
Review Article
Austin J Infect Dis. 2023; 10(4): 1091.
Review on Diagnostic Methods of Infectious Coryza in Chickens
Adugna Girma Lema¹*; Gemechu Tuli²
¹Yemelogi Welel Woreda Agriculture Office, Livestock development department. Kellem Wollega Zone, Oromia, Ethiopia
²Addis Ababa City Administration, Urban Agriculture office, Addis Ababa, Ethiopia
*Corresponding author: Adugna Girma Lema Yemalogi welel Woreda Agriculture Office, Kellem Wollega, Oromia, Ethiopia. Tel: + 251 0911925885 Email: abdiadugna4@gmail.com
Received: September 21, 2023 Accepted: November 04, 2023 Published: November 11, 2023
Summary
The total poultry population in Ethiopia is estimated to be 51.35 million. The Poultry sector is a fastest growing among the animal production activities offers an opportunity to feed the fastest growing human population and provide income resources for poor farmers. There are different diseases which can affect chicken. Among the bacterial diseases, Infectious Coryza (IC) is an acute and contagious respiratory disease of chickens caused by the bacteria, Avibacterium paragallinarum. IC is distributed worldwide and typically transmitted by direct contact, airborne droplets and contamination of drinking water. But Egg transmission does not occur. The initial step in the pathogenesis of infectious coryza is adherence to and colonization of the nasal mucosa. The diagnosis can be based on a history of rapid disease spread, clinical symptoms, and pathological changes caused by IC. Diagnostic methods for IC include direct isolation, the HI test for serovar A and Polymerase Chain Reaction (PCR). Diagnosis of Avibacterium paragallinarum have been proposed as alternatives to conventional serotyping by the Page scheme. The HI test, which is one of the most widely used serological test, is often utilized to detect changes in antibody titers in cases of field infection or vaccination, and is useful for evaluating the prevalence of IC in certain areas. Rapid and accurate detection of respiratory IC has become a challenge because of the involvement of more than one agent with similar clinical signs and lesions, which complicates diagnostic decisions, as well as treatment and control strategies.
Keywords: Avibacterium paragallinarum; Chickens; Diagnostic; Infectious coryza
Introduction
The total poultry population in Ethiopia is estimated to be 51.35 million (CSA, 2013/14) The Poultry sector is a fastest growing among the animal production activities offers an opportunity to feed the fastest growing human population and provide income resources for poor farmers [23]. Commercial poultry production system is highly intensive production system that involves greater than 10,000 birds kept under in door and heavily depends on imported breeds [3,6,26].
Generally, poultry diseases are responsible for a number of adverse economic effects due to mortality and morbidity of chickens, cost of medication, miscarriage in production and international trade ban and public health significance [24]. Poultry disease such as Infectious coryza, Newcastle disease, Coccidiosis, Infectious Bursal disease, Avian Salmonellosis, Avian Colibacillosis and nutritional deficiency are considered to be the most endemic and the one to incur huge economic losses [2].
Respiratory pathogens of chickens continue to cause heavy economic losses to the poultry industry worldwide. Respiratory infections are multifactorial, involving environmental factors and various viral and bacterial agents, either alone or in combination, leading to “respiratory disease complex” [11,20,25,55,59,63].
Among the bacterial Respiratory diseases, Infectious Coryza (IC) is one of the major threats to the poultry industry and the causal agent is a bacterium, Avibacterium paragallinarum new nomenclature of Haemophillus paragallinarum [36] belonging to genus Haemophilus and Pasteurellaceae family. IC is a respiratory disease of chickens primarily affecting upper respiratory tract, including the involvement of nasal passages, serous nasal discharge, sneezing, and depression and slight facial edema, infra orbital and paranasal sinuses. Chicken (Gallus gallus) is the natural host for Av. paragallinarum and birds of all ages are susceptible [49]. Even though the disease is not associated with heavy mortality losses it possesses significant financial liability to chicken farmers [7]. Infectious Coryza is a cosmopolitan disease and the greatest economic losses associated with infectious coryza are attributed to poor growth performance in growing birds and marked reduction (10%–40%) in egg production in layers and breeding fowls [16,40]. This highly contagious disease, which can turn into a chronic respiratory disease when complicated by other pathogens, [9,48]. There are 2 schemes for Av. paragallinarum serotyping, the Page scheme (which recognizes serogroups A–C) and the Kume scheme (which recognizes serogroups A–C and then serovars A-1, A-2, A-3, A-4, B-1, C-1, C-2, C-3, and C-4), and both schemes use the Hemagglutination Inhibition (HI) test [14].
The diagnosis can be based on a history of rapid disease spread, clinical symptoms, and pathological changes caused by IC. Diagnostic methods for IC include direct isolation, the HI test for serovar A and Polymerase Chain Reaction (PCR). Also use polyclonal antisera for immune localization studies of Av. paragallinarum across the upper respiratory tract of birds by immune histochemistry and obtained some novel information about the migration pattern of the pathogen through nasal passages [10]. For direct isolation, the pathogen can be isolated from sterile cotton swabs obtained from the infra orbital sinus, trachea, and air sac. However, the pathogen must be isolated during the acute stage of infection after 1 to 7 days of incubation, which complicates direct isolation [13]. The HI test, which is one of the most widely used serological test, is often utilized to detect changes in antibody titers in cases of field infection or vaccination, and is useful for evaluating the prevalence of IC in certain areas or conducting retrospective/epidemiological studies [32].
• Therefore, the objectives of this review To assess the currently available diagnostic methods of infectious coryza in the poultry industry.
• To assess the advantages and drawbacks of each method.
• To identify the gaps in the diagnostic techniques of infectious coryza in chicken.
• To recommend on further development activities regarding the diagnostic techniques of infectious coryza.
Infectious Coryza
Infectious Coryza (IC) is an acute and contagious respiratory disease of chickens caused by the bacteria, Avibacterium paragallinarum (formerly called Haemophilus paragallinarum) primarily affecting upper respiratory tract, including the involvement of nasal passages, infra orbital and paranasal sinuses [8]. IC is a cosmopolitan disease, which has been reported from all around the world where chickens are raised and the disease has also been reported to affect other avian species than chicken [49,57] with an initial settlement across the nasal passages during naturally acquired infection. Chickens are the natural hosts for the agent Avibacterium paragallinarum [56].
Chickens of all ages are susceptible and the susceptibility increases with age. The organism is widely regarded as one that is a secondary agent which is associated with other primary pathogens such as viruses or mycoplasmas [5,7]. The clinical signs of this disease include nasal discharge, facial swelling, and lacrimation [9,47]. IC is very important and results in poultry economic losses. The economic effect is associated with both a decrease in egg production (10%-40%) and an increase in the culling rate of laying hens [14].
Etiology
Avibacterium paragallinarum is a fastidious gram-negative bacterium in the Pasteurellaceae family, a causative agent of Infectious coryza (IC) in laying and broiler chickens [50]. It was previously known as Haemophilus paragallinarum. The family is known for its pleomorphic, gram negative, non-motile, bacilli and coccobacilli organisms that are able to reduce nitrates and utilize carbohydrates. As many species in the genus, Av. paragallinarum is catalase negative microaerophilic rod [8,37].
Epidemiology
Distribution of the disease : Infectious Coryza is distributed worldwide. Both commercial chickens as well as village chickens appear to be equally at risk. Chickens of all age group are susceptible, yet susceptibility increases with age [15].
Transmission: Infectious Coryza is typically transmitted by direct contact, airborne droplets and contamination of drinking water. But Egg transmission does not occur [14]. The disease can also be introduced when infected birds are brought into the flock. Birds that have recovered from the disease remain carriers of the organism and be a reservoir for transmission via direct contact, airborne droplets, or fomites [9,51]. Transmission can also occur through the exchange of equipment between farms, and also by personnel [41]. Bird to bird transmission is via respiratory rout or by contact with contaminated drinking water [1].
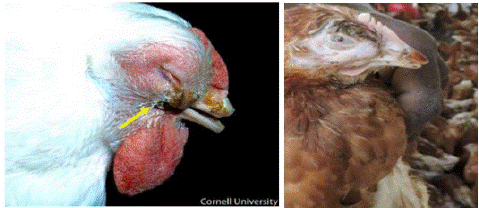
Figure 1: Watery eyes, Conjunctivitis, infra orbital sinusitis/swelling.

Figure 2: Fascial edema.
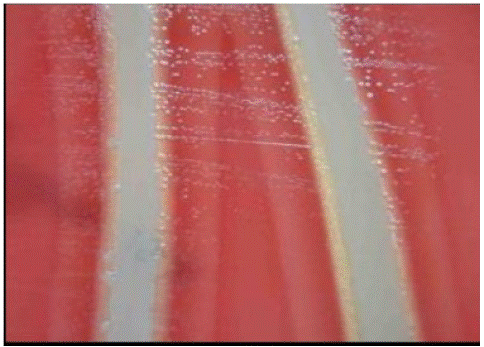
Figure 3: Blood Tryptose Agar (BTA) plate cross streaked with Staphylococcus aureus feeder.
Predisposing factors: Intercurrent respiratory viral and bacterial infections are predisposing factors.Concurrent respiratory agents, including Mycoplasma synoviae, Mycoplasma gallisepticum, Pasteurella species and infectious bronchitis virus, as well as stress factors, can exacerbate disease [9,37].
Physicochemical Property
Avibacterium paragallinarum is a fastidious gram-negative bacterium in the Pasteurellaceae family [14]. It is of paramount importance in the poultry industry because of its worldwide distribution and may contribute to the formation of “respiratory disease complex” under field conditions, thus leading to more severe clinical signs and provokes acute inflammation of the upper respiratory tract and facilitates the growth of other bacterial and viral pathogens [37,48]. Given its slow growth rate and a need for specialized laboratory media and conditions, the organism is difficult to detect by culture, particularly from sites colonized by normal flora [9]. The minimal and maximal temperatures for the growth of the bacterium are 37 and 38°C. It is able to produce acid from maltose, mannitol, sorbitol and sucrose [12].
Serological Variations
Two classification systems applied to Av. paragallinarum are the “Page” and the “Kume” methods. Based on the page method, which employed the use of the plate agglutination method, three different serotypes termed A, B and C was detected. On the other hand, the Kume method is based on hemagglutination and haemagglutination inhibition tests. Accordingly, three different serogroups I, II and III consisting of seven serovars (HA-1 to HA-7) were detected. I.e. HA-1 to HA-3 belonging to serogroup I, HA-4 to HA-6 belonging to serogroup II and HA-7 belonging to serogroup III. Kume’s serogroups I, II and III correspond to Page’s serovars A, C and B respectively. This subsequently led towards the proposal to alter the Kume scheme nomenclature concluding with the nine currently recognized serovars [52].
Pathogenesis
The initial step in the pathogenesis of infectious coryza is adherence to and colonization of the nasal mucosa. An important common mechanism for bacterial pathogenesis is the capacity to adhere to the host cells, which in turn leads to colonization and, finally, to infection of the host cells. Gene hmtp210 of Av. paragallinarum encodes a 210-kDa outer-membrane protein that functions as an HA and has been identified as an important protective antigen.
It has also been proposed that the HA antigen plays a key role in the pathogenicity of Av. Paragallinarum and a trimeric auto transporter adhesin that besides conferring hemagglutination, enables cell adherence and biofilm formation activities [60].
The virulence factors of Av. paragallinarum are not clearly understood. There have been studies that looked at the role of capsule, hemagglutinin, hemocins, lipopolysaccharide, and iron acquisition proteins [54].
Clinical Signs
Infectious Coryza has a short incubation period that develops clinical signs within 1-2 days after inoculation in to the chickens. The most prominent Clinical signs of IC are an acute inflammation of the upper respiratory tract including involvement of nasal discharge and sinuses with a serous to mucoid nasal discharge, sneezing, facial edema and swelling of the face under eye, conjunctivitis, anorexia, and retarded growth in young poultry [9,14,27,33].
Exhibition of clinical signs can vary depending on age and breed, and the duration and severity of these signs can be affected by factors such as poor housing, parasitism, inadequate nutrition, and mixed infection from other infectious diseases such as fowlpox, infectious bronchitis, laryngotracheitis, Mycoplasma gallisepticum, and pasteurellosis [14]. In addition to this, there may be diarrhea, decrease in feed and water consumption and a reduction in egg production in layer chickens. Losses due to persistent mortality are culling where up to 5%. Affected flocks may suffer an egg drop of up to 10%, and to 100% in more serious situations [48].
Pathological Change
Gross Lesions: Grossly acute catarrhal inflammation of mucous membranes of the nasal passages and sinuses, frequently a catarrhal conjunctivitis, subcutaneous edema of the face and wattles are evident of the IC. In acute cases, lesions may be limited to the infraorbital sinuses and there is a copious semifluid exudate from the nostril synovitis [5,13].
Microscopic Lesions
Major histopathological lesions include inflammation of the respiratory mucosa with severe damage to the epithelium and infiltration of heterophils. Furthermore, severe hemorrhages in tissues of the nasal cavity as well as in sinus infraorbitalis [58]. Upon histological examination of the URT, there are usually lesions ranging from necrosis of the respiratory epithelium to marked hypoplasia and squamous metaplasia of the sinuses [1,61].
Diagnosis
The diagnosis can be based on a history of rapid disease spread, clinical symptoms, and pathological changes caused by IC. Molecular, serological and bacteriological methods have been used for the diagnosis and characterisation of Av. paragallinarum in chickens [9,30,48].
Rapid and accurate detection of respiratory IC has become a challenge because of the involvement of more than one agent with similar clinical signs and lesions, which complicates diagnostic decisions, as well as treatment and control strategies [31]. To overcome such difficulties associated with conventional diagnostic methods, alternative approaches such as multiplex Polymerase Chain Reaction (PCR) [33,39,53] have been proved to be useful for specific and sensitive identification of Av. paragallinarum. The test used to establish the serogroups and serovars is the Haemagglutination (HA) and Haemagglutination-Inhibition (HI) tests. There are currently molecular techniques available for the successful and rapid diagnosis of IC. One of these techniques is a species-specific PCR termed HPG2-PCR [34].
Direct Isolation
The diagnosis can be based on a history of rapid disease spread, clinical symptoms and pathological changes caused by IC. Avibacterium paragallinarum mainly isolated from sterile cotton swabs obtained from the infraorbital sinus, trachea and air sac. The swab is then streaked onto or inoculated into Blood Tryptose Agar (BTA) plates and these agar plates were cross streaked by Staphylococcus aureus (feeder culture). The plates were incubated at 37°C for 48 hrs in candle jar. After incubation, the plates were observed for growth. The dew drops colonies used for further biochemical investigation (Akhter et al., 2013) [57].
Molecular Diagnostic Techniques
A species-specific PCR test (HPG-1-PCR and HPG2-PCR) and 16S Ribosomal RNA Sequencing were employed for molecular identification of Av. paragallinarum. HPG2-PCR is sensitive and reliable method for species specific identification of Av. Paragallinarum [40]. Both these PCR’s are specific and sensitive and give positive results with NAD+ -dependent and NAD+ -independent isolates [19].
Serological Diagnostic Tests
For the understanding of prevalence of strains in a particular region, it is essential to characterize and classify organisms on serological level. Hence, Av. paragallinarum were further typed by using Page serotyping scheme [28]. Serological diagnosis of the disease has not been performed because the progress of this disease is rapid and antibodies are not likely to be induced in chickens infected with Av. paragallinarum, particularly with serovar C, even after the disease onset. HI tests are used for identification of Av. paragallinarum; however, the procedure is complicated, and the sensitivity is insufficient. For this, serological classification both conventional Hemagglutination- Inhibition (HI) test as well as modern multiplex PCR (Molecular serotyping) based protocols were followed and described below [29].
Hemagglutination-Inhibition (HI) Test
The Page serotyping scheme was initially developed by using plate or slide agglutination test to recognize the three serovars i.e. serovar A, B and C. However, the use of Hemagglutination-Inhibition (HI) technology has been shown to be a much better method for the identification of Page serovars of Avibacterium paragallinarum isolates around the world. A drawback of the Page scheme is that some isolates could not be typed due to non- agglutination. The maximum serum dilution completely inhibiting hemagglutination was considered as HI titer and the antisera specific to serovar with highest HI titer was considered as serovar of isolate [14]. The Haemagglutination (HA) and Haemagglutination-Inhibition (HI) test, detecting haemagglutinins andthe method used involved treating bacterial cells with potassium thiocyanate (KSCN), followed by sonication. The result was the detection of an additional antigen, together with haemagglutinins that was able to agglutinate fresh and gluteraldehyde-fixed chicken erythrocytes [47]. Three main forms of HI tests have been recently recognized: termed simple, extracted, and treated HI tests.
Simple HI test is based on whole bacterial cells of Page serovar Av. paragallinarum and fresh chicken erythrocytes. Although simple to perform, this HI test can detect antibodies only to serovar A. It has been widely used to detect antibodies in infected as well as vaccinated chickens [45].
Extracted HI test is based on KSCN-extracted and sonicated cells of Av. paragallinarum and glutaraldehyde-fixed chicken erythrocytes. This extracted HI test has been validated mainly by using Page serovar C organisms. The test is capable of detecting a serovar-specific antibody response in Page serovar C-vaccinated chickens. A major weakness of this assay is that the majority of chickens infected with serovar C remain seronegative [47]. Treated HI test is based on hyaluronidase-treated whole bacterial cells of Av. paragallinarum and formaldehyde-fixed chicken erythrocytes. The extracted HI test has not been widely used or evaluated. It has been used to detect antibodies to Page serovars A, B, and C in vaccinated chickens, with only serovar A- and C-vaccinated chickens yielding high titers. It has also been used to screen chicken sera in Indonesia for antibodies arising from infection with serovars A and C [14].
The Kume proposed a new serovar scheme based on the detection of Hemagglutinin (HA) in Av. paragallinarum into three serogroups (A, B, and C), which currently recognizes as nine serovars (A-1, A-2, A-3 (HA-1 to HA-3), A-4(HA-8), B-1 (HA-7), C-1, C-2, C-3 (HA-4 to HA-6), and C-4 (HA-9) However, HA/ HI is a time-consuming technique and it is difficult to serotype accurately to the serovar level, making a molecular technique the preferred alternative [46,47].
Molecular Serotyping by Using Multiplex PCR
The multiplex PCR is based on the amplification of a hypervariable region within the haemagglutinin gene of Av. paragallinarum A and C-serovars. PCR using primer sets around the hypervariable region amplified 0.8, 1.1 and 1.6 kbp fragments for serovars A, B and C, respectively [62]. This region encodes an outer-membrane protein, HMTp210, which serves as a major protective antigen of Av. paragallinarum. HMTp210 gene is an important gene that is responsible to express a 210 kDa outer membrane protein of Av. paragallinarum [43,44].
Based on DNA sequence homology, HMTp210 gene is divided into 3 regions. Out of these 3 regions, region 1 and region 3 are highly conserved between Av. paragallinarum serovars A and C, respectively. On the other hand, the homology of region 2 is around 50% between serovars A and C. Thus, region 2 seems to be a serovar-specific region in HMTp210, which is used for protection against serovars A and C. For these reasons, we focused on region 2 as the target to identification of the serovars of Av. paragallinarum using PCR [53,62]. A molecular technique is less time consuming, which is of benefit to the poultry industry. The homology of region 2 was more than 99.8% within each serovar: 99.9% within serovar A, 100% within serovar B and 99.8% within serovar C. In this protocol, region specific oligonucleotide primers were used to carry out multiplex PCR. The PCR product was further analyzed based on size of amplicon.
Deferential Diagnosis
Infectious Coryza should be differentiated from other common chicken diseases like chronic fowl cholera, Newcastle disease, infectious bronchitis, avian influenza, avian metapneumovirus (swollen head syndrome), mycoplasmosis and infectious laryngotracheitis [14].
Economic Importance
Infectious Coryza is a highly contagious respiratory disease of chickens resulting in high mortality, reduced egg production, and huge economic losses to the poultry industry worldwide [21,35]. The economic impact of the disease is mainly associated with a significant reduction (10%-40%) in egg production, especially on multi-age farms [18,40]. The infection is more commonly observed in intensive poultry farms with multiple age groups, including large-scale egg production and breeding complexes [14].
Treatment
Antibiotic treatment will reduce the severity of the course of the disease. Many drugs have bacteriostatic effect on the organism. Erythromycin, Tetracycline, Fluoroquinolones, Sulphadimetoxine, and Sulphamethazine can be used as treatment medication. Some antibiotics are not suitable for layers producing eggs for human consumption [1,17].
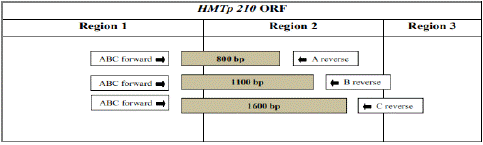
Table 1: Location of primers for multiplex PCR [53].
Prevention and Control
The prevention and control of IC depends on strict biosecurity, use of antiseptics, disinfectants, antibiotics and finally specific vaccines to IC. But the problem is that due to serotype or serovar or strain variation of Av. paragallinarum, this fastidious disease control by using vaccine is sometimes difficult [42].
Conclusion and Recommendations
Infectious Coryza (IC) is an acute and contagious respiratory disease of chickens caused by the bacteria, Avibacterium paragallinarum. The disease is highly contagious and once entered the flock, it is usually a risk for the whole farm. Molecular, serological and bacteriological methods have been used for the diagnosis of Av. paragallinarum in chickens. No suitable serologic test exists; the most commonly used serologic tests are Species specific PCR, hemagglutination Inhibition (HI) and Multiplex PCR. Despite this range of tests, only hemagglutination-inhibition test is the best of that available and widespread use. The presence of infection in flock is largely determined by Haemeagglutination Inhibition (HI) test. The serovar level recognition of Av. paragallinarum is widely carried out using HI test. In general, Rapid and accurate detection of respiratory IC has become a challenge because of the involvement of more than one agent with similar clinical signs and lesions, which complicates diagnostic decisions, as well as treatment and control strategies.
• Thus, the following are recommended based on thFurther development activities regarding the diagnostic techniques of infectious coryza.
Further development on the multiplex PCR using the hypervariable region of the HMTp210 gene is alternative methods to identify the serovar of A. paragallinarum.
References
- Abd El-Ghany W. Evaluation of autogenous Avibacterium paragallinarum bacterins in chickens. Int J Poult Sci. 2011; 10: 56-61.
- Aberra M, Tegene N. Study on the characterization of local chickens found in Southern Ethiopia. In: Proceedings of the annual research review workshop. Hawassa, Ethiopia: Hawassa University, College of Agriculture. 2009; 1-15.
- Abraham L, Yayneshet T. Livestoc. Performance of exotic and indigenous poultry breeds managed by smallholder farmers in northern Ethiopia. Res Rur Dev. 2010; 22: 1 5.
- Akter S, Ali M, Das P, Hossain M. Isolation and identification of Avibacterium paragallinarum, the causal agent of infectious coryza (IC) from layer chickens in Bangladesh. J Bangladesh Agric Univ. 2014; 11: 87-96.
- Akter S. Isolation and identification of Avibacterium paragallinarum from layerchickens [MS thesis] Submitted to Department of Pathology. Bangladesh: Faculty of Veterinary Science. Mymensingh: Bangladesh Agricultural University. 2012; 1-55.
- Alem T. Production and reproduction performance of rural poultry in lowland and midlandagro-ecological zones of central Tigray, Northern Ethiopia. Afr J Agric Res. 2014; 9: 3531-9.
- Ali M, Hossain M, Akter S, Khan M, Hossain M. Pathogenesis of infectious coryza in chickens (Gallus gallus) by Avibacterium paragallinarum isolate of Bangladesh. The agriculturists Sci. 2013; 11: 39-46.
- Anjaneya SD, Singh K, Dhama V, Gowthaman, Chawak MM. Pathogenicity study of field isolates of Avibacterium paragallinarum in experimentally infected birds. Indian J Vet Pathol. 2013; 37: 13-7.
- Askari Badouei M, Sadrzadeh A, Azad N, Blackall P, Madadgar O, Charkhkar S. Isolation and molecular identification of Avibacterium paragallinarum in suspected cases of infectious coryza. Turk J Vet Anim Sci. 2014; 38: 46-9.
- Balouria S, Deshmukh HS, Banga AA, Brar RS, Sodhi S. Early migration pattern of Avibacterium paragallinarum in the nasal passage of experimentally infected chicken and Japanese quail by immunohistochemistry. 2018.
- Bayraktar E, Umar S, Yilmaz A, Turan N, Franzo G, Tucciarone CM, et al. First molecular characterisation of avian Metapneumovirus (aMPV) in Turkish broiler flocks. Avian Dis. 2018; 62: 425-30.
- Blackall P. An update on the diagnosis and prevention of fowl cholera and infectious coryza. Clin Microbiol Rev. 2011; 93: 1522-33.
- Blackall P, Soriano E. Infectious coryza and related bacterial infections. In” Diseases of poultry”. 12th ed. Iowa state university press. 2008; 789-98.
- Blackall PJ, Soriano-Vargas E. Infectious coryza and related bacterial infections. In: Swayne DE, Pattison M, Mc Mullin PF, Bradbury JM, editors. Disease of poultry. 13th ed. 2013.
- Byarugaba D, Minga U, Gwakisa P, Katunguka E, Bisgaard M. Occurrence, isolation and characterization of Avibacterium paragallinarum from poultry farms in Uganda. In: Proceedings of the 11th international symposium on veterinary epidemiology and economics. 2006.
- Chukiatsiri K, Sasipreeyajan J, Blackall PJ, Yuwatanichsampan S, Chansiripornchai N. Serovar identification, antimicrobial sensitivity, and virulence of Avibacterium paragallinarum isolated from chicken in Thailand. Avian Dis. 2012; 56: 359-64.
- Chukiatsiri K, Sasipreeyajan J, Neramitmansuk W, Chansiripornchai N. Efficacy of autogenous killed vaccine of Avibacterium paragallinarum. Avian Dis. 2009; 53: 382-6.
- Chukiatsiri K, Chotinun S, Chansiripornchai N. An outbreak of Avibacterium paragallinarum serovar B in a Thai layer farm. Thai J Vet Med. 2010; 40: 441-4.
- Corney BG, Diallo IS, Wright L, Hewitson G, De Jong A, Tolosa X, et al. Rapid and sensitive detection of Avibacterium paragallinarum in the presence of other bacteria using a 5' Taq nuclease assay: a new tool for diagnosing infectious coryza. Avian Pathol. 2008; 37: 599-604.
- Couto RM, Braga JFV, Gomes SYM, Resende M, Martins NRS, Ecco R. Natural concurrent infections associated with infectious laryngotracheitis in layer chickens. J Appl Poult Res. 2016; 25: 113-28.
- Crispo M, Blackall P, Khan A, Shivaprasad HL, Clothier K, Sentíes-Cué CG, et al. Characterization of an Outbreak of Infectious Coryza (Avibacterium paragallinarum) in Commercial Chickens in Central California. Avian Dis. 2019; 63: 486-94.
- CSA. Agricultural sample survey, Addis Ababa; 2013/14: livestock production in Ethiopia.
- CSA. Agricultural sample survey 2009/10. Report on livestock and livestock characteristics, 2. Stat Bull. 2009; 468.
- Dana N, Vander Dessie T, W Van Arendonk J. Morphological features of indigenous chicken populations of Ethiopia, Ani. Gen Res. 2008; 46: 11-23.
- DastmalchiSaei H, Zavarshani M. Phylogenetic Grouping of Verotoxigenic Escherichia coli (VTEC) Obtained from Sheep and Broiler Chicken in Northwestern Iran. Acta Vet Eurasia. 2018; 44: 53-58.
- Dawit A, Tamrat D, Setotaw T, Devesh R. Draft report on over view and background paper on Ethiopia’s poultry sector relevance for HPAE research in Ethiopia. Addis Ababa. 2008; 1-5.
- Durairajan R, Sharma M, Murugan MS. Detection of Avibacterium paragallinarum in commercial poultry and their antibiogram. Tamil Nadu. J Vet Anim Sci Res. 2013; 9: 332-7.
- El-Sawah A, Soliman YA, Shafey SM. Molecular characterization of Avibacterium paragallinarum strain used in evaluation of coryza vaccine in Egypt. J Am Sci. 2014; 8: 253-63.
- Fedawy HS, Salama SS, Ali A, Rabie NS, El-Kady MF. Phenotypic and genotypic characterization of Avibacterium paragallinarum isolated from layer chicken flocks in Egypt. Am J Res Commun. 2016; 4: 23-34. 30.
- Findik A, Yardimci H. The comparison of agglutination, hemagglutination inhibition and indirect hemagglutination tests in the serological diagnosis of infectious coryza in chickens. Ank Üniv Vet Fakalati Dergesi. 2010; 57: 69-72.
- Galal HM, Tawfek AM, Abdrabou MI, Hessain AM, Alhaaji JH, Kabli SA et al. Recent approaches for control of E. coli and respiratory complex in Middle East. Saudi J Biol Sci. 2018; 25: 1302-7.
- García A, Romo F, Ortiz AM, Blackall PJ. The vaccination-challenge trial: the gold standard test to evaluate the protective efficacy of infectious coryza vaccines. Avian Pathol. 2008; 37: 183-6.
- García-Sánchez A, Morales-Erasto V, Talavera-Rojas M, Robles-González F, Allen MS, Blackall PJ, et al. Phylogenetic relationship of serovar C-1 isolates of Avibacterium paragallinarum. Avian Dis. 2014; 58: 143-6.
- Han MS, Kim JN, Jeon EO, Lee HR, Koo BS, Min KC, et al. The current epidemiological status of infectious coryza and efficacy of PoulShot coryza in chickens. J Vet Sci. 2016; 17: 323-30.
- Heuvelink A, Wiegel J, Kehrenberg C, Dijkman R, Soriano-Vargas E, Feberwee A. Antimicrobial susceptibility of Avibacterium paragallinarum isolates from outbreaks of infectious coryza in Dutch commercial poultry flocks, 2008-2017. Vet Microbiol. 2018; 217: 135-43.
- Mendoza-Espinoza A, Terzolo HR, Delgado RI, Zavaleta AI, Koga Y, Huberman YD. Serotyping of Avibactreiumparagallinarum isolates from Peru. Avian Dis. 2009; 53: 462-5.
- Morales-Erasto V, Falconi-Agapito F, Luna-Galaz GA, Saravia LE, Montalvan-Avalos A, Soriano-Vargas E EE, et al. Coinfection of Avibacteriumparagallinarumand Ornithobacterium rhinotracheale in chickens from Peru. Avian Dis. 2016; 60: 75-8.
- Morales-Erasto VM, Posadas-Quintana F, Fernández-Díaz M, Saravia LE, Martínez-Castañeda JS, Blackall PJ, et al. An evaluation of serotyping of Avibacterium paragallinarum by use of a multiplex polymerase chain reaction. J Vet Diagn Invest. 2014; 26: 272-6.
- Morales-Erasto V, García-Sánchez A, Salgado-Miranda C, Talavera-Rojas M, Robles-Gonzalez F, Blackall PJ, et al. ERIC-PCR genotyping of emergent serovar C-1 isolates of Avibacterium paragallinarum from Mexico. Avian Dis. 2011; 55: 686-8.
- Muhammad TMN, Sreedevi B. Detection of Avibacterium paragallinarum by polimerase chain reaction from outbreaks of infectious coryza of poultry in Andhra Pradesh. Vet World. 2015; 8: 103-8.
- Neubauer C, De Souza-Pilz M, Bojesen AM, Bisgaard M, Hess M. Tissue distribution of haemolyticGallibacteriumanatis isolates in laying birds with reproductive disorders. Avian Pathol. 2009; 38: 1-7.
- Noonkhokhetkong T, Chukiatsiri K, Sasipreeyajan J, Chansiripornchai N. Determination of antimicrobial susceptibility, antimicrobial resistance genes and in vivo testing of antimicrobial susceptibility of Avibacterium paragallinarum. Thai J Vet Med. 2013; 43: 525-31.
- Noro T, Oishi E, Kaneshige T, Yaguchi K, Amimoto K, Shimizu M. Identification and characterization of haemagglutinin epitopes of Avibacterium paragallinarum serovar C. Vet. Vet Microbiol. 2008; 131: 406-13.
- Noro T, Yaguchi K, Amimoto K, Oishi E. Identification and expression of a gene encoding an Epitope that induces hemagglutination inhibition antibody to Avibacterium paragallinarum serovar A. Avian. Avian Dis. 2007; 51: 84-9.
- Nouri A, Banani M, Goudrzi H, Pourbakhsh SA, Mirzaeil SG. Retrospective detection of Avibacterium paragallinarum serovar B in egg yolk materials by PCR. Arch Razi Inst. 2014; 69: 179-83.
- Patil VV, Mishra D, Mane DV. 16S ribosomal RNA sequencing and molecular serotyping of Avibacterium paragallinarum isolated from Indian field conditions. Vet World. 2017; 10: 1004-7.
- Patil VV, Mishra DN, Mane DV. Isolation, characterization and serological study of Avibacterium paragallinarum field isolates from Indian poultry. J Anim Poult Sci. 2016; 5: 13-20.
- Paudel S, Hess M, Hess C. Coinfection of Avibacterium paragallinarum and Gallibacterium anatis in specific-pathogen-free chickens complicates clinical signs of infectious coryza, which can be prevented by vaccination. Avian Dis. 2017; 61: 55-63.
- Priya PM, Vamshi Krishna S. Dinesh Kumar V. Isolation and characterization of Avibacterium paragallinarum from ornamental birds in Thrissur, Kerala. Int J Life Sci. 2012; 1: 87-8.
- Quinn PJ, Markey BK, Leonard FC, Fitzpatrick ES, Fanning S, Hartigan PJ. Veterinary microbiology and microbial disease. Iowa: Wiley-Blackwell. 2011; 451-60.
- Requena D, Chumbe A, Torres M, Alzamora O, Ramirez M, Valdivia-Olarte H, et al. Genome sequence and comparative analysis of Avibacterium paragallinarum. Bioinformation. 2013; 9: 528-36.
- Roodt Y. Towards unraveling the genome of Avibacterium paragallinarum. University of the Free State, Bleomfontein, South Africa. 2009.
- Sakamoto R, Kino Y, Sakaguchi M. Development of a multiplex PCR and PCR-RFLP method for serotyping of Avibacterium paragallinarum. J Vet Med Sci. 2012; 74: 271-3.
- Sandal I, Corbeil LB, Inzana TJ. Haemophilus. In: Thoen, editor. Pathogenesis of bacterial infections in animals. 4th ed. Ames, IA: Wiley-Blackwell. 2010; 387-409.
- Sid H, Benachour K, Rautenschlein S. Co-infection with multiple respiratory pathogens contributes to increased mortality rates in Algerian poultry flocks. Avian Dis. 2015; 59: 440-6.
- Swayne DE, Glisson JR, McDougald LR, Nolan Lisa SDL, et al. Disease of poultry. Blackwell publishing Ltd, Lowa. USA. 2013.
- Thenmozhi V, Malmarugan S. Isolation, identification and antibiogram pattern of avibacterium paragallinarum from Japanase quails. Tamilnadu J Vet Anim Sci. 2013; 9: 253-8.
- Trujillo-Ruíz HH, Shivaprasad HL, Morales-Erasto V, Talavera-Rojas M, Salgado-Miranda C, Salazar-García F, et al. Virulence of serovar C-1 strains of Avibacterium paragallinarum. Avian Dis. 2016; 60: 837-40.
- Umar S, Guerin JL, Ducatez MF. Low pathogenic avian influenza and coinfecting pathogens: a review of experimental infections in avian models. Avian Dis. 2017; 61: 3-15.
- Wang YP, Hsieh MK, Tan DH, Shien JH, Ou SC, Chen CF, et al. The haemagglutinin of Avibacterium paragallinarum is a trimeric autotransporter adhesin that confers haemagglutination, cell adherence and biofilm formation activities. Vet Microbiol. 2014; 174: 474-82.
- Welchman D, King SA, Wragg P, Wood AM, Irvine RM, Pepper WJ, et al. Infectious coryza in chickens in Great Britain. Vet Rec. 2010; 167: 912-3.
- Wu JR, Wu YR, Shien JH, Hsu YM, Chen CF, Shieh HK, et al. Recombinant proteins containing the hypervariable region of the haemagglutinin protect chickens against challenge with Avibacterium paragallinarum. Vaccine. 2011; 29: 660-7.
- Yilmaz H, Altan E, Cizmecigil UY, Gurel A, Ozturk GY, Bamac OE, et al. Phylogeny and S1 gene variation of infectious bronchitis virus detected in broilers and layers in turkey. Avian Dis. 2016; 60: 596-602.