
Review Article
J Hepat Res. 2014;1(3): 1013.
The Contribution of Hepatic NKT Cells to the Pathogenesis of Nonalcoholic Fatty Liver Disease
Tajiri K1* and Shimizu Y2
1Department of Gastroenterology, Toyama University Hospital, Japan
1Gastroenterology Unit, Nanto Municipal Hospital, Japan
*Corresponding author: Tajiri K, Department of Gastroenterology, Toyama University Hospital, 2630 Sugitani, Toyama 930-0194, Japan
Received: July 29, 2014; Accepted: September 13, 2014; Published: September 15, 2014
Abstract
NKT cells are a unique subset of cells, which have both T cell receptors and surface molecules specific to natural killer cells, recognize glycolipid antigens, and have either pro- or anti-inflammatory activity. Recent findings showed that NKT cells play a regulatory role in various diseases involving lipid dysfunction, including Nonalcoholic Fatty Liver Disease (NAFLD), and contribute to the pathogenesis of these diseases. NKT cells are involved in the process of inflammation, due to interactions of CD1d with lipid metabolic and microbiota-derived factors, especially in NAFLD.
Keywords: NKT cell; CD1d; NAFLD, Glycolipid antigen; Gut-microbio
Introduction
Nonalcoholic Fatty Liver Disease (NAFLD) is a leading cause of hepatic dysfunction, leading to cirrhosis and hepatocellular carcinoma [1,2]. Although NAFLD is caused in part by metabolic dysfunction, inflammatory cell infiltration in the livers of patients with NAFLD suggests that immunological mechanisms are also associated with its pathogenesis and progression. However, the contribution of immune responses to the pathogenesis of NAFLD remains unclear. Recently, cells of the innate immune system, especially Natural Killer T (NKT) cells, have been shown to contribute to NAFLD pathogenesis [3-6]. NKT cells recognize glycolipid antigens through CD1d, triggering either pro- or anti-inflammatory activities [7]. The liver contains a large number of NKT cells [8], which are considered a potential participant in metabolic abnormalities [9,10]. This review summarizes and discusses the role of hepatic NKT cells in the pathogenesis of NAFLD, a hepatic manifestation of a systemic metabolic disorder.
Role of Hepatic NKT Cells
The liver contains a large number of cells of the innate immune system, including Kupffer Cells (KCs) and NKT cells [8]. These cells may act to defend against constant exposure to a variety of toxins and antigens secreted by intestinal bacteria [8,11]. NKT cells are a unique subset of cells, which have both T Cell Receptor (TCR) and surface molecules specific to Natural Killer (NK) cells [12]. NKT cells constitute up to 30% of the intrahepatic lymphocytes in mice, and up to 10% in humans [13]. NKT cells can be divided into types 1 and 2, depending on their interactions with CD1d, a non polymorphic glycolipid antigen-presenting molecule structurally related to the class I Major and Histocompatibility Complex (MHC). Type 1 NKT cells, which express an invariant TCR containing Vα14 in mice and Vα24 in humans, recognize glycolipid in conjunction with CD1d, whereas type 2 NKT cells express a diverse repertoire of TCRs [12].
CD1d is a molecule originally identified on thymocytes and antigen presenting cells [14,15]. In normal livers, CD1d is mainly expressed on KCs, but is also expressed on hepatocytes at a very low level. The expression of CD1d molecule on hepatocytes and bile duct epithelium is up regulated in liver diseases, including NAFLD [16,17]. Type 1 NKT cells are lipid antigen-specific lymphocytes that recognize glycolipid antigens presented on CD1d molecules and produce large amounts of T-helper (Th)1 cytokines, including interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α); Th2 cytokines, including Interleukins (IL)-4 and -10; and Th17 cytokines, including IL-17 and IL-22 [7]. Furthermore, type 1 NKT cells can promote fibro genesis involving the Hedgehog (Hh) pathway and cytokines such as Osteopontin (OPN), leading to Hepatic Stellate Cell (HSC) activation [6]. In contrast, type 2 NKT cells, which are more abundant in humans than in mice, are thought to inhibit type 1 NKT cell mediated liver injury [18].
Collectively, hepatic NKT cells have both proinflammatory and anti-inflammatory functions, and play an important regulatory role in the liver.
NKT cell-CD1d interactions in lipid metabolism dysfunction and systemic disorders
The liver has a central role in lipid metabolism, being involved in lipolysis, lipogenesis and fat storage. CD1d deficient mice fed a high-fat or chorine-deficient diet have been shown to develop hepatic steatosis and glucose intolerance, with glucose intolerance mainly induced by decreased hepatic sensitivity to insulin [19]. CD1d deficiency was also shown to aggravate metabolic parameters, such as glucose homeostasis and hepatic lipid metabolism [19]. Furthermore, CD1d deficient mice fed a high-fat diet were more susceptible to weight gain and fatty liver, along with increased adiposity and greater induction of inflammatory genes in the liver [20]. These findings suggest that NKT cells play a protective role in fat storage and onset of inflammation as the first stage of NAFLD. Hepatic NKT cells are rapidly activated by lipids in a CD1d-dependent fashion [21], and dietary fatty acids have been shown to modulate antigen presentation to hepatic NKT cells in a CD1d-dependent manner [22]. CD1d thus can also modulate insulin resistance and play an important role in lipid metabolism, leading to the induction of hepatic inflammation through antigen presentation to NKT cells. In contrast, CD1d function is regulated by Microsomal Triglyceride Transfer Protein (MTP) [23]. MTP deficiency is associated with loss of CD1d function, leading to impaired activation and reduced number and phenotypic alterations of NKT cells [24,25]. MTP is mainly located in the Endoplasmic Reticulum (ER) and plays a central role in transfer of lipids, including phospholipids, triglycerides and cholesterol. In addition, the transmembrane protein ATP-binding cassette transporter G1 has been shown to play a role in the intracellular transport of cholesterol and to regulate NKT cell development and function [26]. Recently, the metabolic regulator Fnip1 was reported crucial for the development of type 1 NKT cells [27]. Fnip1 is an adaptor protein that interacts with AMPK, an energy-sensing kinase that stimulates mitochondrial biogenesis and autophagy in response to low energy conditions [28]. Thus NKT cells are involved in lipid metabolism and energy regulation and may be associated with systemic metabolic abnormalities.
Recent findings have suggested that NKT cells are involved in systemic metabolic disorders. For example, NKT cells were found to be depleted in adipose tissue of obese individuals [29-31], while restoring NKT cells by adoptive transfer improved glucose handling and induced weight loss [31]. These findings suggest that NKT cells protect against diet-induced obesity and glucose intolerance through the regulation of cytokine production [10]. In addition, CD1d restricted NKT cells were shown to exacerbate atherosclerosis through the production of pro-inflammatory cytokines [32,33]. The development and function of NKT cells are thus regulated by various metabolic mechanisms, affecting metabolism itself and inducing various metabolic disorders Figure 1.
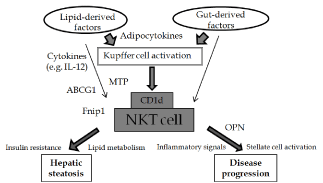
Figure 1: Schematic diagram showing the contribution of intrahepatic NKT cells to the progression of NAFLD. Interactions between NKT cells and CD1d may play important roles throughout NAFLD pathogenesis. ABCG1: ATPBinding Cassette Transporter G1; MTP: Microsomal Triglyceride Transporter Protein; Fnip1: Folliculin-interacting protein 1; OPN: Osteopontin.
Innate immune cells, including NKT cells, can also be activated by microbiota derived antigens through Toll-Like Receptor (TLR) signaling without lipid antigen presentation [34-36]. Recently, however, commensally microbiota was shown to regulate the development and function of CD1d-restricted NKT cells through interactions with lipid antigens [37-39]. Gut microbiota derived lipids and metabolites, as well as cytokines and chemokines secreted in response to microbial recognition, may contribute to systemic NKT cell development [40]. Probiotic antigens may stimulate hepatic NKT cells and restore the number of hepatic NKT cells in mice fed a high-fat diet through interactions between lipid antigens and CD1d, but not through TLR4 signaling [41]. Moreover, NKT cell-mediated inflammation was recently shown to be elaborately regulated by interactions among CD1d, MTP and cytokines [42].
Collectively, NKT cells are regulated, in a complicated manner, by lipid metabolic and gut-microbiota derived factors, resulting in the promotion of or protection against systemic metabolic inflammation.
The contribution of hepatic NKT cells to the progression of NAFLD
The association between NKT cells and NAFLD has been widely analyzed in marine models. Depletion of NKT cells has been reported in ob/ob mice, which are leptin deficient and regarded as a model of obesity-related fatty liver [43,44]. In ob/ob mice, hepatic sensitization toward proinflammatory conditions is induced by endotoxins from the gut, by increased production of adipokines or by ER stress, as seen in human NAFLD [43,45,46]. Increases in adipokines production and ER stress have been found to activate the production of cytokines, especially IL-12, by hepatic KCs, leading to selective depletion of hepatic NKT cells. Hepatic NKT cells were also reported to be decreased in hepatosteatosis through KC- [47] and IL-12- [48] dependent mechanisms. In addition, administration of probiotics has been reported to improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells through reductions in TNF-α production and Nuclear Factor (NF)-κB binding activity [49].
Adoptive transfer of NKT cells or treatment with glycolipid antigens has been shown to reduce hepatic steatosis and improve glucose intolerance in ob/ob mice [50,51]. Moreover, adrenergic activation by nor epinephrine has been reported to induce the expansion of NKT cell populations and improve hepatic steatosis [44]. In wild type mice fed a chorine-deficient or high fat diet, reductions in the numbers of hepatic NKT cells were accompanied by increased Th1 cytokine production [52,53]. In addition, NKT cells were decreased in the livers of patients with relatively mild NAFLD [48]. Collectively, these findings indicate that hepatic NKT cells are preferentially protective during the process of hepatic steatosis through various metabolic factors and cytokines, especially those produced by KCs and associated with gut-derived factors such as endotoxins. However, the role of hepatic NKT cells in the progression of NAFLD has not been clarified, because neither ob/ob mice nor mice fed a high-fat diet develop significant liver fibrosis.
During advanced stages of NAFLD, the number of hepatic NKT cells was increased in the liver. These increases were accompanied by increased activation of the Hh pathway and increased OPN production, leading to the promotion of liver fibrosis through activation of HSCs [4,6]. NKT cells have also been shown to contribute to the pathogenesis of NAFLD in humans. For example, fewer peripheral NKT cells were observed in NAFLD patients than in healthy controls, indicating that peripheral NKT cells were preferentially recruited to the liver [54]. In these patients, the number of NKT cells increased along with the progression of fibrosis. Furthermore, disease progression was accompanied by increased activation of antigen-presenting cells, such as KCs, and increased expression of CD1d [5]. The numbers of intrahepatic NKT cells were increased in the livers of patients with moderate to severe steatosis [55], as well as in the livers of patients with progressive NASH accompanied by Hh pathway activation and OPN production [4,6]. Thus, NKT cells are activated in the livers of patients with NAFLD, at least in those with advanced disease. These cells may contribute to disease progression by interactions with HSCs through the Hh pathway and OPN production.
Results in humans differed somewhat from those in marine models, perhaps due to inter-species differences in adipokines profiles. Serum leptin concentrations have been reported increased in patients with NAFLD [56] but not in mouse models [43]. Moreover, administration of leptin to leptin deficient ob/ob mice has been found to increase the number of NKT cells [43]. Adipokines such as leptin may therefore play a role in regulating the numbers of intrahepatic NKT cells. Alternatively, investigations of simple steatosis are less complete in humans than in mice, because patients with simple steatosis are usually healthy, resulting in a lack of opportunity to analyze the disease. These differences may contribute to discrepancies between mice and humans on the contribution of NKT cells to NAFLD. Furthermore, a recent study in a marine model of NAFLD suggests that T cell I g and mucin domain (Tim)-3/Galectin (Gal)-9 regulates the homeostasis of hepatic NKT cells [57]. Tim-3 positive NKT cells were found to proliferate in the livers of mice fed a high-fat diet, and Gal-9, which is secreted by KCs, was found to induce NKT cell apoptosis [57]. Hepatic NKT cells are thus regulated in various inflammatory conditions. Further investigations are needed to assess the mechanisms underlying the differences in the role of NKT cells in humans and mice.
Collectively, NKT cells are important in lipid metabolic disorders, including in the process of hepatic steatosis and in disease progression in NAFLD, through their interactions with gut-derived factors and HSCs.
Summary
In summary, the numbers of NKT cells in the liver are decreased during early stages of NAFLD by activation of KCs through enhanced production of IL-12. At advanced stages of NAFLD in humans, however, the numbers of NKT cells are increased by up regulation of CD1d expression through increased production of adipokines or gut-derived microbiota. NKT cells may play a protective role during early stage NAFLD (i.e. simple steatosis) by modification of insulin resistance, but act as a progressive factor at an advanced stage (i.e. fibrosis) through increased production of proinflammatory cytokines and OPN and activation of NF-κB activation and Hh, leading to HSC activation. These processes are mainly dependent on interactions of NKT cells with the CD1d molecule in the liver. Changes in the degree or pattern of intrahepatic CD1d expression may therefore influence the numbers or functions of NKT cells.
Conclusion
NKT cells play a regulatory role in the pathogenesis of NAFLD, through interactions with CD1d on antigen-presenting cells. Manipulation of NKT cells may therefore have therapeutic potential.
References
- Younossi ZM, Diehl AM, Ong JP. Nonalcoholic fatty liver disease: an agenda for clinical research. Hepatology. 2002; 35: 746-752.
- Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999; 116: 1413-1419.
- Tajiri K, Shimizu Y. Role of NKT Cells in the Pathogenesis of NAFLD. Int J Hepatol. 2012; 850836.
- Syn WK, Oo YH, Pereira TA, Karaca GF, Jung Y, Omenetti A, et al. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology. 2010; 51: 1998-2007.
- Tajiri K, Shimizu Y, Tsuneyama K, Sugiyama T. Role of liver-infiltrating CD3+CD56+ natural killer T cells in the pathogenesis of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2009; 21: 673-680.
- Syn WK, Agboola KM, Swiderska M, Michelotti GA, Liaskou E, Pang H, et al. NKT-associated hedgehog and osteopontin drive fibrogenesis in non-alcoholic fatty liver disease. Gut. 2012; 61: 1323-1329.
- Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004; 114: 1379-1388.
- Mehal WZ, Azzaroli F, Crispe IN. Immunology of the healthy liver: old questions and new insights. Gastroenterology. 2001; 120: 250-260.
- Huh JY, Park YJ, Ham M, Kim JB. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol Cells. 2014; 37: 365-371.
- Lynch L. Adipose invariant natural killer T cells. Immunology. 2014; 142: 337-346.
- Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003; 3: 51-62.
- Swain MG. Natural killer T cells within the liver: conductors of the hepatic immune orchestra. Dig Dis. 2010; 28: 7-13.
- Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008; 47: 729-736.
- Porcelli SA, Modlin RL. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol. 1999; 17: 297-329.
- Bilsland CA, Milstein C. The identification of the beta 2-microglobulin binding antigen encoded by the human CD1D gene. Eur J Immunol. 1991; 21: 71-78.
- Durante-Mangoni E, Wang R, Shaulov A, He Q, Nasser I, Afdhal N, et al. Hepatic CD1d expression in hepatitis C virus infection and recognition by resident proinflammatory CD1d-reactive T cells. J Immunol. 2004; 173: 2159-2166.
- Tsuneyama K, Yasoshima M, Harada K, Hiramatsu K, Gershwin ME, Nakanuma Y. Increased CD1d expression on small bile duct epithelium and epithelioid granuloma in livers in primary biliary cirrhosis. Hepatology. 1998; 28: 620-623.
- Kumar V. NKT-cell subsets: promoters and protectors in inflammatory liver disease. J Hepatol. 2013; 59: 618-620.
- Kotas ME, Lee HY, Gillum MP, Annicelli C, Guigni BA, Shulman GI, et al. Impact of CD1d deficiency on metabolism. PLoS One. 2011; 6: 25478.
- Martin-Murphy BV, You Q, Wang H, De La Houssaye BA, Reilly TP, Friedman JE, et al. Mice lacking natural killer T cells are more susceptible to metabolic alterations following high fat diet feeding. PLoS One. 2014; 9: 80949.
- Dey N, Szczepanik M, Lau K, Majewska-Szczepanik M, Askenase PW. Stimulatory lipids accumulate in the mouse liver within 30 min of contact sensitization to facilitate the activation of Naïve iNKT cells in a CD1d-dependent fashion. Scand J Immunol. 2011; 74: 52-61.
- Hua J, Ma X, Webb T, Potter JJ, Oelke M, Li Z. Dietary fatty acids modulate antigen presentation to hepatic NKT cells in nonalcoholic fatty liver disease. J Lipid Res. 2010; 51: 1696-1703.
- Brozovic S, Nagaishi T, Yoshida M, Betz S, Salas A, Chen D, et al. CD1d function is regulated by microsomal triglyceride transfer protein. Nat Med. 2004; 10: 535-539.
- Dougan SK, Rava P, Hussain MM, Blumberg RS. MTP regulated by an alternate promoter is essential for NKT cell development. J Exp Med. 2007; 204: 533-545.
- Zeissig S, Dougan SK, Barral DC, Junker Y, Chen Z, Kaser A, et al. Primary deficiency of microsomal triglyceride transfer protein in human abetalipoproteinemia is associated with loss of CD1 function. J Clin Invest. 2010; 120: 2889-2899.
- Sag D, Wingender G, Nowyhed H, Wu R, Gebre AK, Parks JS, et al. ATP-binding cassette transporter G1 intrinsically regulates invariant NKT cell development. J Immunol. 2012; 189: 5129-5138.
- Park H, Tsang M, Iritani BM, Bevan MJ. Metabolic regulator Fnip1 is crucial for iNKT lymphocyte development. Proc Natl Acad Sci USA. 2014; 111: 7066-7071.
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011; 13: 132-141.
- Ji Y, Sun S, Xu A, Bhargava P, Yang L, Lam KS, et al. Activation of natural killer T cells promotes M2 Macrophage polarization in adipose tissue and improves systemic glucose tolerance via interleukin-4 (IL-4)/STAT6 protein signaling axis in obesity. J Biol Chem. 2012; 287: 13561-13571.
- Lynch L, O'Shea D, Winter DC, Geoghegan J, Doherty DG, O'Farrelly C. Invariant NKT cells and CD1d(+) cells amass in human omentum and are depleted in patients with cancer and obesity. Eur J Immunol. 2009; 39: 1893-1901.
- Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012; 37: 574-587.
- Tupin E, Nicoletti A, Elhage R, Rudling M, Ljunggren HG, Hansson GK, et al. CD1d-dependent activation of NKT cells aggravates atherosclerosis. J Exp Med. 2004; 199: 417-422.
- Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol. 2009; 27: 165-197.
- Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003; 4: 1230-1237.
- Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007; 178: 2706-2713.
- Brigl M, Tatituri RV, Watts GF, Bhowruth V, Leadbetter EA, Barton N, et al. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med. 2011; 208: 1163-1177.
- An D, Oh SF, Olszak T, Neves JF, Avci FY, Erturk-Hasdemir D, et al. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell. 2014; 156: 123-133.
- Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012; 336: 489-493.
- Wingender G, Stepniak D, Krebs P, Lin L, McBride S, Wei B, et al. Intestinal microbes affect phenotypes and functions of invariant natural killer T cells in mice. Gastroenterology. 2012; 143: 418-428.
- Zeissig S, Blumberg RS. Commensal microbial regulation of natural killer T cells at the frontiers of the mucosal immune system. FEBS Lett. 2014.
- Liang S, Webb T, Li Z. Probiotic antigens stimulate hepatic natural killer T cells. Immunology. 2014; 141: 203-210.
- Olszak T, Neves JF, Dowds CM, Baker K, Glickman J, Davidson NO, et al. Protective mucosal immunity mediated by epithelial CD1d and IL-10. Nature. 2014; 509: 497-502.
- Li Z, Lin H, Yang S, Diehl AM. Murine leptin deficiency alters Kupffer cell production of cytokines that regulate the innate immune system. Gastroenterology. 2002; 123: 1304-1310.
- Li Z, Oben JA, Yang S, Lin H, Stafford EA, Soloski MJ, et al. Norepinephrine regulates hepatic innate immune system in leptin-deficient mice with nonalcoholic steatohepatitis. Hepatology. 2004; 40: 434-441.
- Guebre-Xabier M, Yang S, Lin HZ, Schwenk R, Krzych U, Diehl AM. Altered hepatic lymphocyte subpopulations in obesity-related murine fatty livers: potential mechanism for sensitization to liver damage. Hepatology. 2000; 31: 633-640.
- Yang L, Jhaveri R, Huang J, Qi Y, Diehl AM. Endoplasmic reticulum stress, hepatocyte CD1d and NKT cell abnormalities in murine fatty livers. Lab Invest. 2007; 87: 927-937.
- Tang T, Sui Y, Lian M, Li Z, Hua J. Pro-inflammatory activated Kupffer cells by lipids induce hepatic NKT cells deficiency through activation-induced cell death. PLoS One. 2013; 8: 81949.
- Kremer M, Thomas E, Milton RJ, Perry AW, van Rooijen N, Wheeler MD, et al. Kupffer cell and interleukin-12-dependent loss of natural killer T cells in hepatosteatosis. Hepatology. 2010; 51: 130-141.
- Ma X, Hua J, Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol. 2008; 49: 821-830.
- Elinav E, Pappo O, Sklair-Levy M, Margalit M, Shibolet O, Gomori M, et al. Adoptive transfer of regulatory NKT lymphocytes ameliorates non-alcoholic steatohepatitis and glucose intolerance in ob/ob mice and is associated with intrahepatic CD8 trapping. J Pathol. 2006; 209: 121-128.
- Margalit M, Shalev Z, Pappo O, Sklair-Levy M, Alper R, Gomori M, et al. Glucocerebroside ameliorates the metabolic syndrome in OB/OB mice. J Pharmacol Exp Ther. 2006; 319: 105-110.
- Kremer M, Hines IN, Milton RJ, Wheeler MD. Favored T helper 1 response in a mouse model of hepatosteatosis is associated with enhanced T cell-mediated hepatitis. Hepatology. 2006; 44: 216-227.
- Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005; 42: 880-885.
- Xu CF, Yu CH, Li YM, Xu L, Du J, Shen Z. Association of the frequency of peripheral natural killer T cells with nonalcoholic fatty liver disease. World J Gastroenterol. 2007; 13: 4504-4508.
- Adler M, Taylor S, Okebugwu K, Yee H, Fielding C, Fielding G, et al. Intrahepatic natural killer T cell populations are increased in human hepatic steatosis. World J Gastroenterol. 2011; 17: 1725-1731.
- Uygun A, Kadayifci A, Yesilova Z, Erdil A, Yaman H, Saka M, et al. Serum leptin levels in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2000; 95: 3584-3589.
- Tang ZH, Liang S, Potter J, Jiang X, Mao HQ, Li Z. Tim-3/galectin-9 regulate the homeostasis of hepatic NKT cells in a murine model of nonalcoholic fatty liver disease. J Immunol. 2013; 190: 1788-1796.