
Research Article
J Immun Res. 2021; 7(1): 1039.
The Pivotal Role of Signal Regulatory Protein α in Exacerbating Pulmonary Fibrosis Complicated with Bacterial Infection
Yamaguchi R¹, Sakamoto A¹, Yamaguchi R², Haraguchi M¹, Narahara S¹, Sugiuchi H¹ and Yamaguchi Y¹*
¹Graduate School of Medical Science, Kumamoto Health Science University, Kumamoto, Japan
²Department of Neuroscience, Kyoto University, Graduate School of Medicine and Faculty of Medicine, Kyoto, Japan
*Corresponding author: Yasuo Yamaguchi, Graduate school of Medical Science, Kumamoto Health Science University, Kitaku Izumi-machi 325, Kumamoto 861-5598, Japan
Received: April 06, 2021; Accepted: May 04, 2021; Published: May 11, 2021
Abstract
The pathogenesis of pulmonary fibrosis remains unknown. However, bacterial infections in patients with idiopathic pulmonary fibrosis are a serious complication that exacerbate the disease. Serum levels of Surfactant Protein D (SPD) are known to be elevated in patients with pulmonary fibrosis, but the role of SPD in pulmonary fibrosis complicated with bacterial infection is unknown. Lipopolysaccharide upregulates Interleukin (IL)-12p40 expression and IL-12p40 promotes Interferon Gamma (IFNγ) production to induce the T helper cell 1 (Th1) immune response via Signal Transducers and Activators of Transcription 4 (STAT4) signaling. A lack of IFNγ shifts the immune response from Th1 to Th2. IL-4 is a profibrotic Th2 cytokine that activates fibroblasts.
Granulocyte-macrophage colony-stimulating factor induced by IL-1 and TNFα during the Th1 immune response upregulates Signal Regulatory Protein α (SIRPα) expression. Interferon Regulatory Factor 1 (IRF1) functions as the promoter activator of IL-12p40 after stimulation with LPS. SPD is a ligand for SIRPα, and SPD/SIRPα ligation activates the Mitogen-Activated Protein Kinase (MAPK)/Extracellular Signal-Related Kinase (ERK) signal cascade; ERK downregulates Interferon Regulatory Factor 1 (IRF1) expression.
Consequently, the SPD/SIRPα signaling pathway decreases IL-12p40 production in human macrophages after exposure to LPS. IL-12p40 is a key immunoregulatory factor in bacterial infection that promotes production of IFNγ by T lymphocytes. Pulmonary fibroblasts are activated by IL-4/IL-4R ligation. IFNγ induces IRF1 via STAT1 signaling, and IRF1 acts as the promoter repressor of IL-4 to attenuate its production. IFNγ also inhibits IL-4R expression. A reduction in IFNγ induced by IL-12p40 deficiency via the SPD/SIRPα signaling pathway enhances IL-4 and IL-4R expression to augment the activity of fibroblasts. This finding indicates that pulmonary fibrosis is exacerbated by SPD/SIRPα signaling during bacterial infection.
Keywords: Signal regulatory protein α; Surfactant protein D; ERK; ROCK; IL-12p40
Abbreviations
CD: Cluster of Differentiation; EGFR: Epidermal Growth Factor Receptor; ERK: Extracellular Signal-Regulated Kinase; GAPDH: Glyceraldehyde 3-Phosphate Dehydrogenase; GM-CSF: Granulocyte- Macrophage Colony-Stimulating Factor; LPS: Lipopolysaccharide; IRF: Interferon Regulatory Factor; Rho: Ras Homolog Gene Family; ROCK: Rho-Associated Coiled-Coil Forming Kinase; siRNA: small interfering RNA; SHP: Src Homology 2-Containing Phosphotyrosine Phosphatase; SIRPα: Signal Regulatory Protein α; SPD: Surfactant Protein D; TLR: Toll-Like Receptor
Introduction
The etiology of pulmonary fibrosis, a multifactorial disease, is unknown. However, viral and bacterial infections may influence disease initiation, exacerbation, and outcome, and bacteria are known to trigger the progression and acute exacerbation of pulmonary fibrosis [1-4]. Innate immunity triggers pulmonary fibrosis via activation of fibroblasts by Toll-Like Receptor 4 (TLR4) [5,6]. Unclear is how fibroblasts and macrophages interact in the innate immune response and facilitate pulmonary fibrosis [7].
Serum levels of Surfactant Protein D (SPD) were reported to be a biomarker of Idiopathic Pulmonary Fibrosis (IPD) because SPD levels are significantly higher in patients with IPD than in controls [8]. Furthermore, patients with high levels of SPD have shorter survival times than those with lower levels [9]. This finding led us to the question whether SPD might play a role in exacerbating pulmonary fibrosis complicated with bacterial infection.
The alveolar cells are composed of alveolar type I and type II cells; the latter type secrete SPD, which is involved in maintaining surface tension of the pulmonary alveolus.
Pulmonary fibrosis elevates levels of SPD by destroying the alveolar basement membrane [10] and causes hyperplasia of alveolar type II cells [11]. However, the question why the severity of pulmonary fibrosis is associated with elevated SPD levels remains unanswered, and the relation between SPD and fibroblast activation is largely unknown.
SPD binds to Signal Regulatory Protein α (SIRPα), a transmembrane protein in macrophages [12]. SIRPα expression is enhanced in Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF)-dependent M1 macrophage [13]. A proinflammatory cytokine such as IL-1 or TNFα induces pathological inflammation during bacterial infection [14] and promotes GM-CSF production [15]. Cluster of differentiation 47 (CD47) is a ligand for SIRPα [16] and is an anti-phagocytic surface marker on blood cells [17]. Indeed, CD47/SIRPα ligation signals, “do not eat me” to phagocytic macrophages [18]. However, the SPD/SIRPα signaling pathway may play other roles in pulmonary fibrosis. In this review, we focus on the molecular function and role of SIRPα in macrophages in pulmonary fibrosis complicated with bacterial infection.
Pulmonary fibrosis is closely associated with fibroblast activation and excess accumulation of extracellular matrix. Studies reported that IL-12p40 levels were elevated in patients with idiopathic pulmonary fibrosis compared with controls [19]. However, the pivotal role of IL-12p40 in the exacerbation of pulmonary fibrosis remains unclear. IL-4 is known to be a fibrotic Th2 cytokine that induces fibroblast activation and proliferation [20], and IL-4 signaling is required for binding to the IL-4 receptor α (IL-4Rα) [21], which is expressed on human fibroblasts [22]. Fibroblasts are activated by IL-4/IL-4Rα via the signal transducers and activators of the transcription 6 (STAT6) signaling pathway [23,24]. IL-4-mediated profibrotic function is influenced by IFNγ, which inhibits IL-4 [25] and IL-4Rα expression [26] to attenuate collagen generation by fibroblasts [27], exerting an anti-fibrotic effect. In contrast, low levels of IFNγ facilitate pulmonary fibrosis [28]. IL-12p40 promotes IFNγ production by T lymphocytes [29], so a lack of IL-12p40 inhibits proliferation of CD4+ T cells and enhances Th2 cytokine responses [30,31]. Therefore, we also review the role of the IL-12p40/IFNγ axis in exacerbation of pulmonary fibrosis complicated with bacterial infection.
Materials and Methods
Ethics statement
The Board of Ethics in Kumamoto Health Science University approved to obtain blood from volunteers in conformity with the declaration of Helsinki after obtaining their informed consent (No. 17046).
Chemicals and reagents
Human recombinant GM-CSF was obtained from Tocris Bioscience, Bristol, UK. Recombinant human surfactant protein D (R&D Systems, Minneapolis, MN), SB203580 (Wako, Kanagawa, Japan), PD98059 (Wako), BIRB796 (Axon Medchem, Groningen, Netherlands), PDTC (BioVision, Mountain View, CA), TMB-8 (Sigma-Aldrich, Ontario, Canada) and Y-27632 (Wako, Osaka, Japan) were obtained to investigate the intracellular signaling pathways involved in SIRPα or IL-12p40 production. Escherichia coli 0111:B4 Lipopolysaccharide (LPS) was purchased from
Sigma-Aldrich (St. Louis, MO).
Induction of GM-CSF-dependent human macrophages
Peripheral Blood Mononuclear Cells (PBMCs) was obtained from heparinized blood samples. PBMCs collected using Lymphoprep gradients (Axis-Shield PoC As, Norway) were suspended with Lymphocyte medium for thawing (BBLYMPH1, Zen-Bio, Inc. Research Triangle Park, NC). The monocytes were stained with CD14-phycoerythrin (PE) mouse anti-human monoclonal antibody (Life technologies, Staley Road Grand Island, NY). The purity of monocytes was determined by Fluorescence Activated Cell Sorting (FACS), showing 87.4 + 1.5 % (mean + SE, n=120, 86.3-89.9). GM-CSF dependent macrophages were obtained after monocytes stimulated with recombinant human GM-CSF on days 1, 3, and 6 of culture. Macrophages (on day 9 of culture) were utilized as GM-CSF dependent macrophages in this study.
Preparation of whole-cell lysates from cell culture
Human macrophages (on day 9 of culture) were stimulated with HNE (5μM) or SP (5μM) for 6 hours and culture medium was carefully removed. Mammalian protein extraction reagent (100μL; M-PER, Thermo Fisher Scientific Inc., Waltham, MA) was pipetted into each well, after which the culture plate was gently shaken for 5 minutes.
The lysate was collected and transferred to a microcentrifuge tube for centrifugation at 12,000g for 10 minutes. The supernatants were used as a whole-cell lysates in this study.
ELISA for IL-12p40
Macrophages were pretreated with SPD (5μM) and stimulated by LPS (10ng) for 6 hours. IL-12p40 levels in whole-cell lysates were measured by ELISA (Abcam, Cambridge, MA). The sensitivity of ELISA for IL-12p40 was 20pg/mL.
RNA interferences with ERK-1, ERK-2, SIRPα, p22phox, β-arrestin 2, EGFR, SHP siRNA
Transfection of macrophages with siRNAs for ERK-1 (50nM), ERK-2 (50nM), SIRPα/γ/δ (50nM), p22phox (50nM), β-arrestin 2 (50nM), EGFR (50nM), SHP (50nM) or control siRNA-A (Santa Cruz Biotechnology, Santa Cruz, CA) was performed day 7-8 of cell culture using Lipofectamine (Life Technologies, Carlsbad, CA). IL- 12p40 protein levels in whole-cell lysates or cell-culture supernatants were measured by ELISA.
Western blotting for SIRPα
Adherent macrophages (on 3 day of culture) pretreated with PD98059 (An inhibitor of MAPK/MEK: 1μM), TMB-8 (A calcium antagonist: 10μM), SB203580 (a p38 MAPK inhibitor: 10μM) or PDTC (A NF-κB inhibitor: 10μM) were stimulated with GM-CSF (10ng) for 6 hours. The levels of SIRPα in whole-cell lysates were detected by western blotting. The proteins in the whole-cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (ATTO Corporation, Tokyo, Japan) and transferred onto polyvinylidene fluoride membranes (Thermo Fisher Scientific) for immunoblotting. The membranes were incubated with 0.2 × 103 mg/L mouse anti-human SIRPα (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour at room temperature, washed, and incubated with alkaline phosphatase-conjugated anti-mouse IgG (Santa Cruz Biotechnology) diluted to 1:5000. Then the membranes were incubated with chemiluminescence enhancer (Immun-Star, Bio- Rad Laboratories, Hercules, CA) and exposed to XAR film (Kodak, Rochester, NY). After the film was developed, bands were quantified with a densitometer and ImageQuant software (Molecular Dynamics, Sunnydale, CA). Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) was also detected by western blotting with an anti-GAPDH antibody (Santa Cruz Biotechnology) and SIRPα protein levels were normalized to GAPDH.
Statistical analysis
Results are expressed as the mean (SE). Differences between two groups were analyzed using a t-test for independent means, and differences between more than two groups were compared by analysis of variance. When the F ratio was found to be significant, mean values were compared using a post hoc Bonferroni test. P<0.05 was considered to indicate significance in all analyses.
Results and Discussion
Signal regulatory protein α expression is induced by GMCSF
The Signal Regulatory Protein (SIRP) family, which consists of type I transmembrane glycoproteins, is composed of SIRPα, SIRPβ, and SIRPγ. The expression of SIRPα is restricted to myeloid cells, including monocytes, macrophages, and dendritic cells, and SIRPα promotes anti-inflammatory responses [32]. Indeed, studies reported that knockdown of SIRPα enhanced the susceptibility for endotoxin shock [33]. We were interested in this inhibitory mechanism of SIRPα signaling and investigated SIRPα expression in macrophages induced by GM-CSF. The GM-CSF receptor (GM-CSFR) is composed of α and β subunits and stimulates the Ras signaling pathway [34]. Ras is activated by proto-oncogene tyrosine-protein kinase Src (Src) [35], which also starts to phosphorylate adaptor proteins such as Src Homology region 2 domain-containing Phosphatase 2 (SHP-2), Growth Factor Receptor-Bound Protein-2 (Grb-2), and Src Homology and Collagen Homology (SHC). Ras interacts with an effector, the Raf serine/threonine kinase family, leading to activation of the Ras/Raf/MEK/ERK signaling pathway [36]. We found that PD98059, an inhibitor of mitogen-activated protein kinase kinase (MAPKK/MEK), inhibited SIRPα expression by GM-CSF and that PDTC, an NF-κB inhibitor and antioxidant, partially blunted it (Figure 1). GM-CSF stimulates SIRPα production via the Ras/Raf/ MEK/ERK signaling pathway. Figure 2 depicts the cellular signal transduction of SIRPα expression via GM-CSF/GM-CSFR ligation.
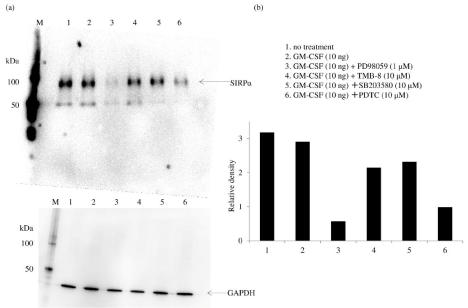
Figure 1: Effect of PD98059, TMB-8, SB203580, and PDTC on signal regulatory protein α expression by human macrophages Adherent macrophages (on day 3 of
culture) were pretreated with PD98059 (1μM), TMB-8 (10μM), SB203580 (10μM), or PDTC (10μM) and stimulated with granulocyte-macrophage colony-stimulating
factor (GM-CSF; 10ng) for 6 hours. Then, whole-cell lysates of macrophages and the positive control were analyzed by Western blotting (a). The density of each
signal regulatory protein α band was normalized to that of glyceraldehyde 3-phosphate dehydrogenase (b). Samples were tested in triplicate, and three separate
experiments were performed. a) Representative Western blot. b) Densitometry data were obtained from the cells of three volunteers in each experiment (mean ±
SE).
*P <0.05; **P <0.01; NS: Not Significant.
Abbreviation: GM-CSF: Granulocyte Macrophage-Colony-Stimulating Factor.
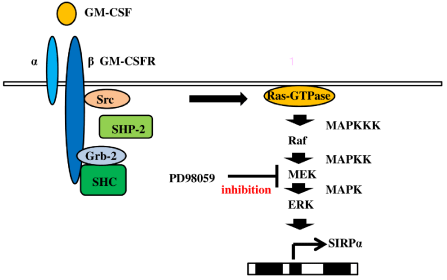
Figure 2: Mechanism of signal regulatory protein α expression in human macrophages by granulocyte-macrophage colony-stimulating factor After stimulation
of human macrophages with Granulocyte Macrophage-Colony-Stimulating Factor (GM-CSF), Src homology region 2 domain-containing phosphatase 2 (SHP-
2) activates Src and interacts with Growth Factor Receptor-Bound protein-2 (Grb-2). Formation of the SHC/Grb-2 complex promotes the activation of Ras.
Furthermore, signal regulatory protein α (SIRPα) production is mediated by the Ras/Raf/MEK-extracellular signal-regulated kinase (ERK) signaling pathway.
PD98059 (an ERK inhibitor) blunts SIRPα expression after stimulation with GM-CSF.
Abbreviations: ERK: Extracellular Signal-Regulated Kinase; GM-CSF: Granulocyte-Macrophage Colony-Stimulating Factor; ß GM-CSFR: ß subunit of the GM-CSF
Receptor; Grb-2: Growth factor Receptor-Bound protein-2; MAPK: Mitogen-Activated Protein Kinase; MAPKK: Mitogen-Activated Protein Kinase Kinase; MAPKKK:
Mitogen-Activated Protein Kinase Kinase Kinase; SHP-2: Src Homology Region 2 domain-containing Phosphatase 2; SIRPα: Signal Regulatory Protein α.
Fibroblast migration and activation
Fibroblasts and myofibroblasts are found in patients with pulmonary fibrosis [37]. Repeated lung epithelial injury leads to tissue repair or abnormal fibrotic tissue formation, and fibroblasts migrate to inflamed areas and differentiate into myofibroblasts.
Fibroblasts from patients with idiopathic pulmonary fibrosis express CC Chemokine Receptor 7 (CCR7), one ligand of which is CC chemokine ligand 21 (CCL21). CCL21/CCR7 signaling promotes the migration and proliferation of fibroblasts [38]. IL-4 upregulates the expression of CCR7 [39]. After lung injury, levels of Hyaluronan (HA) also are elevated in the airway [40]. HA is a ligand for CD44 [41, 42], which is expressed in fibroblasts [43]. This HA/CD44 signal induces activation of the potent profibrotic growth factor, TGFβ, in pulmonary fibrosis [44]. Additionally, studies reported that IL-4Rpositive cells have high levels of CD44 expression [45]. IFNγ is known to reduce IL-4 [25] and IL-4R expression [26]. It also suppresses Th2 cell development to attenuate IL-4 production and promotes Interferon Regulatory Factor 1 (IRF1) expression via STAT1 signaling. Studies found that IRF1 acts as the promotor repressor of IL-4, inhibiting its expression [46,47]. IRF1 also is known to be induced by IL-12 via the STAT4 signaling pathway [48]. IFN-γ is an inducer of the Th1 immune response and is reported to be upregulated by IL-12 [49]. Figure 3 depicts the inhibitory mechanism of IL-4 expression by IFNγ. The IL-12p40/IFNγ axis may be important for IL-14 expression. Indeed, IL-12 deficiency decreases production of IFNγ [50]. This finding indicates that IL-12 regulates IL-4 and IL-4R expression via the IL-12p40/IFNγ signaling pathway.
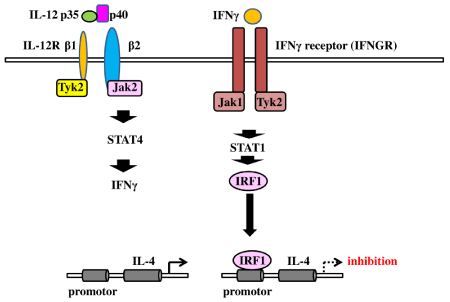
Figure 3: Inhibitory effect of Interleukin (IL)-4 expression by the IL-12p40/interferon gamma axis IL-12/IL-12R ligation activates the signal transducers and activators
of transcription 4 (STAT4) signal to induce interferon gamma (IFNγ) production. IFNγ promotes Interferon Regulatory Factor 1 (IRF1) expression. IRF1 acts as the
promotor repressor of IL-4 to inhibit its production.
Abbreviations: IFN: Interferon γ; IL: Interleukin; IRF1: Interferon Regulatory Factor; STAT 4: Signal Transducers and Activators of Transcription 4.
Inhibitory effect of the SIRPα signal on IL-12p40 expression
As mentioned above, the etiology of pulmonary fibrosis remains unclear [51]. Although viral infection is reported to be an important factor for the development of the disease [52], the role of bacterial infection in lung fibrosis has not been extensively researched.
However, the presence of bacteria in bronchoalveolar lavage from patients with idiopathic pulmonary fibrosis [53] and the exacerbation of pulmonary fibrosis after pneumonia suggest that bacterial infection may be involved in exacerbating the disease [54]. Interestingly, septrin (co-trimoxazole), an antibiotic, was reported to reduce disease progression of pulmonary fibrosis [55].
TLR4 is expressed on fibroblasts [56], and fibroblast activation by TLR4 signaling enhances TGFβ response and facilitates fibrosis [57]. Bacterial infection induces IL-1 and TNFα production to promote GM-CSF expression, and GM-CSF facilitates SIRPα expression on macrophages. Therefore, in this review we discuss the effects of LPS exposure on SIRPα in human macrophages and how SIRPα may exacerbate pulmonary fibrosis. Studies showed that SPD binds to SIRPα to inhibit inflammatory responses [58].
Previously, we reported that SPD/SIRPα signaling reduced IL- 12p40 production after exposure of human macrophages to LPS [59]. Figure 4 shows that treatment with SPD decreases IL-12p40 production in response to LPS in a dose-dependent manner, and RNA silencing for SIRPα/β/γ significantly blunts this response.
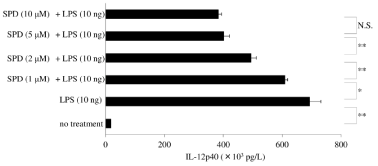
Figure 4: Inhibitory effect of surfactant protein D on IL-12p40 production in macrophages exposed to LPS Granulocyte-Macrophage Colony-Stimulating Factor
(GM-CSF)-stimulated macrophages (day 9 of culture) were pretreated with surfactant protein D (SPD; 0, 1, 2, 5, or 10μM) for 30 minutes and then stimulated with
lipopolysaccharide (10ng) for 6 hours. IL-12p40 protein was measured in whole-cell lysates by enzyme-linked immunosorbent assay. Data were obtained from the
cells of three donors and represent the mean ± SE.
*P <0.05; **P <0.01; NS: Not Significant.
Abbreviations: LPS: Lipopolysaccharide; SPD: Surfactant Protein D.
SPD/SIRPα-mediated cellular signaling is known to be involved in the inhibition of IL-12p40 expression because attenuation of IL-12p40 levels after LPS stimulation by SPD was significantly reduced by Y-27632 (a Rho-associated coiled-coil forming kinase (ROCK) inhibitor) but not by RNA silencing of SHP. Moreover, the restoration of IL-12p40 production in macrophages transfected with ERK1/2 siRNA was blunted by Y-27632 (Figure 5). Therefore, SPD inhibits the production of IL-12p40 after stimulation with LPS via the SIRPα/ROCK/ERK signaling pathway. Indeed, ERK is known to suppress IL-12p40 production mediated by LPS [60], and IL-12p40 expression after exposure to LPS is known to be regulated by ERK or p38MAPK. Neither SB203580 (A p38α/β MAPK inhibitor) nor BIRB796 (A p38δ/γ MAPK inhibitor) has an inhibitory effect on IL- 12p40 expression by SPD/SIRPα signaling (Figure 6). ERK1/2 and p38MAPK are activated by MEK and MKK, respectively. In addition, SPD/SIRPα signaling is reported to inhibit p38MAPK activation [61]. SIRPα activation recruits downstream signals of Src Homology region 2 domain-containing Phosphatase-1 (SHP-1) and SHP-2 [62]. However, RNA silencing for SHP-2 does not blunt the reduction of IL-12p40 expression in macrophages stimulated with LPS after the treatment of SPD (Figure 7).
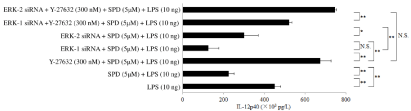
Figure 5: Inhibitory effect of ERK1/2 small interfering RNA on the restoration of IL-12p40 production in human macrophages by Y-27632 Granulocyte-Macrophage
Colony-Stimulating Factor (GM-CSF)-stimulated macrophages (day 9 of culture) transfected with extracellular signal-regulated kinase (ERK)1/2 small interfering
RNA (siRNA) were pretreated with Y-27632 (300nM) and subsequently treated with surfactant protein D (SPD; 5μM). Then, the cells were stimulated with
lipopolysaccharide (10ng) for 6 hours, and the IL-12p40 protein levels in whole-cell lysates were determined by Enzyme-Linked Immunosorbent Assay (ELISA).
The IL-12p40 protein level in whole-cell lysates was determined by ELISA after stimulation of macrophages with SPD (5μM), ERK-1 siRNA, ERK-2 siRNA, or
Y-27632 (300nM). Data were obtained from the cells of three individuals and represent the mean ± SE.
*P <0.05; **P <0.01; NS: Not Significant.
Abbreviations: ERK: Extracellular Signal-Regulated Kinase; LPS: Lipopolysaccharide; SPD: Surfactant Protein D.
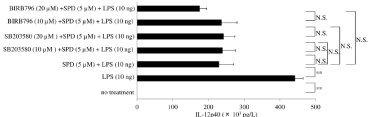
Figure 6: Effect of SB203580 or BIRB796 on interleukin (IL)-12p40 production by surfactant protein D-pretreated macrophages after exposure to lipopolysaccharide
Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF)-stimulated macrophages (day 9 of culture) were pretreated with SB203580 (10μM or 20μM) or
BIRB796 (10μM or 20μM) and were subsequently treated with Surfactant Protein D (SPD; 5μM). Then, cells were stimulated with lipopolysaccharide (10ng) for
6 hours, and IL-12p40 protein levels were determined in whole-cell lysates by enzyme-linked immunosorbent assay. Data were obtained from the cells of three
individuals and represent the mean ± SE.
**P <0.01; NS: Not Significant.
Abbreviations: LPS: Lipopolysaccharide; SPD: Surfactant Protein D.
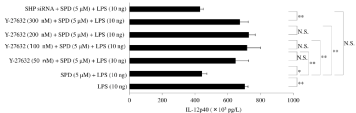
Figure 7: Effect of Y-27632 on interleukin (IL)-12p40 production by surfactant protein D-pretreated macrophages after exposure to lipopolysaccharide Granulocyte-
Macrophage Colony-Stimulating Factor (GM-CSF)-stimulated macrophages (day 9 of culture) were pretreated with Y-27632 (0, 50, 100, 200, or 300nM) and
subsequently treated with surfactant protein D (SPD; 5μM). Then, cells were stimulated with lipopolysaccharide (LPS; 10ng) for 6 hours, and IL-12p40 protein
levels were determined in whole-cell lysates by Enzyme-Linked Immunosorbent Assay (ELISA). In addition, macrophages transfected with SHP small interfering
RNA were stimulated with LPS (10ng) for 6 hours, and IL-12p40 was determined by ELISA. Data were obtained from the cells of three individuals and represent
the mean ± SE.
*P <0.05; **P <0.01; NS: Not Significant.
Abbreviations: LPS: Lipopolysaccharide; siRNA: small interfering RNA; SHP siRNA: Src Homology 2-containing Phosphotyrosine Phosphatase; SPD: Surfactant
Protein D.
SHP-2 interacts with EGFR [63,64] and positively regulates the oxidative burst of macrophages [65]. β-arrestin 2 is known to recruit SHP-1 and SHP-2 [66]. Therefore, we investigated the role of EGFR, p22phox, and β-arrestin 2 in reducing IL-12p40 expression by SPD/ SIRPα signaling after exposure to LPS. However, RNA silencing for β-arrestin 2, p22phox, or EGFR did not blunt the inhibitory effect of SP-D on IL-12p40 production by macrophages in response to LPS (Figure 8).
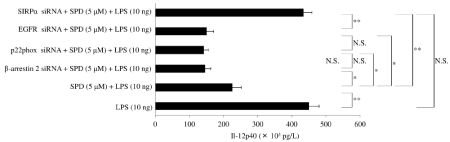
Figure 8: Effect of small interfering RNA for signal regulatory protein α, β-arrestin2, p22phox, or EGFR on interleukin (IL)-12p40 production by surfactant protein
D-pretreated macrophages after exposure to lipopolysaccharide Granulocyte-macrophage colony-stimulating factor (GM-CSF)-stimulated macrophages (day 9 of
culture) were treated with surfactant protein D (5μM) and stimulated with lipopolysaccharide (10ng) for 6 hours. Then, the IL-12p40 level in whole-cell lysates was
determined by enzyme-linked immunosorbent assay. Data were obtained from the cells of three individuals and represent the mean ± SE.
*P <0.05; **P <0.01; NS: Not Significant.
Abbreviations: EGFR: Epidermal Growth Factor Receptor; LPS: Lipopolysaccharide; siRNA: small interfering RNA; SIRPα: Signal Regulatory Protein α; SPD:
Surfactant Protein D.
How does the SPD/SIRPα signal affect IL-12p40 expression after LPS stimulation?
IRF1 is reported to bind to the promoter region of IL-12p40 and to induce transcription of IL-12p40 [67]. IRF1 may have other roles in IL-12p40 expression. For example, studies reported that IL- 12p40 production was inhibited in IRF1-deficient macrophages after stimulation with LPS [68]. Tumor Necrosis Factor (TNF) Receptor- Associated Factor-6 (TRAF6), an adaptor molecule of TLR4, interacts and phosphorylates STAT1 [69,70], and STAT1 was reported to promote activation of IRF1 [71]. IRF1 acts as the promoter activator of IL-12p40. Most importantly, IRF1 is known to bind to RelA (p65) and be required for NF-κB activation [72,73]. We found that SPD inhibited IL-12p40 production in macrophages stimulated with LPS. GM-CSF also upregulated SIRPα expression via the Ras/Raf/ MEK/ERK signaling pathway. Indeed, PD98059 (an ERK inhibitor) inhibited SIRPα expression. ERK signals affect IRF1 expression: Interestingly, MEK signals are known to reduce IRF1 expression [74]. MAPK ERK is phosphorylated by MAPKK/MEK. This finding indicates that ERK signaling activated by SIRPα influences the reduced expression of IRF1. Figure 9 depicts the proposed mechanism of the inhibitory effect of SPD/SIRPα signaling on IL-12p40 production in human macrophages after exposure to LPS.
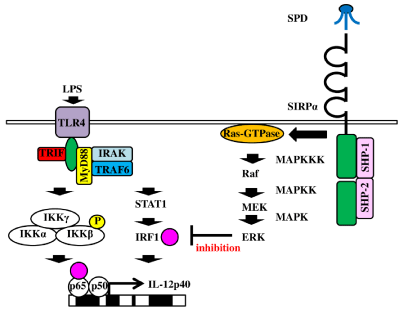
Figure 9: 9: Proposed mechanism of the inhibitory effect of the surfactant protein D/signal regulatory protein α signaling pathway on interleukin (IL)-12p40 production
by macrophages stimulated with lipopolysaccharide After stimulation of human macrophages with lipopolysaccharide, Interferon Regulatory Factor 1 (IRF1)
is upregulated via the tumor necrosis factor receptor-associated factor-6/signal transducers and activators of transcription 1 signaling pathway and binds to
the promoter region of IL-12p40. Surfactant protein D/signal regulatory protein α ligation induces signal transduction of Ras/Raf/MEK/ERK. ERK reduces IRF1
expression, leading to inhibition of IL-12p40 production.
Abbreviations: ERK: Extracellular Signal-Regulated Kinase; IRF1: Interferon Regulatory Factor; GM-CSF: Granulocyte-Macrophage Colony-Stimulating Factor;
ß GM-CSFR: ß subunit of the GM-CSF Receptor; Grb-2: Growth Factor Receptor-Bound Protein-2; IKK: IκB Kinase; IRAK: Interleukin-1 Receptor-Associated
Kinase; LPS: Lipopolysaccharide; MAPK: Mitogen-Activated Protein Kinase; MAPKK: Mitogen-Activated Protein Kinase Kinase; MAPKKK: Mitogen-Activated
Protein Kinase Kinase Kinase; MEK: Mitogen-Activated Protein Kinase Kinase; Raf: Rapidly Accelerated Fibrosarcoma; Ras-GTPase; SHP-1 or -2: Src Homology
Region 2 Domain-Containing Phosphatase 1 or 2; SIRPα: Signal Regulatory Protein α; SPD: Surfactant Protein D; STAT1: Signal Transducers And Activators Of
Transcription 1; TLR4: Toll-Like Receptor 4; TRAF6: Tumor Necrosis Factor (TNF) Receptor-Associated Factor-6; TRIF: TIR-Domain-Containing Adapter-Inducing
IFNβ.
The pivotal role of ROCK for SPD/SIRPα signaling pathway through ERK
Several factors influence ERK signaling, including ROCK [75]. RhoA is activated by SIRPα signaling [76,77]), and ROCK is an effector of RhoA. Rho/ROCK induces activation of MAPKK kinase (MEKK)-1 to activate ERK signaling [78,79], and ERK is known to repress IRF1 transcription factor. Most importantly, ROCK also is reported to be required for nuclear translocation of ERK [80,81]. ERK is known to repress inflammatory gene expression by regulating IκB kinase activity [82]. Inhibition of IL-12p40 production after LPS stimulation by SPD was restored by the ROCK inhibitor Y-27632, but this effect of Y-27632 was blunted by RNA interference for ERK1/2 (Figure 5). This finding indicates that SPD/SIRPα attenuates IL- 12p40 expression by IRF1 and that this attenuation is regulated by the ROCK/ERK signaling pathway.
Conclusion
Serum levels of SPD are elevated in patients with pulmonary fibrosis. Here, we reviewed the role of SPD in pulmonary fibrosis complicated with bacterial infection. Proinflammatory cytokines such as IL-1 and TNFα are secreted during bacterial infection to promote GM-CSF production, and GM-CSF upregulates SIRPα. SPD, a ligand for SIRPα, reduces IL-12p40 expression. Although IL-12p40 is a potent inducer of IFNγ, the lack of IL-12p40 induced by SPD/ SIRPα signaling reduces IFNγ production, leading to a reduction of IRF1, the promoter activator of IL-12p40. Reduced IFNγ levels also shift the immune response from Th1 to Th2, which increases IL-4 production and activates fibroblasts. Although IFNγ reduces IL-4R expression, decreased IFNγ levels increase IL-4R expression, resulting in enhanced activity of fibroblasts through the IL-4/IL-4R signaling pathway. Consequently, SPD/SIRPα signaling leads to the activation of fibroblasts in pulmonary fibrosis complicated with bacterial infection. We propose that SIRPα has a possible exacerbating effect on pulmonary fibrosis after bacterial infection.
Highlights
• SIRPα is upregulated by GM-CSF via the Ras/Raf/MEK/ ERK signaling pathway.
• ERK induced by the SPD/SIRPα signal represses IRF1.
• IRF1 acts as the promoter activator of IL-12p40.
• IL-12p40 enhances IFNγ production, but lack of IL-12p40 decreases it.
• IFNγ induces IRF1 via the STAT1 signal, and IRF1 functions as the promoter repressor of IL-4.
• Elevated levels of IL-4 induced by paucity of IFNγ enhance the activity of fibroblasts.
Acknowledgement
This study was supported by a Kumamoto Health Science University special fellowship grant (No. 2018-C-02).
References
- Han MK, Zhou Y, Murray S, Tayob N, Noth I, Lama VN, et al. Lung microbiome and disease progression in idiopathic pulmonary fibrosis: an analysis of the COMET study. Lancet Respir Med. 2014; 2: 548-556.
- Knippenberg S, Ueberberg B, Maus R, Bohling J, Ding N, Tarres MT, et al. Streptococcus pneumoniae triggers progression of pulmonary fibrosis through pneumolysin. Thorax. 2015; 70: 636-646.
- Huang Y, Ma S-F, Espindola MS, Vij R, Oldham JM, Huffnagle GB, et al. Microbes Are Associated with Host Innate Immune Response in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2017; 196: 208-219.
- O’Dwyer DN, Ashley SL, Gurczynski SJ, Xia M, Wilke C, Nicole R. et al. Lung Microbiota Contribute to Pulmonary Inflammation and Disease Progression in Pulmonary Fibrosis. Am J Respir Crit Care Med. 2019; 99: 1127-1138.
- Burke JP, Cunningham MF, Watson RWG, Docherty NG, Coffey JC, O’Connell PR. Bacterial lipopolysaccharide promotes profibrotic activation of intestinal fibroblasts. Br J Surg. 2010; 97: 1126-1134.
- Bhattacharyya S, Wang W, Qin W, Cheng K, Coulup S, Chavez S, et al. TLR4-dependent fibroblast activation drives persistent organ fibrosis in skin and lung. JCI Insight. 2018; 3: e98850.
- Linthout SV, Miteva K, Tschöpe C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res. 2014; 102: 258-269.
- Hant FN, Ludwicka-Bradley A, Wang H-J, Li N, Elashoff R, Tashkin DP, et al. Surfactant protein D and KL-6 as serum biomarkers of interstitial lung disease in patients with scleroderma. J Rheumatol. 2009; 36: 773-780.
- Barlo NP, van Moorsel CHM, Ruven HJT, Zanen P, van den Bosch JMM, Grutters JC. Surfactant protein-D predicts survival in patients with idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2009; 26: 155-161.
- Strieter RM. What differentiates normal lung repair and fibrosis? Inflammation, resolution of repair, and fibrosis. Proc Am Thorac Soc. 2008; 5: 305-310.
- Pardo A, Selman M. Molecular mechanisms of pulmonary fibrosis. Front Biosci. 2002; 7; d1743-761.
- Fournier B, Andargachew R, Robin AZ, Laur O, Voelker DR, Lee WY, et al. Surfactant protein D (Sp-D) binds to membrane-proximal domain (D3) of signal regulatory protein α (SIRPα), a site distant from binding domain of CD47, while also binding to analogous region on signal regulatory protein β (SIRPβ). J Biol Chem. 2012; 287: 19386-1998.
- Zhang Y, Sime W, Juhas M, Sjölander A. Crosstalk between colon cancer cells and macrophages via inflammatory mediators and CD47 promotes tumour cell migration. Eur J Cancer. 2013; 49: 3320-3334.
- Dube PH, Revell A, Chaplin DD, Lorenz RG, Miller VL. A role for IL-1 alpha in inducing pathologic inflammation during bacterial infection. Proc Natl Acad Sci U S A. 2001; 98: 10880-10885.
- Hamilton JA. GM-CSF in inflammation and autoimmunity. Trends Immunol. 2002; 23: 403-408.
- Vernon-Wilson EF, Kee WJ, Willis AC, Barclay AN, Simmons DL, Brown MH. CD47 is a ligand for rat macrophage membrane signal regulatory protein SIRP (OX41) and human SIRPalpha 1. Eur J Immunol . 2000; 30: 2130-2137.
- Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000; 288: 2051-2054.
- Matozaki T, Murata Y, Okazawa H, Ohnishi H. Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway. Trends Cell Biol. 2009; 19: 72-80.
- Tsoutsou PG, Gourgoulianis KI, Petinaki E, Germenis A, Tsoutsou AG, Mpaka M, et al. Cytokine levels in the sera of patients with idiopathic pulmonary fibrosis. Respir Med. 2006; 100: 938-945.
- Huaux F, Liu T, McGarry B, Ullenbruch M, Phan SH. Dual roles of IL-4 in lung injury and fibrosis. J Immunol. 2003; 70: 2083-2092.
- Andrews A-L, Holloway JW, Holgate ST, Davies DE. IL-4 Receptor α Is an Important Modulator of IL-4 and IL-13 Receptor Binding: Implications for the Development of Therapeutic Targets. J Immunol. 2006; 176: 7456-7461.
- Doucet C, Jasmin C, Azzarone B. Unusual interleukin-4 and -13 signaling in human normal and tumor lung fibroblasts. Oncogene. 2000; 19: 5898-5905.
- Atamas SP, Luzina IG, Dai H, Wilt SG, White B. Synergy between CD40 ligation and IL-4 on fibroblast proliferation involves IL-4 receptor signaling. J Immunol. 2002; 168: 1139-1145.
- Aoudjehane L, Pissaia AJr, Scatton O, Podevin P, Massault P-P, Chouzenoux S, et al. Interleukin-4 induces the activation and collagen production of cultured human intrahepatic fibroblasts via the STAT-6 pathway. Lab Invest. 2008; 88: 973-985.
- Elser B, Lohoff M, Kock S, Giaisi M, Kirchhoff S, Krammer PH, et al. IFNgamma represses IL-4 expression via IRF-1 and IRF-2. Immunity. 2002; 17: 703-712.
- So E-Y, Park H-H, Lee C-E. IFN-γ and IFN-α Posttranscriptionally Down- Regulate the IL-4-Induced IL-4 Receptor Gene Expression. J Immunol. 2000; 165: 5472-5479.
- Serpier H, Gillery P, Salmon-Ehr V, Garnotel R, Georges N, Kalis B, et al. Antagonistic effects of interferon-gamma and interleukin-4 on fibroblast cultures. J Invest Dermatol. 1997; 109: 158-162.
- Chen ES, Greenlee BM, Wills-Karp M, Moller DR. Attenuation of lung inflammation and fibrosis in interferon-gamma-deficient mice after intratracheal bleomycin. Am J Respir Cell Mol Biol. 2001; 24: 545-555.
- Ma X, Chow JM, Gri G, Carra G, Gerosa F, Wolf SF, et al. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J Exp Med. 1996; 183: 147-157.
- Eriksson U, Kurrer MO, Sebald W, Brombacher F, Kopf M. Dual Role of the IL-12/IFN-γ Axis in the Development of Autoimmune Myocarditis: Induction by IL-12 and Protection by IFN-γ. J Immunol. 2001; 167: 5464-5469.
- Wang S-Z, Bao Y-X, Rosenberger CL, Tesfaigzi Y, Stark JM, Harrod KS. IL-12p40 and IL-18 Modulate Inflammatory and Immune Responses to Respiratory Syncytial Virus Infection. J Immunol 2004; 173: 4040-4049.
- Zen K, Guo Y, Bian Z, Lv Z, Zhu D, Ohnishi H, et al. Inflammation-induced proteolytic processing of the SIRPα cytoplasmic ITIM in neutrophils propagates a proinflammatory state. Nat Commun. 2013; 4: 2436.
- Kong X-N, Yan H-X, Chen L, L Dong L-W, Wen Yang W, Liu Q, et al. LPSinduced down-regulation of signal regulatory protein α contributes to innate immune activation in macrophages. J Exp Med. 2007; 204: 2719-2731.
- Guidez F, Li AC, Horvai A, Welch JS, Glass CK. Differential Utilization of Ras Signaling Pathways by Macrophage Colony-Stimulating Factor (CSF) and Granulocyte-Macrophage CSF Receptors during Macrophage Differentiation. Mol Cell Biol. 1998; 18: 3851-3861.
- Bunda S, Heir P, Srikumar T, Cook JD, Burrell K, Kano Y, et al. Src promotes GTPase activity of Ras via tyrosine 32 phosphorylation. Proc Natl Acad Sci U S A.2014; 111: E3785-E3794.
- Leicht DT, Balan V, Kaplun A, Singh-Gupta V, Kaplun L, Dobson M, et al. Raf kinases: function, regulation and role in human cancer. Biochim Biophys Acta. 2007; 1773: 1196-1212.
- Larsson O, Diebold D, Fan D, Peterson M, Nho RS, Bitterman PB, et al. Fibrotic myofibroblasts manifest genome-wide derangements of translational control. PLoS One. 2008; 3: e3220.
- Pierce EM, Carpenter K, Jakubzick C, Kunkel SL, Evanoff H, Flaherty KR, et al. Idiopathic pulmonary fibrosis fibroblasts migrate and proliferate to CC chemokine ligand 21. Eur Respir J. 2007; 29: 1082-1093.
- Seneviratne SL, Black AP, Jones L, di Gleria K, Bailey AS, Ogg GS. Interleukin-4 promotes human CD8+ T cell expression of CCR7. Immunology. 2007; 120: 66-72.
- Simpson MA, de la Motte C, Sherman LS, Weigel PH. Advances in Hyaluronan Biology: Signaling, Regulation, and Disease Mechanisms. Int J Cell Biol. 2015; 2015: 690572.
- Lesley J, Hascall VC, Tammi M, Hyman R. Hyaluronan binding by cell surface CD44. J Biol Chem. 2000; 275: 26967-26975.
- Misra S, Hascall VC, Markwald RR, Ghatak S. Interactions between Hyaluronan and Its Receptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer. Front Immunol. 2015; 6: 201.
- Acharya PS, Majumdar S, Jacob M, Hayden J, Mrass P, Weninger W, et al. Fibroblast migration is mediated by CD44-dependent TGFβ activation. J Cell Sci. 2008; 121: 139-1402.
- Tatler AL, Jenkins G. TGF-β activation and lung fibrosis. Proc Am Thorac Soc. 2012; 9: 130-136.
- van Panhuys N, Tang S-C, Prout M, Camberis M, Scarlett D, Roberts J, et al. In vivo studies fail to reveal a role for IL-4 or STAT6 signaling in Th2 lymphocyte differentiation. Proc Natl Acad Sci USA. 2008; 105: 12423-12428.
- Elser B, Lohoff M, Kock S, Giaisi M, Kirchhoff S, Krammer PH, et al. IFNgamma represses IL-4 expression via IRF-1 and IRF-2. Immunity. 2002; 17: 703-712.
- Maruyama S, Kanoh M, Matsumoto A, Kuwahara M, Yamashita M, Asano Y. A novel function of interferon regulatory factor-1: inhibition of Th2 cells by down-regulating the Il4 gene during Listeria infection. Int Immunol. 2015; 27: 143-152.
- Coccia EM, Passini N, Battistini A, Pini C, Sinigaglia F, Rogge L. Interleukin-12 induces expression of interferon regulatory factor-1 via signal transducer and activator of transcription-4 in human T helper type 1 cells. J Biol Chem. 1999; 274: 6698-6703.
- Zhu R, Diem S, Araujo LM, Aumeunier A, Denizeau J, Philadelphe E, et al. The Pro-Th1 cytokine IL-12 enhances IL-4 production by invariant NKT cells: relevance for T cell-mediated hepatitis. J Immunol. 2007; 178: 5435-5442.
- Magram J, Connaughton SE, Warrier RR, Carvajal DM, Wu CY, Ferrante J, et al. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996; 4: 471-481.
- Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunology 2009; 2: 103-121.
- Naik PK, Moore BB. Viral infection and aging as cofactors for the development of pulmonary fibrosis. Expert Rev Respir Med. 2010; 4: 759-771.
- Pesci A, Ricchiuti E, Ruggiero R, De Micheli A. Bronchoalveolar lavage in idiopathic pulmonary fibrosis: what does it tell us? Respir Med. 2010; 104: S70-S73.
- Chioma OS, Drake WP. Role of Microbial Agents in Pulmonary Fibrosis. Yale J Biol Med. 2017; 90: 219-227.
- Eiró N, González L, González LO, Fernandez-Garcia B, Andicoechea A, Barbón E, et al. Toll-like receptor-4 expression by stromal fibroblasts is associated with poor prognosis in colorectal cancer. J Immunother. 2013; 36: 342-349.
- Shulgina L, Cahn AP, Chilvers ER, Parfrey H, Clark AB, Wilson ECF, et al. Treating idiopathic pulmonary fibrosis with the addition of co-trimoxazole: a randomised controlled trial. Thorax. 2013; 68: 155-162.
- Eiró N, González L, González LO, Fernandez-Garcia B, Andicoechea A, Barbón E, et al. Toll-like receptor-4 expression by stromal fibroblasts is associated with poor prognosis in colorectal cancer. J Immunother. 2013; 36: 342-349.
- Bhattacharyya S, Kelley K, Melichian DS, Tamaki Z, Fang F, Su Y, et al. Tolllike receptor 4 signaling augments transforming growth factor-β responses: a novel mechanism for maintaining and amplifying fibrosis in scleroderma. Am J Pathol. 2013; 182: 192-205.
- Gardai SJ, Xiao Y-Q, Dickinson M, Nick JA, Voelker DR, Greene KE, et al. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003; 115: 13-23.
- Yamaguchi R, Sakamoto A, Yamamoto T, Ishimaru Y, Narahara S, Sugiuchi H, et al. Surfactant Protein D Inhibits Interleukin-12p40 Production by Macrophages Through the SIRPα/ROCK/ERK Signaling Pathway. Am J Med Sci. 2017; 353: 559-567.
- Feng GJ, Goodridge HS, Harnett MM, Wei XQ, Nikolaev AV, Higson AP, et al. Extracellular Signal-Related Kinase (ERK) and p38 Mitogen-Activated Protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J Immunol. 1999; 163: 6403-6412.
- Qin Y, Liu J, Liu J, Hu F. Collectins in urinary tract and kidney diseases. Int Urol Nephrol. 2018; 50: 695-703.
- Bunda S, Burrell K, Heir P, Zeng L, Alamsahebpour A, Kano Y, et al. Inhibition of SHP2-mediated dephosphorylation of Ras suppresses oncogenesis. Nat Commun. 2015; 6: 8859, 2015.
- Tomic S, Greiser U, Lammers R, Kharitonenkov A, Imyanitov E, Ullrich A, et al. Association of SH2 domain protein tyrosine phosphatases with the epidermal growth factor receptor in human tumor cells. Phosphatidic acid activates receptor dephosphorylation by PTP1C. J Biol Chem. 1995; 270: 21277-21284.
- Agazie YM, Hayman MJ. Molecular mechanism for a role of SHP2 in epidermal growth factor receptor signaling. Mol Cell Biol. 2003; 23: 7875- 7886.
- Li XJ, Goodwin CB, Nabinger SC, Richine BM, Yang Z, Hanenberg H, et al. Protein-tyrosine phosphatase Shp2 positively regulates macrophage oxidative burst. J Biol Chem. 2015; 290: 3894-3909.
- Yu M-C, Su L-L, Zou L, Liu Y, Wu N, Ling Kong L, et al. An essential function for beta-arrestin 2 in the inhibitory signaling of natural killer cells. Nat Immunol. 2008; 9: 898-907.
- Maruyama S, Sumita K, Shen H, Kanoh M, Xu X, Sato M, et al. Identification of IFN Regulatory Factor-1 Binding Site in IL-12 p40 Gene Promoter. J Immunol. 2003; 170: 997-1001.
- Salkowski CA, Kopydlowski K, Blanco J, Cody MJ, McNally R, Vogel SN. IL- 12 is dysregulated in macrophages from IRF-1 and IRF-2 knockout mice. J Immunol. 1999; 163: 1529-1536.
- Rhee SH, Jones BW, Toshchakov V, Vogel SN, Fenton MJ. Toll-like receptors 2 and 4 activate STAT1 serine phosphorylation by distinct mechanisms in macrophages. J Biol Chem. 2003; 278: 22506-22512.
- Luu K, Greenhill CJ, Majoros A, Decker T, Jenkins BJ, Mansell A. STAT1 plays a role in TLR signal transduction and inflammatory responses. Immunol Cell Biol. 2014; 92:761-769.
- Sato M, Taniguchi T, Tanaka N. The interferon system and interferon regulatory factor transcription factors -- studies from gene knockout mice. Cytokine Growth Factor Rev. 2001; 12: 133-142.
- Ohmori Y, Schreiber RD, Hamilton TA. Synergy between interferon-gamma and tumor necrosis factor-alpha in transcriptional activation is mediated by cooperation between signal transducer and activator of transcription 1 and nuclear factor kappaB. J Biol Chem. 1997; 272: 14899-14907.
- Piaszyk-Borychowska A, Széles L, Csermely A, Chiang H-C, Wesoły J, Lee C-K , et al. Signal Integration of IFN-I and IFN-II With TLR4 Involves Sequential Recruitment of STAT1-Complexes and NFκB to Enhance Proinflammatory Transcription. Front Immunol. 2019; 10: 1253.
- Komatsu Y, Christian SL, Ho N, Pongnopparat T, Licursi M, Hirasawa K. Oncogenic Ras inhibits IRF1 to promote viral oncolysis. Oncogene. 2015; 34: 3985-3993.
- Hensel N, Baskal S, Walter LM, Brinkmann H, Gernert M, Claus P. ERK and ROCK functionally interact in a signaling network that is compensationally upregulated in Spinal Muscular Atrophy. Neurobiol Dis. 2017; 108: 352-361.
- Janssen WJ, McPhillips KA, Dickinson MG, Linderman DJ, Morimoto K, Xiao YQ, et al. Surfactant proteins A and D suppress alveolar macrophage phagocytosis via interaction with SIRP alpha. Am J Respir Crit Care Med. 2008; 178: 158-167.
- Kim S-Y, Kim S, Bae D-J, Park S-Y, Lee G-Y, Park G-M, et al. Coordinated balance of Rac1 and RhoA plays key roles in determining phagocytic appetite. PLoS One. 2017; 12: e0174603.
- Yujiri T, Sather S, Fanger GR, Johnson GL. Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science. 1998; 282: 1911-1914.
- Li H, Ung CY, Ma XH, Li BW, Low BC, Cao ZW, et al. Simulation of crosstalk between small GTPase RhoA and EGFR-ERK signaling pathway via MEKK1. Bioinformatics. 2009; 25: 358-364.
- Liu Y, Suzuki YJ, Day RM, Fanburg BL. Rho kinase-induced nuclear translocation of ERK1/ERK2 in smooth muscle cell mitogenesis caused by serotonin. Circ Res. 2004: 95: 579-586.
- Zhao Y, Lv M, Lin HS, Cui Y, Wei XQ, Qin YH, et al. Rho-associated protein kinase isoforms stimulate proliferation of vascular smooth muscle cells through ERK and induction of cyclin D1 and PCNA. Biochem Biophys Res Commun. 2013; 432: 488-493.
- Maeng Y-S, Min J-K, Kim JH, Yamagishi A, Mochizuki N, Kwon J-Y, et al. ERK is an anti-inflammatory signal that suppresses expression of NF-kappaBdependent inflammatory genes by inhibiting IKK activity in endothelial cells. Cell Signal. 2006; 18: 994-1005.