methodological score was 21 out of 28 and this was observed in the prospective pilot study by Heldens et al [14]. The lowest methodological score was 17 out of 28 and this was observed in the retrospective cohort study by Huang et al [16]. West et al. had a methodological score of 20 out of 28 [15].
Discussion
Overview & quality of included studies
One of the difficulties in assessing the available literature on exercise prehabilitation remains the heterogeneity of study subjects, caused by variations in cancer treatment algorithms between tumour groups, and notable differences in the magnitude of the surgical insult. This is the first systematic review, focussing specifically on patients with gastrointestinal and thoracic cancers, to assess the use of exercise prehabilitation during neo-adjuvant cancer treatment. The intention was to assess the benefit of exercise in a relatively homogenous group of patients but the paucity of studies meeting the inclusion criteria highlighted the novelty of this emerging area of research.
All of the included studies demonstrated some improvement in the main primary outcome of physical fitness, measured using either CPET-derived variables or maximum walking distance and muscle strength. In addition, all three studies demonstrated that an exercise prehabilitation programme in the setting of neoadjuvant cancer treatment for patients with gastrointestinal and thoracic cancer was both feasible and safe. The quality of the included studies was variable. No randomised controlled trials were identified that could be included in this review. There were two prospective pilot studies and one retrospective cohort study [14-16]. Only one study, by West et al had a control group and also used blinding at the point of data collection [15]. The number of subjects in each of the three studies was small (13 to 39 participants). Two of the three included studies involved patients with rectal cancer and the third study involved patients undergoing oesophago-gastrectomy, complex colorectal surgery and thoracic cancer surgery. Adherence to the exercise prehabilitation programmes was good, although the adherence rates were higher when the programme was supervised within a hospital setting. Only one of the studies assessed the use of exercise prehabilitation during neoadjuvant cancer treatment. The two prospective studies assessed exercise following the completion of neoadjuvant cancer treatment but prior to surgical resection [14,15]. Given the well-established deleterious effects of these treatments on physical fitness and outcomes following surgery, this highlights a specific area where further studies are required [18-20].
Type of measurements and outcomes
The primary outcome in all three studies was to assess changes in physical fitness through the implementation of an exercise prehabilitation programme either during or after neoadjuvant cancer treatment. However, physical fitness was measured in different ways in each of the different studies. In two of the studies, CPET-derived variables were used. Unlike testing individual organ systems, CPET testing provides an objective global assessment of a patient’s fitness [21]. CPET is commonly used for evaluating perioperative risk for many different types of surgery including major intra-abdominal surgery [22,23]. In patients having major elective surgery, a lower anaerobic threshold is associated with an elevated mortality postsurgery [24]. Furthermore, many studies have shown a consistent relationship between physical fitness, defined by CPET-derived variables, and postoperative outcomes [25,26]. The use of CPET in the perioperative setting has significantly increased over the past two decades and its use for assessing physical fitness in patients participating in an exercise prehabilitation programme was therefore justified. Two of the included studies used CPET-derived variables to measure their primary outcome [15,16]. The only study which assessed exercise prehabilitation during neoadjuvant treatment did not include any CPET measurements [14]. Whilst it is unclear why CPET-derived variables were not measured in this study, there is evidence to support the use of muscle strength as a measure of physical fitness [27].
High adherence rates and no adverse events following exercise programmes would support the conclusion that such interventions are both feasible and safe. Whether there are truly ‘responders’ and ‘nonresponders’ to exercise programmes as reported by Huang remains uncertain, particularly given the discrepancies in the levels of patient supervision. Undoubtedly there will be variations amongst patients in terms of co-morbidities, baseline physical fitness and compliance that will affect the overall impact of an exercise programme, but this might be taken to support a more individually tailored and structured programme that incorporates these factors. Post-operative outcomes, health-related quality of life and perception of fatigue were included in these studies but all were considered as secondary outcomes.
There has been increasing evidence to suggest that exercise prehabilitation following neoadjuvant cancer therapy is safe and feasible in numerous different cancer types, predominantly in breast cancer [28-30] and rectal cancer [15], however this finding is new with regard to gastrointestinal and thoracic cancer as shown in this review.
Limitations
The limitations of this review centre on the paucity and low quality of the eligible clinical studies which also exhibited significant heterogeneity. There were no randomised controlled trials identified and the numbers of subjects involved were small. Although there was some overlap between the studies, a meta-analysis was not performed.
Conclusions
There is some preliminary evidence supporting the use of exercise prehabilitation in patients undergoing neo-adjuvant treatment followed by surgery for cancer. However, whilst inherently logical, there is a conspicuous lack of robust data to support exercise programmes in a way that might help guide the optimal timing, duration, location and frequency of these interventions. In addition, the positive impact of exercise may be broad, including, but not limited to, physical fitness, mood, activity levels, quality of life, postoperative morbidity and mortality and long-term cancer survival. Future studies to define these optimal outcome measures and develop guidelines for the use of exercise prehabilitation in cancer patients would be beneficial.
References
- Carli F, Charlebois P, Stein B, Feldman L, Zavorsky G, Kim DJ, et al. Randomized clinical trial of prehabilitation in colorectal surgery. Br J Surg. England; 2010; 97: 1187–1197.
- West MA, Loughney L, Barben CP, Sripadam R, Kemp GJ, Grocott MPW, et al. The effects of neoadjuvant chemoradiotherapy on physical fitness and morbidity in rectal cancer surgery patients. Eur J Surg Oncol. 2014; 40: 1421–1428.
- Jack S, West MA, Raw D, Marwood S, Ambler G, Cope TM, et al. The effect of neoadjuvant chemotherapy on physical fitness and survival in patients undergoing oesophagogastric cancer surgery. Eur J Surg Oncol. 2014; 40: 1313–1320.
- Moros MT, Ruidiaz M, Caballero A, Serrano E, Martínez V, Tres A. Effects of an exercise training program on the quality of life of women with breast cancer on chemotherapy. Rev Med Chil. 2010; 138: 715–722.
- Thomas R, Holm M, Al-Adhami A. Physical activity after cancer: an evidence review of the international literature. Br J Med Pract. 2014; 7: a708.
- Adamsen L, Quist M, Andersen C, Møller T, Herrstedt J, Kronborg D, et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: randomised controlled trial. BMJ. 2009; 339: b3410.
- Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol. 1984; 56: 831–838.
- Meyerhardt J a, Giovannucci EL, Holmes MD, Chan AT, Chan J a, Colditz G a, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol [Internet]. 2006; 24: 3527–3534.
- Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005; 293: 2479–2486.
- Granger CL, McDonald CF, Berney S, Chao C, Denehy L. Exercise intervention to improve exercise capacity and health related quality of life for patients with Non-small cell lung cancer: A systematic review. Lung Cancer. 2011; 72: 139–153.
- Trac MH, McArthur E, Jandoc R, Dixon SN, Nash DM, Hackam DG, et al. Macrolide antibiotics and the risk of ventricular arrhythmia in older adults. CMAJ. 2016; 188: e120–129.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998; 52: 377–384.
- Moher D, Liberati A, Tetzlaff J AD. PRISMA 2009 Flow Diagram. The PRISMA statement. 2009. p. 1000097.
- Heldens AFJM, Bongers BC, de Vos-Geelen J, van Meeteren NLU, Lenssen AF. Feasibility and preliminary effectiveness of a physical exercise training program during neoadjuvant chemoradiotherapy in individual patients with rectal cancer prior to major elective surgery. Eur J Surg Oncol. 2016; 1–9.
- West MA, Loughney L, Lythgoe D, Barben CP, Sripadam R, Kemp GJ, et al. Effect of prehabilitation on objectively measured physical fitness after neoadjuvant treatment in preoperative rectal cancer patients: a blinded interventional pilot study. Br J Anaesth. England; 2015; 114: 244–251.
- Huang GH, Ismail H, Murnane A, Kim P, Riedel B. Structured exercise program prior to major cancer surgery improves cardiopulmonary fitness: a retrospective cohort study. Support Care Cancer. 2016; 24: 2277–2285.
- Kothmann E, Danjoux G, Owen SJ, Parry A, Turley AJ, Batterham AM. Reliability of the anaerobic threshold in cardiopulmonary exercise testing of patients with abdominal aortic aneurysms. In: Anaesthesia. 2009. p. 9–13.
- Awad S, Tan BH, Cui H, Bhalla A, Fearon KCH, Parsons SL, et al. Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin Nutr. 2012; 31: 74–77.
- Tan BHL, Brammer K, Randhawa N, Welch NT, Parsons SL, James EJ, et al. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Eur J Surg Oncol. 2015; 41: 333–338.
- Sahai SK. Perioperative assessment of the cancer patient. Best Pract Res Clin Anaesthesiol. Elsevier Ltd; 2013; 27: 465–480.
- Levett DZH, Grocott MPW. Cardiopulmonary exercise testing, prehabilitation, and Enhanced Recovery After Surgery (ERAS) [Test d’effort cardiopulmonaire, préadaptation et récupération rapide après la chirurgie (RRAC)]. Can J Anesth. Springer New York LLC. 2015; 62: 131–142.
- Wilson RJT, Davies S, Yates D, Redman J, Stone M. Impaired functional capacity is associated with all-cause mortality after major elective intra-abdominal surgery. Br J Anaesth. 2010; 105: 297–303.
- Hennis PJ, Meale PM, Hurst RA, O’Doherty AF, Otto J, Kuper M, et al. Cardiopulmonary exercise testing predicts postoperative outcome in patients undergoing gastric bypass surgery. Br J Anaesth. 2012; 109: 566–571.
- Older P, Smith R, Courtney P, Hone R. Preoperative evaluation of cardiac failure and ischemia in elderly patients by cardiopulmonary exercise testing. Chest. 1993; 104: 701–704.
- Smith TB, Stonell C, Purkayastha S, Paraskevas P. Cardiopulmonary exercise testing as a risk assessment method in non cardio-pulmonary surgery: a systematic review. Anaesthesia. 2009; 64: 883–893.
- West M, Jack S, Grocott MPW. Perioperative cardiopulmonary exercise testing in the elderly. Best Pract Res Clin Anaesthesiol. 2011; 25: 427–437.
- Strasser B, Steindorf K, Wiskemann J, Ulrich CM. Impact of resistance training in cancer survivors: A meta-analysis. Med Sci Sports Exerc. 2013; 45: 2080–2090.
- Hornsby WE, Douglas PS, West MJ, Kenjale A a, Lane AR, Schwitzer ER, et al. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol. 2014; 53: 65–74.
- Jones LW, Fels DR, West M, Allen JD, Broadwater G, Barry WT, et al. Modulation of circulating angiogenic factors and tumor biology by aerobic training in breast cancer patients receiving neoadjuvant chemotherapy. Cancer Prev Res. 2013; 6: 925–937.
- Rao R, Cruz V, Peng Y, Harker-Murray A, Haley BB, Zhao H, et al. Bootcamp during neoadjuvant chemotherapy for breast cancer: A randomized pilot trial. Breast Cancer Basic Clin Res. 2012; 6: 39–46.