
Mini Review
Gerontol Geriatr Res. 2021; 7(2): 1054.
Enhanced Thymopoiesis as an Alternative Therapeutic Option for COVID-19
Genebat M1,2#, Tarancón-Díez L3#, Calderón A1, Muñoz-Fernández MA3 and Leal M4*
1Department of Internal Medicine, Hospital Fátima, Sevilla, Spain
2Department of Emergency, Virgen del Rocío University Hospital, Sevilla, Spain
3Immunology Section, Laboratorio Inmuno-Biología Molecular (LIBM), Hospital General Universitario Gregorio Marañón, Madrid, Spain; Instituto de Investigación Sanitaria Gregorio Marañón (IISGM), Madrid, Spain
4Infectious Diseases and Immunology Unit, Internal Medicine Department, Hospital Viamed Santa Ángela de la Cruz, Sevilla, Spain
#Contributed Equally to this Work
*Corresponding author: Leal M, Infectious Diseases and Immunology Unit, Internal Medicine Department, Hospital Viamed Santa Ángela de la Cruz, Sevilla, Spain
Received: April 20, 2021; Accepted: May 17, 2021; Published: May 24, 2021
Introduction
The pandemic caused by SARS-CoV-2 infection (COVID-19 disease) has expanded worldwide. Currently, it is well known that advanced age is an independent predictor of mortality and severe clinical outcome, apart from other comorbidities [1]. Underlying molecular and cellular mechanisms that could explain the severe clinical outcome among elderly subjects are not well known [2], although it has been described that both immunosenescence and a low-level systemic inflammation (inflamm-aging) could also play a relevant role [3,4].
Now a days, apart from an effective vaccine development, research efforts are focused on therapeutic approaches that could minimize both the viral replication and the further inflammatory cascade driving to respiratory distress and multiorgan failure; however, up to now, no specific therapy for SARS-CoV-2 infection has been established [5]. Awaiting for definitive and conclusive results from prospective clinical trials, antiviral and immunomodulatory drugs currently employed in SARS-CoV-2-infected subjects are based on their biological plausibility according to the mechanism of action or in vitro efficacy, but not in a definitive scientific evidences.
Taken altogether, alternative hypothesis about underlying mechanisms driving to an impaired clinical outcome in COVID-19 disease are required. In this sense, even the universally accepted role of the cytokine storm has been questioned [6]. Hence, the greater hypothesis is considered the greater and more beneficial therapeutic options could be tested. Recently, our group has suggested that thymic dysfunction could play a relevant role in the impaired clinical outcome observed in elderly SARS-CoV-2-infected subjects [7]. Thus, the main objective of the present opinion paper is to explore a new therapeutic option for COVID-19 disease, based on enhancing thymic function.
Thymic Function in Adulthood
It has been traditionally accepted that thymic function begins its involution in childhood and continues in adolescence under the effect of sexual hormones, so that just thymic vestiges are observed in the elderly. However, it is well known that thymic function may be present and enhanced in different clinical scenarios. First of all, Mackall et al. [8] reported for the first time how thymic function increased in children and adults with chemotherapy-induced lymphopenia. This observation has been confirmed in other clinical settings [9,10], including HIV-infected subjects under suppressive antiretroviral therapy (Figure 1). Additionally, it has been shown that even in the elderly certain thymopoiesis remains (Figure 2) and it is directly associated with T-cell homeostasis and survival in healthy elderly subjects [11-13].
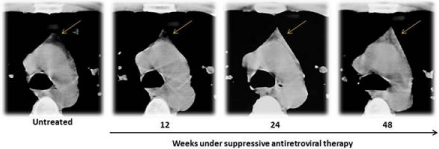
Figure 1: Visualization of thymus slices of a 28-year-old HIV-1 infected patient by Computed Tomography (CT). Enhanced and amplified CT scans at the level
of pulmonary artery are shown at baseline and after 12, 24 and 48 weeks under highly active antiretroviral treatment. Thymus outline and density (indicated by
yellow arrows at the top centre of each frame) increased progressively after treatment introduction. Images taken by the Laboratorio de Inmunoviología, Instituto
de Biomedicina de Sevilla (IBiS), Hospital Universitario Virgen del Rocío de Sevilla (Spain).
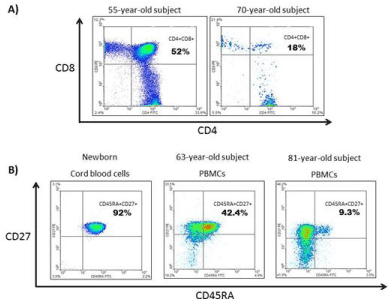
Figure 2: Flow cytometer plots. Percentage of double positive thymocites (CD4+CD8+) obtained from 55-year-old and 70-year-old subjects. Thymic cells obtained
after cardiac surgery and tissue disgregation (A). Plots showing the percentage of thymocites (CD4+CD8+) and, obtained from two different adults: thymus samples
after cardiac surgery. Frequency of naïve CD4+ T-cells based on the expression of CD45RA+ and CD27+ from a newborn cord blood cells, and peripheral blood
mononuclear cells of two adults: a 63-year-old and 81-year-old subjects (B). Experiments were performed in a FACSCanto (BD Biosciences) and analysed using
Flowjo 8.7.7 (TreeStar, San Carlos, California, USA). Data from Laboratorio de Inmunoviología, Instituto de Biomedicina de Sevilla (IBiS), Hospital Universitario
Virgen del Rocío de Sevilla (Spain).
According to these evidences, thymic function could increase in different clinical scenarios when lymphopenia occurs (chemotherapy, HIV-infection), trying to compensate the lack of T-cells. Besides, thymic function may also be active in healthy elderly subjects, playing a relevant role in the maintenance of the naïve T-cell pool and survival of these people.
Thymic Function and COVID-19 Disease
The potential role of thymic function in the control of acute infections, including SARS-CoV-2, is not well known. Hypothetically, thymic function could have a dual positive role regarding acute infections: 1) First of all, through a direct action against novel antigens (in this case, SARS-CoV-2) by a triple function: elimination of selfreactive cellular clones, generation of naive T-cells and expansion of T-cell receptor repertoire; 2) Secondly, modulating the inflammatory response by regulatory T-cells.
Regarding COVID-19 disease, lymphopenia is developed by around 80% of subjects affecting T-cell, B-cell and NK lymphocytes. Additionally, these affected subjects also show increased exhaustion biomarkers such as PD-1 or TIM-3; the increased levels of those biomarkers could be associated with plasma proinflammatory citokines (IL-6, IL-10, TNFa) and an impaired clinical outcome [14,15].
However, the reason why lymphopenia occurs in the context of severe COVID-19 disease remains still unknown. Different hypothesis have been proposed: direct viral infection against thymus, peripheral T-cell redistribution due to cell migration affecting and colonizating organs including lungs, bone marrow and thymus, despite no ACE receptors (angiotensine converting enzyme, the main SARS-CoV-2 cell receptor) have been described in that lymphoid organ [16]. However, the origin of the distinct lymphopenia that characterizes COVID-19 disease is unknown. We have recently hypothesized that thymic function could play a relevant role in this context [7], an attractive approach that could have subsequent clinical implications.
Enhancing Thymopoiesis: The Role of Alpha-1-Thymosine
In the context of COVID-19 disease, and mainly in elderly subjects because of the greater severity of SARS-CoV-2 infection in this population, the maintenance of a residual thymic function may be critical regarding the further clinical outcome. Moreover, this thymic function may be therapeutically enhanced in order to increased thymopoiesis and improved the cellular immune response to the novel antigen, modifying the natural course of COVID-19 disease (Figure 3A).
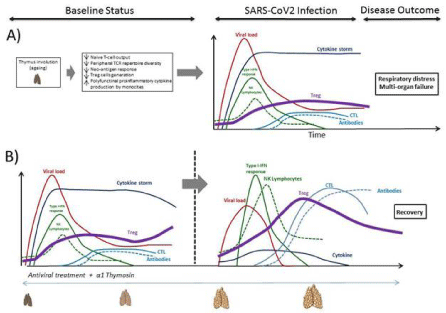
Figure 3: Figurative kinetics of immune system response against SARS-CoV-2, disease evolution and outcome in a status of thymus involution or impairment due
to ageing (modified from Genebat et al. Ageing and disease In press) (A). Potential immunomodulatory effect of a1thymosin (a1Thy) as adjuvant in the adaptive
and innate immune system restoration and SARS-CoV-2 specific cytotoxic T-response increase (B). The effect of a1Thy could reverse the thymus involution into a
status in which thymic function is preserved. Treg: Regulatory T-cell. NK lymphocyte: Natural Killer lymphocyte. CTL: Cytotoxic T lymphocyte. TCR: T-cell Receptor.
The human alpha-1-thymosine (a1Thy) is segregated by the epitelial cells of the thymic tissue and widely distributed through secondary lymphoid organs [17]. The potential immunologic effects of a1Thy are: 1) to mitigate the exacerbated inflammatory response favouring the production of regulatory T-cells, that can diminish the production of proinflammatory cytokines [18]; and 2) to favour antigen presentation increasing type I and II MHC expression on antigen-presentation cells [19].
Accordingly to the above commented immunologic effects, clinical use of a1Thy has been tested in different scenarios, with an excellent safety profile [20,21]. Focusing in SARS-CoV-2 infection, it has been recently reported a favourable clinical approach using a1Thy in the context of severe COVID-19 disease, showing a clinical benefit and an immune recovery based on a T-cell count increase [22,23], suggesting that thymus capacity could be partially preserved (Figure 3B). The mechanisms by which a1Thy can participate as an immunomodulatory coadjuvant in therapy and vaccine designs against COVID-19 remain still unknown; however, some evidences has indicated a direct inhibitory property of a1Thy against ACE receptor [24].
Conclusion
In conclusion, we consider that thymic function is critical to control SARS-CoV-2 infection, mainly in the elderly when thymic function is impaired but not absent. Based on the immunologic properties of a1Thy and its preliminary results in different clinical scenarios including COVID-19 disease, prospective studies are required in order to determine the mechanisms and confirm the efficacy of this therapeutic approach as an immunomodulatory adjuvant in therapies against SARS-CoV-2 infection.
References
- Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020; 584: 430-436.
- Mueller AL, McNamara MS, Sinclair DA. Why does COVID-19 disproportionately affect older people? Aging. 2020; 12: 9959-9981.
- Brooke RT, Fahy GM. Reversing immunosenescence for prevention of COVID-19. Aging. 2020; 12: 11161-11162.
- Meftahi GH, Jangravi Z, Sahraei H, et al. The possible pathophysiology mechanism of cytokine storm in elderly adults with COVID-19 infection: the contribution of “inflame-aging”. Inflamm Res. 2020; 69: 825-839.
- Vijayvargiya P, Esquer Garrigos Z, Castillo Almeida NE, et al. Treatment considerations for COVID-19: a critical review of the evidence (or lack thereof). Mayo Clin Proc. 2020; 95: 1454-1466.
- Remy KE, Mazer M, Striker DA, et al. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI insight. 2020; 5: 140329.
- Genebat M, Tarancon-Diez L, De Pablo-Bernal RS, et al. Coronavirus disease (COVID-19): a perspective from immunonosenescense. Aging Dis 2021; 121: 3-6.
- Mackall CL, Fleisher TA, Brown MR, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995; 332: 143-149.
- Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998; 396: 690-695.
- Franco JM, Rubio A, Martínez-Moya M, et al. T-cell repopulation and thymic volume in HIV-1-infected adult patients after highly active antiretroviral therapy. Blood. 2002; 99: 3702-3706.
- Ferrando-Martinez S, Franco JM, Hernandez A, et al. Thymopoiesis in elderly human is associated with systemic inflammatory status. Age. 2009; 31: 87- 97.
- Ferrando-Martínez S, Romero-Sánchez MC, Solana R, et al. Thymic function failure and C-reactive protein levels are independent predictors of all-cause mortality in healthy elderly humans. Age. 2013; 35: 251-259.
- Ferrando-Martínez S, Ruiz-Mateos E, Hernández A, et al. Age-related deregulation of naive T cell homeostasis in elderly humans. Age (Dordr). 2011; 33: 197-207.
- Coles AJ, Azzopardi L, Kousin-Ezewu O, et al. Keratinocyte growth factor impairs human thymic recovery from lymphopenia. JCI Insight. 2019; 4: e125377.
- Diao B, Wang C, Tan Y, et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front Immunol. 2020; 11: 827.
- Hamming I, Timens W, Bulthuis MLC, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004; 203: 631-637.
- Goldstein AL, Badamchian M. Thymosins: chemistry and biological properties in health and disease. Expert Opin Biol Ther. 2004; 4: 559-573.
- Romani L, Bistoni F, Perruccio K, et al. Thymosin alpha1 activates dendritic cell tryptophan catabolism and establishes a regulatory environment for balance of inflammation and tolerance. Blood. 2006; 108: 2265-2274.
- Yao Q, Doan LX, Zhang R, et al. Thymosin-alpha1 modulates dendritic cell differentiation and functional maturation from human peripheral blood CD14+ monocytes. Immunol Lett. 2007; 110: 110-120.
- Zhang D, Zhou Y, Cheng Q. Effects of combined thymosin and hydrocortisone on immune response in septic mice. Int J Clin Exp Med. 2015; 8: 12989- 12994.
- Pica F, Gaziano R, Casalinuovo IA, et al. Serum thymosin alpha 1 levels in normal and pathological conditions. Expert Opin Biol Ther. 2018; 18: 13-21.
- Wu M, Ji J-J, Zhong L, et al. Thymosin a1 therapy in critically ill patients with COVID-19: A multicenter retrospective cohort study. Int Immunopharmacol. 2020; 88: 106873.
- Liu Y, Pang Y, Hu Z, et al. Thymosin alpha 1 (Ta1) reduces the mortality of severe COVID-19 by restoration of lymphocytopenia and reversion of exhausted T cells. Clin Infect Dis. 2020; 71: 2150-2157.
- Kharazmi-Khorassani J, Asoodeh A, Tanzadehpanah H. Antioxidant and Angiotensin-Converting Enzyme (ACE) inhibitory activity of thymosin alpha-1 (Tha1) peptide. Bioorg Chem. 2019; 87: 743-752.