
Research Article
J Immun Res. 2015;2(2): 1017.
CD161-positive CD4 T Cells in Rheumatoid Joint
Chrong-Reen Wang*
Department of Internal Medicine, National Cheng Kung University Medical College, Tainan, Taiwan, Republic of China
*Corresponding author: Chrong-Reen Wang, MD, PhD, Section of Rheumatology and Immunology, National Cheng Kung University Hospital and Department of Internal Medicine, National Cheng Kung University Medical College, No.1 University Road, Tainan 70101, Taiwan, Republic of China
Received: February 11, 2015; Accepted: February 26, 2015; Published: February 27, 2015
Abstract
Background: Activation of T cells participates in the pathogenesis of rheumatoid arthritis (RA) as demonstrated by effective clinical responses to abatacept treatment. Recent studies determine the significance of CD161 expression on CD4+T cells as the effector and regulator in autoimmunity; however, the implication of CD161+CD4+ T lymphocytes in RA remains to be fully defined.
Methods: Expression of CD161 was examined in circulating lymphocytes in 140 RA patients and 140 age-and sex-matched healthy controls, and analyzed in paired peripheral blood and synovial fluid samples from 18 late-stage RA patients with further functional characterization of synovial CD161+CD4+ T cells.
Results: There were no differences in circulating frequencies of CD161+ T cells and expression levels of CD161 in different T cell subsets. Increased CD161 expression levels were found in synovial T cells, especially in the CD4 subset. Functional analysis of synovial CD161+CD4+ T cells revealed significant intracellular IFN-γ expression upon PMA/ionomycin activation and cytotoxicity against target cells with IL-15 stimulation.
Conclusions: Synovial CD161+CD4+ T cells with pro-inflammatory features like IFN-γ production and cytotoxicity ability might participate in the RA pathogenesis
Keywords: CD161; CD4+ T cell; IFN-γ; IL-15; Rheumatoid arthritis; T cell activation
Abbreviations
DMARD: Disease-Modifying Anti-Rheumatic Drug; ESR: Erythrocyte Sedimentation Rate; FITC: Fluorescein Isothiocyanate; IL: Interleukin; IFN: Interferon; mAb: Monoclonal Antibody; MNC: Mononuclear cell; NK: Natural Killer; PB: Peripheral Blood; PE: Phycoerythrin; PMA: Phorbol Myristate Acetate; RA: Rheumatoid Arthritis; SF: Synovial Fluid; TCR: T cell receptor; Th: T helper
Introduction
Rheumatoid arthritis (RA), an autoimmune disease affecting diarthrodial joints and leading to disability, is associated with systemic complications and socioeconomic costs [1]. In rheumatoid joint, abundant T cells accumulate in synovial milieu with activated CD4+ T cells predominant in infiltrating leukocytes [1, 2]. Activation of T cells participates in the RA pathogenesis as demonstrated by effective clinical responses to abatacept treatment, a soluble fusion protein selectively modulating the co-stimulatory signals in activation processes [1, 3]. The NKR-P1 receptor is initially identified as the surface NK1.1 antigen in rodent natural killer (NK) cells and later shown to be expressed in human with CD161 (NKRP1A) expressed by NK cells, both αβ and γ δ Τ cell receptor (TCR)-expressing T cells and NKT cells [4, 5]. Notably, recent studies determine the significance of CD161 expression on CD4+ T cells as the effector and regulator with further analysis of their role in disease states like autoimmunity [6]. The function of CD161+CD4+ T cells in experimental autoimmune encephalomyelitis is considered to be T helper 1 (Th1)-driven, and increased frequencies of CD161+CD4+ T cells have been identified in lesion sites of patients with psoriasis and Crohn’s disease, both belonging to the Th1-related autoimmunity [7-9]. In RA, there are infiltrating CD161+CD4+T cells in synovium, and expression levels of CD161 within CD4+CD25hiCD127loCD45RA- Treg cells are higher in synovial fluid (SF) than peripheral blood (PB) specimen [10, 11]. Furthermore, circulating CD4+CD161+ T cells have been reported to be decreased in newly diagnosed RA patients, and there are increased frequencies of CD4+CD161+ T cells with the immune phenotype skewing toward Th1 cells in SF from late-stage rheumatoid joints [12]. Nevertheless, the implication of CD161+CD4+ T lymphocytes in rheumatoid joint is yet to be fully defined, especially in the functional characterization.
In this study, the expression levels of CD161 were examined in circulating lymphocytes in a considerable number of subjects, 140 RA patients and 140 age- and sex-matched healthy individuals, and analyzed in paired PB and SF samples from 18 patients with late-stage rheumatoid joints. Although there were no differences in circulating frequencies of CD161+ T cells and expression levels of CD161 in different T cell subsets, increased CD161 expression levels were found in synovial T cells, especially in the CD4 subset. Further characterization of synovial CD161+CD4+ T cells revealed significant intracellular IFN-γ expression upon PMA/ionomycin activation and cytotoxicity against target cells with IL-15 stimulation.
Material and Methods
Patients and samples
One hundred and forty RA patients (125 female and 15 male, ages 24 to 76, mean 53.7 ± 12.9) with 75.7% seropositivity were recruited into this study [13]. All of these patients were under the treatment with disease-modifying anti-rheumatic drugs (DMARDs). Their erythrocyte sedimentation rate (ESR) varied from 1 to 108 with mean 25.94 ± 17.47 mm/hr during the collection of PB samples (abnormal values in 56.4% patients; local normal references, no more than 15 mm/hr for male and no more than 20 mm/hr for female). Another 140 age-and sex-matched healthy individuals were enrolled as a control group. Venous blood samples were drawn from patients and controls. Furthermore, SF and PB specimens were simultaneously obtained from 18 patients with late-stage rheumatoid joints (15 female, 3 male, ages 45 to 76, mean 60.4 ± 10.4). The Institutional Review Board of National Cheng Kung University Hospital approved this study.
Preparation of mononuclear cells (MNCs) and flow cytometry analysis
MNCs were purified from heparin-anticoagulated venous blood and joint fluid samples by Histopaque (Sigma-Aldrich, St. Louis, MO) gradient centrifugation as described previously [2]. Double fluorescence staining was used for the surface phenotype analysis, and monoclonal antibodies (mAbs) against human antigens CD3, CD4, CD8 and CD161 (clone DX12) were purchased from BD Biosciences (San Jose, CA). Purified MNCs were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD3, anti-CD4 or anti-CD8 and phycoerythrin (PE)-conjugated anti-CD161. These cells were incubated with mAbs for 30 mins on ice in the dark. After washing, the stained cells were analyzed with a FACS Calibur flow cytometer with the Cell Quest software program (BD Biosciences). The control isotype mAb (BD Biosciences) staining was included in each sample. Calculated frequencies of CD161+ T cells were expressed by the percentages of CD161 and CD3 double-positive cells in total lymphocytes, andCD161 expression levels in different T cell subsets were expressed by the percentages of total CD3+, CD4+ or CD8+ T lymphocytes.
Intracellular cytokine staining
The MNCs from joint fluid were stimulated with PMA (25 ng/ ml) plus ionomycin (500 ng/ml) for 4 hr with the addition of 2 μM monesin (GolgiStop, BD Biosciences) in the culture, and stained by Cy-Chrome-conjugated anti-CD4 and FITC-conjugated anti- CD161 after harvest as described previously [14, 15]. After fixation with 1% paraformaldehyde (Sigma-Aldrich), these cells were stained with PE-conjugated anti-IL-4 (BD Biosciences) or anti-IFN-γ (BD Biosciences) in staining buffer containing 0.1% saponin (Sigma- Aldrich), and were further subjected to flow cytometry analysis. The control isotype mAb (BD Biosciences) staining was also included in each sample.
Purification of CD161+CD4+ T cells
The MNCs from SF samples were first stained with FITCconjugated anti-human CD4. After washing, these cells were incubated with magnetic particles coated anti-FITC MicroBeads (Miltenyi Biotec, Bergish Gladbach, Germany) and then passed through a positive selection column in the Magnetic Cell Sorter (Miltenyi Biotec) as described previously [2]. The column was removed from the separator and the positive fraction of CD4+ T cells was flushed out. The MicroBeads were released by incubation with release reagents, and these cells were further stained with PEconjugated The MNCs from SF samples were first stained with FITCconjugated anti-human CD4. After washing, these cells were incubated with magnetic particles coated anti-FITC MicroBeads (Miltenyi Biotec, Bergish Gladbach, Germany) and then passed through a positive selection column in the Magnetic Cell Sorter (Miltenyi Biotec) as described previously [2]. The column was removed from the separator and the positive fraction of CD4+ T cells was flushed out. The MicroBeads were released by incubation with release reagents, and these cells were further stained with PEconjugated anti-human CD161. After washing, they were incubated with magnetic particles coated with anti-PE MicroBeads (Miltenyi Biotec) and further passed through a positive selection column in the Magnetic Cell Sorter. Finally, the CD161+CD4+ T cells were obtained by flushing the positive selection column removed from the separator and incubating with release reagents.
In vitro stimulation with IL-15
Purified CD161+CD4+T cells were cultured in the presence of 100 ng/ml recombinant human IL-15 (R&D systems, Minneapolis, MN) or medium alone as the control for 18 hr in 96-well flat-bottom plates [16], and these cells were harvested for the further tests in cytotoxicity against target cells.
Cytotoxicity assay
One hundred thousand target K562 cells (NHRI cell bank, Miaoli, Taiwan) were incubated in 48-well flat-bottom plates with the addition of IL-15-activated or medium alone-treated effector CD161+CD4+ T cells at 1, 3 or 10 times numbers of the target cells. Negative controls were defined as target cells only without the addition of effector cells in the culture. After the incubation for 4 hr at 370C, cells were harvested and further subjected to flow cytometry analysis. Propidium iodide (Sigma-Aldrich) was added in a final concentration of 2 mg/ml just before the analysis. Specific killing was calculated as percentages of dead cells in the negative control subtracted from percentages of dead cells in the sample [17].
Statistical analysis
Data in this study were presented as mean ± standard deviation. The statistical analyses were carried out by using non-parametric tests; including the Mann-Whitney rank sum test for comparisons between RA patients and healthy controls, and the Wilcoxon signed ranks test for comparisons between different groups of RA patients. Pearson correlation coefficient with linear regression analysis was performed to correlate between ESR values or age and CD161 frequencies in PB samples from RA patients. Differences between cytotoxicity in IL- 15- and medium alone-stimulated effector against different ratios of target cells were compared by repeated-measures analysis of variance. A P-value less than 0.05 were considered to be statistically significant in this study.
Results
No differences in circulating CD161+ T cell frequencies and CD161 expression levels in different T cell subsets in RA patients
At first, frequencies of circulating CD161+ T cells in total lymphocytes were examined, and there were no differences in frequencies of CD161+ T cells between RA patients and healthy controls (Figure 1A, patients versus control, 12.92 ± 4.78 % versus 13.37 ± 4.76 %, P = 0.3245). In RA patients, the frequencies of these cells were marginally correlated with ESR values (Figure 1B, γ = 0.1721, P =0.0421), and no correlation was found between CD161+ T cell frequencies and age ( γ = 0.0067, P = 0.9373). There were no differences in CD161+T cell frequencies regarding the seropositivity in RA patients (seropositive versus seronegative, 12.91 ± 4.45% versus 12.94 ± 4.28%, P = 0.9808). Next, CD161 expression levels were checked in CD3, CD4 and CD8 T cell subsets. No differences were found in expression levels in different subsets (patients versus control, 20.18 ± 6.78 % versus 21.14 ± 6.88 % for CD3, P = 0.3484; 20.80 ± 7.45 % versus 19.98 ± 7.47 % for CD4, P = 0.3216; 21.02 ± 10.37 %versus 22.39 ± 9.04 % for CD8, P = 0.2956), and further analyses regarding the ESR value and RA seropositivity also failed to reveal any correlation and differences, respectively (ESR, for CD3, P = 0.4614, for CD4, P = 0.6891, for CD8, P = 0.9465; seropositive versus seronegative, for CD3, P = 0.4614, for CD4, P= 0.7373, for CD8, P = 0.9597).
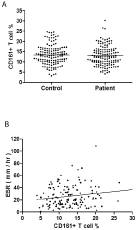
Figure 1: Circulating CD161+ T cells in RA patients and healthy controls.
A. No differences in frequencies of CD161 and CD3 double-positive cells
in total lymphocytes from peripheral blood samples of 140 RA patients and
140 healthy controls (P = 0.3245). B. Correlation found between ESR values
and CD161+ T cell frequencies in 140 RA patients (γ = 0.1721, P = 0.0421).
Increased CD161 expression levels in synovial CD4+ T lymphocytes
The CD161 expression levels in CD3, CD4 and CD8 T cell subsets were further compared between PB and SF samples (Figure 2). In SF samples, there were significantly increased expression levels of CD161 in CD3 and CD4 T cell subsets (SF versus PB, 24.43 ± 7.54 % versus 19.40 ± 7.33 % for CD3, P = 0.0187; 31.95 ± 9.70 % versus 16.30 ±92 % for CD4, P = 0.0015), whereas significantly decreased expression levels of CD161 in CD8 subset were found (SF versus PB, 11.06 ± 5.19 % versus 22.31 ± 10.02 %, P = 0.0011). In Figure 3A, there is a representative figure with CD161 expression on CD4+T cells in PB and SF samples from a RA patient.
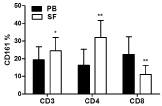
Figure 2: Expression levels of CD161 in different T cell subsets in paired
peripheral blood (PB) and synovial fluid (SF) samples from 18 RA patients (*P
= 0,0187 for CD3; **P = 0,0015 for CD4, P = 0,0011 for CD8).
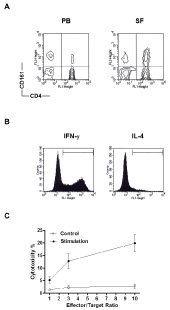
Figure 3: Functional characterization of synovial CD161+CD4+ T cells. A.
A representative figure with CD161 expression (the vertical axis) on CD4
cells (the horizontal axis) in paired peripheral blood (PB) and synovial fluid
(SF) samples from a RA patient. B. A representative intracellular IFN-γ and
IL-4 staining graph from a RA patient. The area limited by each horizontal
bar is defined by the positive staining with an isotype-matched mAb as the
negative control in each sample. C. The cytotoxicity in IL-15- or medium
alone-stimulated synovial CD161+CD4+ effector cells against K562target
cells in different effector/target cells ratios (cytotoxicity, IL-15 versus medium
alone, P = 0.0028).
Functional characterization of synovial CD161+CD4+ T cells
Since there was a significant expansion in the CD161 portion of synovial CD4+ T cells, the functional characterization of this population was carried out firstly by examining the intracellular cytokine expression pattern. Synovial MNCs from 5 patients were stimulated with PMA/ionomycin and further stained with IL-4 and IFN-γ. There were high expression levels of IFN-γ but only marginal IL-4 levels in the CD161+CD4+ portion (a representative figure in Figure 3B; expression percentages, 52.92 ± 6.0 % for IFN-γ and 2.66 ± 1.45 % for IL-4). These cells were further purified from joint fluid of 3 RA patients by Magnetic Cell Sorter. Although no obvious cytotoxicity was observed in the co-culture of target K562 cells with medium alone-treated control effector cells, the significant cytolytic effects were observed in IL-15-stimulated effector cells against target cells (Figure 3C, IL-15 versus medium alone, 5.19 ± 1.35 % versus 1.34 ± 0.35 % for E:T = 1, 12.74 ± 3.00 % versus 2.33 ± 0.69 % for E:T = 3, 19.88 ± 3.37 % versus 2.80 ± 0.80 % for E:T = 10, P = 0.0028).
Discussion
In addition to be expressed in human T cells, CD161 is expressed in a majority of NK cells, innate lymphoid cells critical for host defense against pathogens and antitumor immunity [6, 18]. IL-2 and IL-15 are well-known cytokine activators on NK cells with Jak/STAT, PI3K and MAPK signaling pathways downstream of the IL-2/IL-15 receptor, contributing to the enhancement of their functional effects like cytokine production, proliferation, survival and cytotoxicity [18-20]. In rheumatoid joint, despite a much lower IL-2 concentrations, IL-15 is routinely found with considerable amounts, and involved in the activation processes of CD4+ T cell such as the induction of CD40 ligand expression [2]. In NK cells, since CD161 participates in triggering their cytotoxicity and IL-15 enhances the cytotoxic process, it is reasonable to speculate that in vitro addition of IL-15 can induce the cytolytic activity of synovial CD161+CD4+ T lymphocytes [19, 21]. Indeed, in this study, in vitro stimulation with IL-15 could significantly induce the cytotoxicity of synovial CD161+CD4+ effector T cells. However, there was no increase in CD161 expression levels on synovial T cells under such a stimulation (data not shown), suggesting that the CD161 molecule is not simply an activation marker. Notably, there are elevated circulating frequencies of CD4+CD28 null T cells in RA patients, and their expansion is correlated with extra-articular manifestations [10, 22]. This T cell population, expressed with CD161, has pro-inflammatory features like IFN-γ production and cytotoxicity capacity, involving in the RA pathogenesis [23]. Interestingly, NKT cells are a specialized T cell subset using monospecific TCR to recognize lipids presented by CD1d, and these cells, with rapid production of cytokines like IFN-γ and IL-4 upon activation, have either protective or harmful roles in autoimmune disorders [24]. In circulating healthy human T lymphocytes, as CD161 is expressed in 21.14 ± 6.88 % by this study but NKT cells compose less than 1 % in a previous report [25], the major portion of CD161+ T cells should be a distinct lineage. Furthermore, in this study, significant IFN-γ but only marginal IL-4 intracellular concentrations (52.92 ± 6.0 % for IFN-γ and 2.66 ± 1.45 % for IL- 4) were demonstrated in activated synovial CD161+CD4+T cells, conflicting with the capable IL-4 production ability in NKT cells.
In particular, the Th1-like cells, either a CD4+ or CD4- phenotype and with NK1.1 surface antigen in some inbred mouse strains, can produce significant amounts of IFN-γ and constitute the majority of NKT cells [5, 24]. In my previous study, by induction of collageninduced arthritis (CIA) in the C57BL/6 strain of mice bearing the NK1.1 marker, there were increased frequencies of NK1.1+ T lymphocytes identified in synovial MNCs from severe inflamed joints; however, the intraperitoneal administration of anti-mouse NK1.1 mAb (clone PK136) to deplete NK1.1+ cells before priming with the type II collagen failed to ameliorate the arthritis scores in these mice [26, 27]. Nevertheless, selective in vivo removal of NK1.1+CD4+ T cells instead of depleting other NK1.1-positive cell populations like NK and NK1.1+CD4- T cells would be a better approach in examining the pathogenic role of this T cell subset in rheumatoid joint.
In addition to compare the phenotype and function between CD161+ and CD28 null subsets in circulating and peripheral CD4+ T cells from RA patients, further efforts are required to fully characterize the CD161+ T cells in rheumatoid joint. Since IL-17-secreting CD4+ T cells, a Th subset involved in the RA pathogenesis, belong to the CD161-positive fraction, this molecule is considered recently to be a hallmark of human Th17 cells [6, 28, 29]. Besides, in the adult circulation, CD161 expression is associated with a memory phenotype in CD4+ T cells with a significant portion of these cells displaying the CD62L+CCR7+ central memory phenotype [6, 30]. However, in this study, further examinations on the IL-17 producing capacity and memory phenotype were lacking in synovial CD161+ T cells. Besides, discrepancies were found in CD161 expression levels in circulating CD4+ T lymphocytes from RA patients [10, 12]. As compared with healthy individuals, CD161 expression levels in CD4+ T cells were higher in 36 seropositive patients with unknown therapeutic status in one study, whereas frequencies of CD161 cells within the CD4 subset were decreased in 35 patients without therapy from another report. Nevertheless, by recruiting 140 RA patients under DMARDs therapy into this study, there were no differences in the expression levels of CD161 in circulating CD4+T cells irrespective of their seropositivity. Notably, the number of CD161+CD4+ T cells has been reported to be correlated inversely with CRP levels in treatment-naïve patients from a previous report, while the values of ESR, an inflammation indicator monitoring RA, were found to be marginally in parallel with the circulating frequencies of CD161+ T cells in treated patients from the present study [12, 31]. Since normalization of decreased peripheral CD161+CD4+ T lymphocytes has been identified in RA patients after the regular treatment with methotrexate, raising the possibility that the underlying therapeutic modality has influence on the expression levels of CD161 [12]. Future work to analyze the changes in expression levels of CD161 in synovial T cells before and after the DMARDs treatment, biologics in particular, would contribute to the elucidation of pathogenic mechanisms regarding the activation of T cells in RA.
In conclusion, in rheumatoid joint, higher expression levels of CD161 in synovial T cells were found with a significant expansion in the CD4 subset. Synovial CD161+CD4+ T cells with proinflammatory features like IFN-γ production and cytotoxicity ability might participate in the RA pathogenesis.
Acknowledgement
This work was supported by grants NSC89-2314-B-006-142, 90- 2314-B-006-094, 91-2314-B-006-019, and MOST 103-2314-B-006- 058-MY3 from Ministry of Science and Technology, Taiwan, Republic of China. I thank Ms. Chiung-Ru Wu and Dr. Ming-Fei Liu, National Cheng Kung University Hospital Internal Medicine Department, for technical assistances and specimen collection, respectively. There is no conflict of interest in this study.
References
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. See comment in PubMed Commons below N Engl J Med. 2011; 365: 2205-2219.
- Wang CR, Liu MF. Regulation of CCR5 expression and MIP-1alpha production in CD4+ T cells from patients with rheumatoid arthritis. See comment in PubMed Commons below Clin Exp Immunol. 2003; 132: 371-378.
- Caporali R, Bugatti S, Cavagna L, Antivalle M, Sarzi-Puttini P. Modulating the co-stimulatory signal for T cell activation in rheumatoid arthritis: could it be the first step of the treatment? See comment in PubMed Commons below Autoimmun Rev. 2014; 13: 49-53.
- Kirkham CL, Carlyle JR. Complexity and Diversity of the NKR-P1:Clr (Klrb1:Clec2) Recognition Systems. See comment in PubMed Commons below Front Immunol. 2014; 5: 214.
- Tajiri K. CD1d-restricted natural killer T cells in metabolic disorders. J Immun Res 2014; 1: 4.
- Fergusson JR, Fleming VM, Klenerman P. CD161-expressing human T cells. See comment in PubMed Commons below Front Immunol. 2011; 2: 36.
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. See comment in PubMed Commons below Nature. 2003; 421: 744-748.
- Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. See comment in PubMed Commons below J Exp Med. 2008; 205: 1903-1916.
- Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. See comment in PubMed Commons below J Exp Med. 2009; 206: 525-534.
- Warrington KJ, Takemura S, Goronzy JJ, Weyand CM. CD4+,CD28- T cells in rheumatoid arthritis patients combine features of the innate and adaptive immune systems. See comment in PubMed Commons below Arthritis Rheum. 2001; 44: 13-20.
- Afzali B, Mitchell PJ, Edozie FC, Povoleri GA, Dowson SE, Demandt L, et al. CD161 expression characterizes a subpopulation of human regulatory T cells that produces IL-17 in a STAT3-dependent manner. Eur J Immunol 2013; 43: 2043-2054.
- Chalan P, Kroesen BJ, van der Geest KS, Huitema MG, Abdulahad WH, Bijzet J. Circulating CD4+CD161+ T lymphocytes are increased in seropositive arthralgia patients but decreased in patients with newly diagnosed rheumatoid arthritis. PLoS One 2013; 8: e79370.
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62: 2569-2581.
- Liu MF, Wang CR. Increased Th17 cells in flow cytometer-sorted CD45RO-positive memory CD4 T cells from patients with systemic lupus erythematosus. See comment in PubMed Commons below Lupus Sci Med. 2014; 1: e000062.
- Bessler H, Bergman M, Sirota L, Salman H, Djaldetti M. Lipofundin affects cytokine release by peripheral blood mononuclear cells from healthy individuals. J Immun Res 2014; 1: 1013.
- Robertson MJ, Soiffer RJ, Wolf SF, Manley TJ, Donahue C, Young D, et al. Response of human natural killer (NK) cells to NK cell stimulatory factor (NKSF): cytolytic activity and proliferation of NK cells are differentially regulated by NKSF. See comment in PubMed Commons below J Exp Med. 1992; 175: 779-788.
- Oumouna M, Jaso-Friedmann L, Evans DL. Flow cytometry-based assay for determination of teleost cytotoxic cell lysis of target cells. See comment in PubMed Commons below Cytometry. 2001; 45: 259-266.
- Yokoyama WM. Natural killer cell immune responses. See comment in PubMed Commons below Immunol Res. 2005; 32: 317-325.
- Romee R, Leong JW, Fehniger TA. Utilizing cytokines to function-enable human NK cells for the immunotherapy of cancer. See comment in PubMed Commons below Scientifica (Cairo). 2014; 2014: 205796.
- Mishra A, Sullivan L, Caligiuri MA. Molecular pathways: interleukin-15 signaling in health and in cancer. See comment in PubMed Commons below Clin Cancer Res. 2014; 20: 2044-2050.
- Lanier LL, Chang C, Phillips JH . Human NKR-P1A. A disulfide-linked homodimer of the C-type lectin superfamily expressed by a subset of NK and T lymphocytes. See comment in PubMed Commons below J Immunol. 1994; 153: 2417-2428.
- Namekawa T, Wagner UG, Goronzy JJ, Weyand CM. Functional subsets of CD4 T cells in rheumatoid synovitis. See comment in PubMed Commons below Arthritis Rheum. 1998; 41: 2108-2116.
- Pieper J, Johansson S, Snir O, Linton L, Rieck M, Buckner JH, et al. Peripheral and site-specific CD4(+) CD28(null) T cells from rheumatoid arthritis patients show distinct characteristics. See comment in PubMed Commons below Scand J Immunol. 2014; 79: 149-155.
- Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. See comment in PubMed Commons below Nat Rev Immunol. 2013; 13: 101-117.
- Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. See comment in PubMed Commons below J Exp Med. 2002; 195: 625-636.
- Wang CR. Involvement of natural killer T cells in C57BL/6 mouse model of collagen-induced arthritis. See comment in PubMed Commons below J Microbiol Immunol Infect. 2003; 36: 15-20.
- Inglis JJ, Criado G, Medghalchi M, Andrews M, Sandison A, Feldmann M, et al. Collagen-induced arthritis in C57BL/6 mice is associated with a robust and sustained T-cell response to type II collagen. See comment in PubMed Commons below Arthritis Res Ther. 2007; 9: R113.
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. See comment in PubMed Commons below Cell. 2006; 126: 1121-1133.
- Hoe E, Toh ZQ, Marimla R, Balloch A and Licciardi PV. Friend or Foe? – The Role of TH17 Immunity inHost Protection. J Immun Res 2015; 2: 1015.
- Takahashi T, Dejbakhsh-Jones S, Strober S. Expression of CD161 (NKR-P1A) defines subsets of human CD4 and CD8 T cells with different functional activities. See comment in PubMed Commons below J Immunol. 2006; 176: 211-216.
- Abelson B, Sokka T, Pincus T. Declines in erythrocyte sedimentation rates in patients with rheumatoid arthritis over the second half of the 20th century. J Rheumatol 2009; 36: 1596-1599.