
Case Report
Ann Hematol Oncol. 2022; 9(4): 1404.
Disseminated Strongyloidiasis in a Patient Treated with Rituximab and Ibrutinib, a Case Report
Vic S¹*, Belaz S², Decaux O¹, Robert-Gangneux F² and Revest M³
1Service d’hématologie Clinique, CHU de Rennes, Université de Rennes, Rennes, France
2Laboratoire de Parasitologie et Mycologie, CHU de Rennes, Université de Rennes, Rennes, France
3Service des Maladies Infectieuses, CHU de Rennes, Université de Rennes, Rennes, France
*Corresponding author: Samuel VIC, Service d’hématologie Clinique, CHU Rennes, 2 rue Henri Le Guilloux 35033 Rennes Cedex 9, France
Received: August 17, 2022; Accepted: September 16, 2022; Published: September 23, 2022
Abstract
We report the case of a woman treated by rituximab and ibrutinib for a marginal-zone lymphoma, presenting diarrhea, dyspnea, retiform purpura, hemolytic anemia and hypereosinophilia revealing a disseminated strongyloidiasis. Diagnosis was done by stool parasitic examination, serology was negative. She was treated by ivermectin. All the symptoms disappeared, including hemolytic anemia. To our knowledge, this is the first report of a disseminated strongyloidiasis in a patient treated by ibrutinib. Hemolytic anemia was not immune-mediated, and possibly related to the infection as it regressed under anti-parasitic treatment.
Keywords: Strongyloidiasis; Lymphoma; Rituximab; Ibrutinib; Hemolytic anemia
Abbreviations
ANCA: Anti-Neutrophil Cytoplasmic Antibodies; BTK: Bruton Tyrosine Kinase; CCP: Cyclic Citrullinated Peptide; CT: Computed Tomography; CRP: C-Reactive Protein; G6PD: Glucose-6-Phosphate Dehydrogenase; HTLV-1: Human T-Lymphotropic Virus 1; ICU: Intensive Care Unit; IV: Intravenously; LDH: Lactate Dehydrogenase; SC: Subcutaneously; WBC: White Blood Cell
Introduction
Strongyloides stercoralis is an intestinal helminth that infects humans through contact with soil contaminated by human feces containing strongyloid larvae. The disease is widely distributed in tropical and subtropical regions, and it is estimated that 30 to 100 million people are infected worldwide [1]. Strongyloidiasis is asymptomatic in more than half of cases, where eosinophilia or a positive serologic test may be the only finding [2]. The parasite has a long-term persistence in the host leading to infection that could last for decades. While strongyloidiasis is mainly a chronic clinically inapparent infection in immunocompetent hosts, immunosuppressed patients are at risk of life-threatening hyperinfection syndrome due to uncontrolled rhabiditoid larvae production, and differentiation into strongyloid larvae, which can translocate through the gut. Larvae migration through tissues carries intestinal bacteria, leading to sepsis, and death in 60 to 70% of cases [3]. The main risk factor for strongyloidiasis hyperinfection is systemic corticosteroid use, but other immunosuppressed backgrounds such as infection by the Human T-Lymphotropic Virus 1 (HTLV-1), hematological malignancies, and transplantation are described [4]. Even if some studies suggested to screen and treat every immunosuppressed patient or candidate to immunosuppression if they may have been exposed to S. stercoralis [5], there are no guidelines for the prevention of infection in patients treated for hematologic diseases. It is commonly recommended to treat every patient coming from a high-risk area and scheduled to start a prolonged corticosteroid or another “highly immunosuppressive” therapy [6]. Data regarding other hematologic treatments, especially recent ones such as targeted therapies, are scarce, and there is no consensus on whether systematic screening or prophylactic treatment should be done. In this article, we report a case of disseminated strongyloidiasis in a patient treated by rituximab and ibrutinib for a lymphoma.
Case Description
The patient was a 70-year-old woman with a splenic marginalzone lymphoma diagnosed one year before, on November 2020. She was included in a clinical trial evaluating a combination of rituximab and ibrutinib: rituximab 375mg/m² Intravenously (IV) on day 1 and then 1400mg Subcutaneously (SC) on days 8-15-21 for the first cycle, then 1400mg SC every 28 days for the following cycles; and a daily administration of oral ibrutinib 560mg. She received a total of 5 cycles from February to July 2021. Treatment was efficient with a complete remission, and well tolerated. The last rituximab injection was performed on the 5th of July 2021, and then she continued to take daily ibrutinib at the same dose. She did not take any other immunosuppressive treatment.
She was admitted to the emergency department on the 21st of December 2021 for diarrhea, nausea and vomiting, lasting for 3 weeks. She presented severe asthenia with clinical signs of extracellular dehydration. She had no fever. Laboratory tests showed a normal electrolyte balance and renal function. C-Reactive Protein (CRP) was 9 mg/L. Hemoglobin level was 11.5 g/dL, platelets 308 G/L, White Blood Cell (WBC) count 11.5 G/L with a high neutrophil count at 8.2 G/L, and no eosinophil excess. An abdominal Computed Tomography (CT) scan was performed, showing uncomplicated colitis. Standard stool culture and search for Clostridium difficile toxins were negative. Levofloxacin was prescribed and ibrutinib was stopped, without any efficacy on diarrhea. Nine days after her admission, she developed abdominal retiform purpura (Figure 1) and dyspnea with desaturation requiring oxygen until 10L/min. Thoracic CT scan showed a diffuse infiltrative pneumonitis with ground-glass opacities and moderate bilateral pleural effusion (Figure 2). SARS-CoV-2 test by polymerase chain reaction was negative on nasopharyngeal swab, urinary antigen testing for Streptococcus pneumoniae and Legionella pneumophila were negative. Biological analysis showed an acute anemia with hemoglobin level at 7.6 g/dL. WBC count was 20 G/L with still a neutrophil excess (16 G/L) and normal eosinophil count (0.06 G/L). Levofloxacin was switched to ceftriaxone, she received 2 packed red blood cells and was transferred to the Intensive Care Unit (ICU).
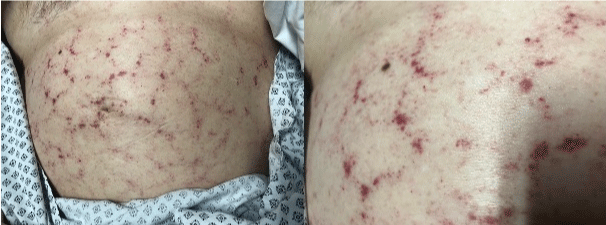
Figure 1: Retiform purpura.
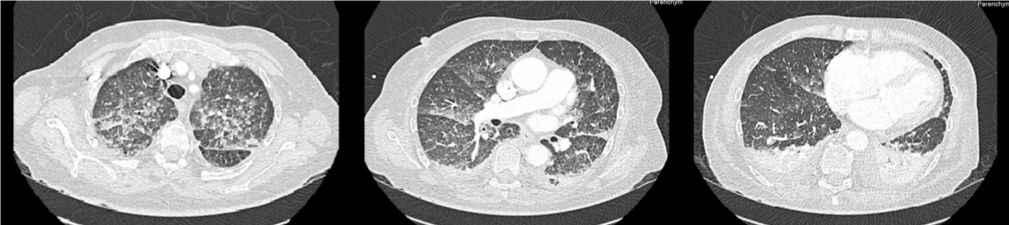
Figure 2: Thoracic CT-scan.
In ICU, her respiratory function slowly improved with decreasing oxygen needs. Four days after ICU admission, WBC count showed hypereosinophilia at 0.77 then 1.04 G/L. Anemia persisted between 8 and 9 g/dL, with signs of hemolysis (haptoglobin < 0.1 g/dL, LDH 436 UI/L, reticulocytes count 206 G/L). Abdominal purpura and diarrhea persisted. She was transferred to the dermatology department. Serological assays for anti-nuclear, Anti-phospholipid, Anti-Neutrophil Cytoplasmic (ANCA), Anti-Cyclic Citrullinated Peptide (CCP) antibodies and rheumatoid factor were all negative. Total hemolytic complement was normal. Protein electrophoresis showed hypoalbuminemia and hypogammaglobulinemia. Regarding the hemolytic anemia: direct antiglobulin test was negative, immunophenotyping of circulating lymphocytes did not detect any clonal population, and searches for paroxysmal nocturnal hemoglobinuria and Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency were negative. Finally, a stool examination was performed on two specimen three days apart using direct examination, concentration with Bailanger, diphasic merthiolate-formaldhyde concentration ad agar-plate culture. Results showed numerous S. stercoralis rhabditiform larvae, filariform larvae, eggs and adults (Figure 3), leading to the diagnosis of disseminated strongyloidiasis. No other parasites have been detected. The serology (Bordier IVD® Strongyloides ratti ELISA assay, Bordier Affinity Products, Switzerland) was negative in relation to the recent administration of rituximab. Patient’s history was re-evaluated and she reported a travel to Madagascar and to the French West Indies 10 and 20 years before, respectively. She was treated with ivermectin 200 μg/kg/day for two days. Digestive and respiratory state rapidly improved, skin lesions disappeared, hemolytic anemia and eosinophilia corrected. Five days after the initial treatment, retiform purpura reappeared with an increasing eosinophil count until 1.43 G/L. A second course of ivermectin was started at the same dose for 10 consecutive days, and all symptoms resolved. The patient was discharged and was seen at the consultation unit one month after treatment. She did not present any digestive, respiratory or skin symptoms. Hemoglobin level was 10.4 g/dL with no hemolysis signs, and eosinophil count was normal.
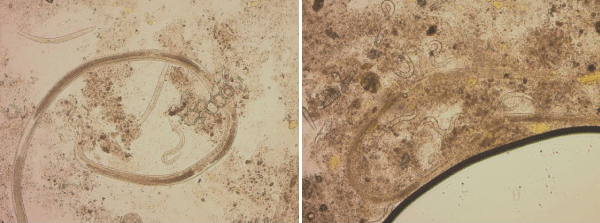
Figure 3: Stool direct examination (x10). Strongyloides stercoralis rhabditiform larvae, and adults.
Discussion
Disseminated strongyloidiasis is a rare and potentially lifethreatening infection, which every hematologist should be aware of. It is well established that patients undergoing long and “high dose” corticosteroids (e.g 10 to 15 mg prednisone-equivalent daily for ≥ 4 weeks) must be screened and treated if they present any risk factor [6], but recommendations regarding other immunosuppressive therapies are less strong, especially for more recent ones such as rituximab and ibrutinib. Cases of S. stercoralis infections in patients treated by rituximab for hematologic malignancies have already been reported [7-9]. However, to our knowledge, this is the first case of disseminated strongyloidiasis associated with ibrutinib. Even if the patient in our case description received both treatments, the first symptoms of the parasitic infection (diarrhea) occurred four months after the last rituximab injection, while she was still taking daily ibrutinib only. We cannot conclude about the accountability of rituximab or ibrutinib alone in this infection, but the combination of both therapies, added to the immunosuppressive state due to the hematologic malignancy by itself, may be considered combined risk factors for severe strongyloidiasis. Regarding ibrutinib, it is interesting to notice that the clinical practice guidelines in Portugal recommend an antiparasitic treatment before ibrutinib introduction, if epidemiological evaluations suggest the risk of strongyloidiasis [10]. This is the only recommendation we could find about the associated risk with a Bruton Tyrosine Kinase (BTK) inhibitor.
Another interesting point in this clinical presentation is the presence of hemolytic anemia. This symptom is very uncommon in disseminated strongyloidiasis, though the association has been suggested in another case-report [11]. More frequently, auto-immune hemolytic anemia is the underlying disease, requiring a treatment by corticosteroids, and disseminated strongyloidiasis appears secondarily, induced by the immunosuppressive therapy [12-13]. However, in our case, hemolytic anemia was not mediated by an autoimmune phenomenon (direct anti-globulin test was negative). The fact that the hemoglobin level returned to normal and the hemolytic markers disappeared with the treatment of strongyloidiasis suggests that hemolysis was induced by the infection itself.
Finally, this case raises the question of patient screening. While some authors plead for serologic screening before the onset of longterm immune suppressive therapies, the present case demonstrates the uselessness of serology afterwards. Indeed, in our patient, serology remained negative despite massive larvae loads. Molecular detection of S. stercoralis in stool is also possible using new marketed assays [14,15], but in the setting of hyperinfection, they have poor added value, as larvae detection using standard methods (Baerman funnel technique or agar-plate cultures) is easy. Whereas hypereosinophilia is inconstant in chronically infected immunocompetent patients [2], any hypereosinophilia associated with atypical skin signs should stress the search for strongyloidiasis.
Conclusion
We herein report the case of a disseminated strongyloidiasis presenting some singularities. Firstly, BTK-inhibitors are yetunreported risk factors for strongyloidiasis, and to our knowledge, this is the first report of such a case. Secondly, she had a hemolytic anemia at the acute stage of the parasitic disease, which completely disappeared after treatment by ivermectin. Screening of patients who lived or have traveled in endemic areas should be standard recommendation, as far as they are immunocompromised.
References
- Greaves D, Coggle S, Pollard C, Aliyu SH, Moore EM. Strongyloides stercoralis infection. BMJ. 2013; 347: f4610–f4610.
- Autier B, Boukthir S, Degeilh B, Belaz S, Dupuis A, et al. Clinical value of serology for the diagnosis of strongyloidiasis in travelers and migrants: A 4-year retrospective study using the Bordier IVD® Strongyloides ratti ELISA assay. Parasite. 2021; 28: 79.
- Buonfrate D, Requena-Mendez A, Angheben A, Muñoz J, Gobbi F, et al. Severe strongyloidiasis: a systematic review of case reports. BMC Infect Dis. 2013; 13: 78.
- Schär F, Trostdorf U, Giardina F, Khieu V, Muth S, et al. Strongyloides stercoralis: Global Distribution and Risk Factors. Brooker S, editor. PLoS Negl Trop Dis. 2013; 7: e2288.
- Muñoz J, Gomez-Junyent J, Bisoffi Z, Buonfrate D, Zammarchi L, et al. Evidence-Based Guidelines for Screening and Management of Strongyloidiasis in Non-Endemic Countries. The American Journal of Tropical Medicine and Hygiene. 2017; 97: 645–52.
- Malpica L, Moll S. Practical approach to monitoring and prevention of infectious complications associated with systemic corticosteroids, antimetabolites, cyclosporine, and cyclophosphamide in nonmalignant hematologic diseases. Hematology. 2020; 2020: 319–27.
- Sahu KK, Mahagaokar K, Patel B, Winokur D, Suzuki S, et al. Strongyloides stercoralis hyperinfection syndrome in mantle cell lymphoma in posttransplant setting. Ann Hematol. 2021; 100: 1089–91.
- Wilkin A, Palavecino E, Guerrero-Wooley R, Aranda-Aguirre E, Li W. Case Report: Strongyloides stercoralis Hyperinfection in a Patient with Chronic Lymphocytic Leukemia. The American Journal of Tropical Medicine and Hygiene. 2017; 97: 1629–31.
- Incani RN, Hernández M, González ME. Hyperinfection by Strongyloides stercoralis probably associated with Rituximab in a patient with mantle cell lymphoma and hyper eosinophilia. Rev Inst Med trop S Paulo. 2010; 52: 221–4.
- Carda JP, Santos L, Mariz JM, Monteiro P, Gonçalves HM, et al. Management of ibrutinib treatment in patients with B-cell malignancies: clinical practice in Portugal and multidisciplinary recommendations. Hematology. 2021; 26: 785–98.
- Ajise AG, Hope AA, Rahman HM. AN Unusual Cause of Refractory Coombs- Positive Autoimmune Hemolytic Anemia (Aiha) and Wheezing. Chest. 2009; 136: 30S.
- Praharaj I, Sujatha S, Ashwini MA, Parija SC. Co-infection with Nocardia asteroides Complex and Strongyloides stercoralis in a Patient with Autoimmune Hemolytic Anemia. Infection. 2014; 42: 211–4.
- Lemos LB, Qu Z, Laucirica R, Fred HL. Hyperinfection syndrome in strongyloidiasis: Report of two cases. Annals of Diagnostic Pathology. 2003; 7: 87–94.
- Autier B, Gangneux JP, Robert-Gangneux F. Evaluation of the AllplexTM GIHelminth (I) Assay, the first marketed multiplex PCR for helminth diagnosis. Parasite. 2021; 28: 33.
- Hartuis S, Lavergne RA, Nourrisson C, Verweij J, Desoubeaux G, et al. The Novodiag® Stool parasites assay, an innovative high-plex technique for fast detection of protozoa, helminths and microsporidia in stool samples: a retrospective and prospective study. Parasite. 2022; 29: 27.