
Research Article
Gastrointest Cancer Res Ther. 2017; 2(1): 1015.
Extraintestinal Gastrointetsinal Stromal Tumor: Is it Biologically Different from Gastrointestinal Stromal Tumor
Priya V, Kumari N and Krishnani N*
Senior Resident, Department of Pathology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India
*Corresponding author: Niraj Kumari, Additional Professor, Department of Pathology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India
Received: January 17, 2017; Accepted: February 16, 2017; Published: February 17, 2017
Abstract
Extraintestinal gastrointestinal stromal tumors (EGIST) are rare tumorsoccurring at many different locations. Their clinicopathological and genotypic profile vary from the GISTs and has not been well described. Clinicopathological and genotype profiles of all EGISTs received within a period of 8 years were evaluated and compared with GIST. Genotyping for KIT and PDGFRA were done by PCR–Sanger sequencing method. Six cases of EGIST (4 mesenteric, 2 retroperitoneal) were encountered along with 74 GISTs. Four cases had epithelioidand/or mixed morphology. CD117 was positive in 100%, DOG1 in 66.7% and desmin in 50% of EGISTs. Mutation rate was 100% in EGIST and 58.8% in gastrointestinal GISTs. All EGISTs were of high malignant potential except one which was of intermediate malignant potential. Median recurrence free survival was lower in EGIST (24 months) than GIST (79 months). EGIST is distinct from the GIST by predominance of epithelioid morphology, higher malignant potential, higher desmin expression and high mutation rate thus indicating a need of specific risk stratification system for EGIST.
Keywords: EGIST; Gastrointestinal stromal tumor; KIT; PDGFRA
Introduction
Gastrointestinal stromal tumour (GIST) is the most common mesenchymal neoplasm of the gastrointestinal tract (GIT) that arises from the interstitial cells of Cajal (ICC) or a stem cell-like subset of KIT-positive spindle cells around the myenteric plexus [1]. GIST can occur in any part of GIT including stomach, duodenum, rectum and extraintestinal areas such as omentum, mesentry, peritoneum etc. Extraintestinal GISTs (EGISTs) are rare tumors forming <1% of all GISTs [2]. They most commonly occur in omentum and mesentery with less common reported sites being prostate, scrotum, pancreas, gallbladder, liver, rectovaginal septum and gynecological organs, and pleura [3-7]. EGISTs often show an epithelioid or mixed morphology and frequently bear PDGFRA mutations [2].
Materials and Methods
The study included all consecutive resected GISTs received in the Department of Pathology at tertiary care referral hospital over a period of 8 years. The clinical features, laboratory and followup data were recorded from the Hospital Information System (HIS) and patient’s case files. All cases were reviewed for gross, microscopic and immunohistochemical (IHC) features. Apanel of antibodies including CD117, DOG1, CD34, SMA, S100, desmin and vimentin were available in all cases required for the diagnosis of GIST. Positive staining of a marker was defined as moderate to intense cytoplasmic staining in at least >10% tumour cells. DNA extraction was done from formalin fixed paraffin embedded tissues comprising of more than 80% tumor cells. Mutation analysis was done by PCR-Sanger sequencing method for KIT exons 11, 9, 13 and 17, and PDGFRA exons 18 and 12. After amplification the products were checked on 2% agarose gel electrophoresis followed by post-PCR purification and Sanger sequencing.
Results
Six EGISTs were received in a total of 80 GISTs within a period of 8 years accounting for 0.75% of all GISTs with four cases occurring in retroperitoneum and 2 cases in mesentery. The median agewas similar in which was 56 years in EGIST patients (range 39- 65) and 57 years in GIST patients (range 16-80), all the 6 cases were symptomatic with 66.7% presenting with abdominal pain (4 patients) and one case each with palpable lump and history of gastrointestinal bleeding. The tumour size of EGIST ranged from 2.5 – 28cm with a mean size of 12.9cm and median of 10cm, which was larger than GIST (mean size – 10.1cm, median – 8.7cm). Necrosis was present in 4 cases. Skenoidfibres were not seen in any of the EGIST. Epithelioid or mixed morphology was present in 4 cases (66.7%) and spindle cell morphology in 2 cases (33.3%) of EGIST (Figure 1a,1b). The clinicopathological features of EGIST and GIST are compared in Table 1.
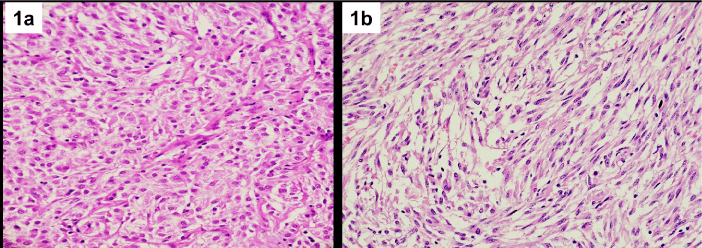
Figure 1: Extraintestinal GIST displaying epithelioid cell morphology (1a);
and spindle cell morphology (1b). Hematoxylin and eosin stain; 400X
Magnification.
Clinicopathological feature
EGIST
(n=6)
Gastrointestinal GIST
(n=74)
Median age (range) in years
56(39-65)
53.5(16-64)
Male: Female ratio
2:1
3.1:1
Clinical features
Abdominal pain
Palpable abdominal lump
GI bleeding
4
4
1
29
33
33
Tumour size (range) in cm
12.9(2.5-28)
10.1(1.5-30)
Cell Type
Spindle
Epithelioid
Mixed
2
1
3
50
6
18
Risk group
None
Very Low
Low
Intermediate
High
0
0
0
1
5
1
14
14
13
42
Mitosis
<5/50 HPF
>5/50 HPF
1
5
39
35
Necrosis
4
29
Lymph node metastasis
1
10
Distant metastasis
1
1
Skenoidfibres
0
11
Table 1: Comparison clinicopathological features of EGIST and GIST.
Immunohistochemically CD117 was positive in all 6 cases of EGIST. DOG1, CD34, SMA and desmin showed positivity in 4/6, 3/6, 3/6, and 3/6 cases of EGISTs (Figure 2a,2b). Comparison of IHC profile of EGIST and GIST is mentioned in Table 2. Five cases (83.3%) of EGIST were of high and one (16.7%) of intermediate malignant potential, whereas 56.7% (42 of 74 cases) of GISTs were of high malignant potential. Comparison of risk stratification groupsof EGIST and GIST is given in Table 3.
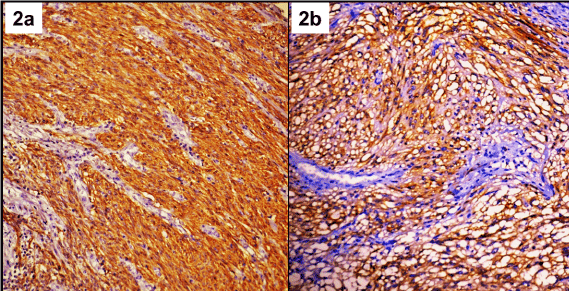
Figure 2: Strong expression of DOG1 (2a); and CD117 (2b) in EGIST.
(Immunohistochemistry; 400X Magnification).
IHC Markers
EGIST (%)
Gastrointestinal GIST (%)
CD117
100
93.2
DOG1
66.7
93.2
CD34
50
60.8
SMA
50
39.1
Desmin
50
13.5
Table 2: Immunohistochemical expression in GIST and EGIST.
Risk groups
Stomach (n=38)(%)
Small intestine (n=33)(%)
Large intestine (n=3)(%)
Extra-intestinal (n=6)(%)
None
1 (100)
0
0
0
Very low
4 (100)
0
0
0
Low
7 (50)
7 (50)
0
0
Intermediate
8 (57.1)
5 (35.7)
0
1 (7.1)
High
18 (38.3)
21 (44.6)
3 (6.4)
5 (10.6)
Table 3: Comparison of risk groups in EGIST and GIST.
Mutation rate in EGISTs was 100% compared to the GIST where it was 58.8%. Mutations in EGIST included KIT exon 11 mutations in three cases (del 557-559, insertion 575-576, substitution leu 576 pro); KIT exon 9 mutations in two cases (duplication 502-503, substitution phe 504 ser) and 1 case of PDGFRA exon 18 mutation (substitution D842E) (Figure 3a,3b). Median recurrence free survival in EGIST was 24.4 months whereas in GIST it was 79 months. Recurrences were observed in 2 cases of EGIST after 11 months and 20 months of diagnosis. One of them had KIT exon 11 deletion which is known to have an aggressive behavior while the other had PDGFRA18 D842E substitution which is said to be resistant to imatinib therapy. The association of mutation profile with clinicopathological features of GIST and EGIST is mentioned in Table 4.
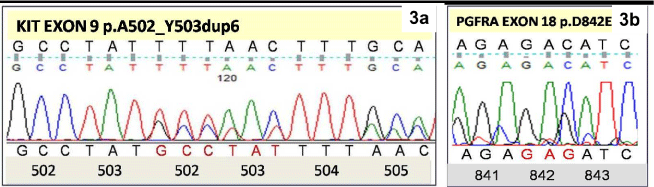
Figure 3: Electrophoretogram of KIT exon 9showing A502-503Y duplication
(3a); and PDGFRA exon 18 showing D842E point mutation (3b).
Location
Mitosis
(/50HPF)
Risk group
Recurrence
Exons
Type of mutation
Mesentery
32
High
No
KIT 11
Complex insertion 575-576
Mesentery
71
High
No
KIT 9
PT PheAla504serine
Retroperitoneum
82
High
Yes
KIT 11
Del557-559
Retroperitoneum
7
High
No
KIT 11
PT Leu576Pro
Retroperitoneum
10
High
No
KIT 9
Duplication c502-503
Retroperitoneum
<1
Intermediate
Yes
PDGFRA 18
PT D842E
Table 4: Mutation profile of EGIST.
Discussion
EGIST is a rare disease accounting for ~10 % of all GISTs [4,8,9]. In the present study EGIST constituted 0.75% of all GISTs. Morphologically it resembles gastric GISTs in having predominantly epithelioid or mixed cell type, however behavior wise they resemble small intestinal or colonic GISTs with all of them being intermediate to high malignant potential.
The CD117 positivity was 100% in EGIST similar to other studies on EGIST, which show CD117 expression varying from 92.2 to 100% [3,4,10]. DOG1 expression was 66.6% in the present study while it was 100% in EGIST in the study by Yi et al [4]. SMA positivity (50% vs. 39.1%) and desmin positivity (50% vs. 13.5%) were higher in EGIST as compared to GIST. Patnayak et al found 20% desmin positivity in EGIST in their study [11]. Diffuse strong and consistent CD117 positivity in these tumours demonstrate the origin of these tumors to be from ICC like cells or a multipotent progenitor cell with differentiation along the lines of ICC.
The median recurrence free survival was low in EGIST with 2 cases having local recurrence within 11 and 20 months in spite of imatinib therapy. One of them had a PDGFRA mutation, which is known to be resistantto therapy while the other had KIT exon 11 deletion which is also a poor prognostic feature.
Mutation profiles of EGIST in this study showed mutation in all three common genes (KIT exons 11 and 9 and PDGFRA exon 18). In studies by Yi et al and Yamomoto et al the mutation frequency in EGISTwas similar to mutations encountered in GIST [3,4]. One of the notable differencein the present study was that EGIST had higher mutation frequency (100%) as compared to GIST.
According to the risk stratification criteria given by Mittienen et al which was basically formulated for gastrointestinal GIST, all cases of EGIST in the present study were of intermediate to high malignant potential with recurrence in 2 of 6 cases [12]. The different IHC profile and 100% mutation rate point towards EGIST being biologically distinct fromGIST. Though numbers of cases in the present study are too small to arrive at a definite conclusion, studies on larger number of EGISTs with long followup may be required to prove whether the same risk stratification criteria of GIST holds true for EGISTs as well.
Conclusion
EGIST accounted for <1% of all GISTs and has distinct morphological, immunohistochemical and genetic profile from GIST by harboring predominance of epithelioid morphology, higher malignant potential, higher desmin expression and high mutation rate thus indicating a need of specific risk stratification system for EGIST.
References
- Wahem A, Schaefer IM, Schüler P, et al. Gastrointestinal stromal tumors. Int J Colorectal Dis. 2012; 27: 689–700.
- Wong NACS. Gastrointestinal stromal tumors–an update for histopathologists. Histopathology. 2011; 59: 807–821.
- Yamamoto H, Oda Y, Kawaguchi K, et al. C-kit and PDGFRA mutations in extra gastrointestinal stromal tumor (gastrointestinal stromal tumor of the soft tissue). Am J Surg Pathol. 2004; 28: 479–488.
- Yi JH, Park B-B, Kang JH, et al. Retrospective analysis of extra-gastrointestinal stromal tumors. World Journal of Gastroenterology: WJG. 2015; 21: 1845-1850.
- Kang SH, Kim MJ, Park MG, et al. Extra gastrointestinal stromal tumor presenting as a scrotal mass: an unusual case. Asian J Androl. 2007; 9: 275-279.
- Bolanaki H, Delladetsima I, Argyropoulou P, et al. Primary Malignant Gastrointestinal Stromal Tumor (GIST) of the Gallbladder: Report of a Case. J Gastrointest Cancer. 2012; 43: S151-155.
- Lam M, Corless CL, Goldblum JR, et al. Extra gastrointestinal Stromal Tumors Presenting as Vulvovaginal/Rectovaginal Septal Masses: A Diagnostic Pitfall. International Journal of Gynecological Pathology. 2006; 25: 288-292.
- Du CY, Shi YQ, Zhou Y, et al. The analysis of status and clinical implication of KIT and PDGFRA mutations in gastrointestinal stromal tumor (GIST). J Surg Oncol. 2008; 98: 175–178.
- Cho MY, Sohn JH, Kim JM, et al. Current trends in the epidemiological and pathological characteristics of gastrointestinal stromal tumors in Korea, 2003-2004. J Korean Med Sci. 2010; 25: 853–862.
- Reith JD, Goldblum JR, Lyles RH, et al. Extra gastrointestinal (soft tissue) stromal tumors: an analysis of 48 cases with emphasis on histologic predictors of outcome. Mod Pathol. 2000; 13: 577–585.
- Patnayak R, Jena A, Parthasarathy S, et al. Primary extra gastrointestinal stromal tumors: a clinicopathological and immunohistochemical study: a tertiary care center experience. Indian J Cancer. 2013; 50: 41–45.
- Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005; 29: 52-68.