
Review Article
Austin J Infect Dis. 2022; 9(1): 1066.
A Brief Review of Parasitic Castration in Aquatic Snails and Its Contribution in Control of Diverse Vector Snail Populations and Trematodiases in Man and Animals
Choubisa SL*
Department of Advanced Science and Technology, NIMS University Rajasthan, India
*Corresponding author: Shanti Lal Choubisa, Department of Advanced Science and Technology, National Institute of Medical Science and Research, NIMS University Rajasthan, Jaipur, Rajasthan 303121, India
Received: June 07, 2022; Accepted: July 11, 2022; Published: July 18, 2022
Abstract
Snails are invertebrate gastropod molluscs inhabited both the terrestrial and aquatic habitats. Most of these animals are dioeciously and their sexes are separated. In many monoecious (hermaphrodite) snail species, individuals have both male and female gonads. But in pulmonate monoecious snail species individuals have only single gonad called “ovotestis” which contains both testicular and ovarian tissues. In general, aquatic snail species are intermediate hosts of digenean trematode parasites of vertebrates including man and animals. In addition, aquatic snails are also act as vector of diverse trematodiases, such as schistosomiasis, clonorchiasis, opisthorchiasis, fascioliasis, amphistomiasis, etc. The prevalence of these digenetic trematode parasitic diseases in diverse geographical provinces is depending on the population of vector or intermediate host snail species. In fact, the various larval stages of digenean trematode parasites, such as sporocysts, rediae and cercariae are developed and multiplied asexually in the organ of hepatopancreas and/or gonads of host snails. These parasitic trematode larvae also act as castrators for snails and potential to prevent or block partially or completely their reproduction called “parasitic castration”. In this biological process, trematode larvae destroy the gonads in two ways, one is mechanically and the other physiologically. Parasitic castration is also induces sex conversion, gigantism and alteration the gene expression in brain of snails. In present communication, the most common vector snail species, different forms of trematode larvae and their basic biology and mode of parasitic castration in aquatic snails and its contribution in control of diverse vector snail populations and spreading of trematodiases are considered and brief and critically reviewed. Simultaneously, research gaps have also been highlighted for further advance research work. This review is helpful in understanding of biology or mechanism of parasitic castration and its contributory role the balancing of aquatic ecosystem.
Keywords: Digenean Trematode Parasites; Aquatic Ecosystem; Ovotestis; Molluscs; Parasitic castration; Snails; Trematode larvae; Trematodiases; Vector
Abbreviations
AC: Acini; C: Cercaria; CAC: Compressed Acinus; DAC: Disorganization of Acinus; DG: Digestive Gland; DGT: Digestive Gland Tubule; DO: Developing Oocyte; GE: Germinal Epithelium; LTP: Larval Trematode Parasites; O: Ovary; OC: Oocyte; OG: Oogonia; PG: Prostate Gland; S: Spermatozoa; SC: Sertoli Cell; SG: Spermatogonia; SP: Sporocysts; T: Testis; TP: Tunica Propria.
Introduction
Snails are unsegmented coelomate invertebrate animals belonging to Phylum Mollusca and Class Gastropoda and inhabited both the terrestrial and aquatic habitats. Most of the snail species are dioeciously and their sexes are separated. In the monoecious or hermaphrodite snail species, individuals have both male and female gonads. But in the aquatic monoecious pulmonate snails have only single gonad called “ovotestis” which contains both testicular and ovarian tissues. Although these snails are mostly herbivores, some of them are scavengers and omnivores. But some freshwater snail species, such as Ramshorn snails (Planorbis spp.), Tadpole snails (Physa and Physella spp.), Turret snails (Melanoides tuberculata) and Apple snails (Pila globosa and Pomacea spp) are invasive, pest and harmful for aquatic vegetation and agriculture crops [1-4]. Among the diverse freshwater snail species, the most common and widely distributed snail species are Faunus ater, Lymnaea acuminata f. patula, L. acuminata f. chlamys, L. acuminata f. typica, L. acuminata f. rufescens, L. luteola f. australis, L. Luteola f. typica, L. Luteola f. impura, Melania (Plotia) scabra, Melanoides striatella tuberculata, Planorbis (Indoplanorbis) exustus, Thiara (Tarebia) lineata, Thiara scabra var. choubisai, Vivipara bengalensis race gigantic, V. bengalensis race mandiensis and Pila spp (Figures 1a-n). Based on morphology, habit and habitats, these species could be identified. The genus of these snails remains the same, but their species are varied in different geographical regions or countries.
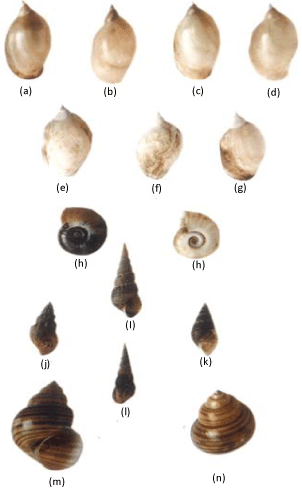
Figure 1: The most common freshwater vector snail species. Lymnaea
acuminata f. patula (Figure a), L. acuminata f. chlamys (Figure b), L.
acuminata f. typica (Figure c), L. acuminata f. rufescens (Figure d), L.
luteola f. australis (Figure e), L. luteola f. typical (Figure f), L. luteola f.
impure (Figure g), Planorbis (Indoplanorbis) exustus (Figure h), Faunus
ater (Figure i), Melania (Plotia) scabra (Figure j), Thiara (Tarebia) lineate
(Figure k), Melanoides striatella tuberculata (Figure l), Vivipara bengalensis
race gigantica (Figure m) and V. bengalensis race mandiensis (Figure n).
Among the freshwater snail species, apple snail (P. globosa) is one of the largest molluscs. It has wide ranges of habitats and found in ponds, pools, tanks, lakes, marshes, rice fields and sometimes even in streams and rivers [1, 10]. They are also amphibious and well adapted for life in water and on land. Some snail species are found to be stenotopic and able to tolerate only a narrow range of environmental changes. But many species are also eurytopic and have wide range of their distribution. Aquatic snails are also good bio-indicators for the assessment of paleo-environment, different kinds of aquatic habitats, trophic stages of lentic ecosystems and endemic of trematodiases [11- 13]. Because of their feeding habits, most of the aquatic snail species are act as intermediate hosts of wide range of digenean trematode parasites of vertebrates include man and animals. Indeed, these aquatic snails complete the life cycle of these helminth parasites and harbour their asexual reproductive stages, such as sporocyst, redia and cercaria and rarely metacercariae [14, 15]. Metacercariae are the infective stage and causes trematodiases in the final vertebrate hosts. Therefore, these aquatic snails are also considered to be act as vectors for diverse trematodiases, such as schistosomiasis, clonorchiasis, opisthorchiasis, fascioliasis, paragonomiasis, amphistomiasis, paraamphistomiasis, etc. The prevalence and spreading of trematodiasis are directly proportion to the population, density and distribution of vector snail species [5].
In many countries, due to being vector and pest, many snail species are destroyed and eliminated by using of different types of molluscicides. But there is a high risk of threat or damage to nontarget aquatic organisms, vegetations and aquatic ecosystem due to repeated use of molluscicides and there are greater chances of ecological balance in aquatic environment. But during the evolution, such a unique adaptive strategy, “parasitic castration” has been developed in aquatic vector snail species, which does not harm the aquatic ecosystem and does not cause any damage to the aquatic animals and vegetations. In fact, parasitic castration controls the population of vector snails and reduces spreading of trematodiases. Parasitic castration also induces gigantism, sex changes or reversion and alteration the gene expression in brain of snails. In present communication, the most common vector snail species, diverse trematode larvae and their basic biology and mode of parasitic castration in aquatic snails and its contribution in control of population of diverse vector snail species, spreading of trematodiases and ecological balance in aquatic ecosystem are considered and brief and critically reviewed. Simultaneously, research gaps are also highlighted for further research study. The review is useful in understanding of biology or mechanism of parasitic castration and its significant contribution.
Larval Trematodes and Their Biology in Brief
Aquatic snails, serve as hosts for at least two stages in the lifecycle of digenetic trematodes parasites of vertebrates. As soon as the miracidium larva (infective stage) hatches from the eggs of digenean trematode parasites, it penetrates the soft tissues of snails to be transformed into the next larval stage called sporocyst. The latter gives rise either to daughter sporocysts or rediae depending on the developmental pattern of trematode species. Morphology of sporocyst is not uniform and varied greatly that either produces cercarial larvae directly or give rise to redial larvae which in turn produce a large number of cercariae [14,15]. Cercaria larva is non-feeding and free swimming stage and finally these larvae transformed to an infective stage called metacerariae in cyst form. Based on morphology, movement and behaviour, these intra-molluscan stages (sporocysts, rediae and cercariae) could be easily identified and are well studied by several workers [16-31]. The most common and widely distributed trematode cercariae are furcocercous, echinostome, amphistome, xephidiocercous, gymnocephalous and monostome [14,15]. Among the metacercariae, aspidogaster, echinostome, opisthorchid, plagiorchiid, strigeid, etc. are the most common metacercariae and reported from different geographical provinces [5,32].
Interesting, as the advancement of these intra-molluscan larvae their various organ systems, such as nervous, excretory (flame cells), digestive, reproductive, etc. are also progressive and well developed at the cercarial and metacercarial stages. These organ systems in sporocysts, rediae, cercarae, metacercarae and juvenile trematodes are well studied in both in-vivo and in-vitro by several workers [33- 39]. However, the digestive and reproductive systems are almost in non-functional. For getting a continuous energy needed for growth, development and multiplication, sporocyst and redial larvae depend on extra-cellular digestion [39]. In sporocyst, the digestive system is completely absent; therefore, these larvae absorbed food nutrients through general body surface. However, redial stage is nutritionally more advanced over the sporocyst larvae. Digestive system in redia is physiologically is partially active or functional and consisting of mouth, pharynx, pharyngeal gland and single elongated blind gut. Redia has been found to use both the routs to obtain nutrition through tegument and caecal gut. This is a physiological adaptation in radial larvae for getting maximum amount of energy required for growth, development and multiplication of germ cells into numerous free swimming cercarial larvae [14,15,5]. For getting continuous energy, these trematode larvae parasitized in those organs of snail hosts having ample amount reserve food nutrients, glycogen, lipids, proteins, etc. and these organs are haepatopancreas and reproductive (gonads) [39]. But, how miracidium larvae trace out these organs is still mysterious and has not yet been proven conclusively. However, it may possible due to presence of numerous sensory papillae on their anterior region of the body.
The seasonal occurrence or infection, behaviours and hostspecificity of diverse trematode larvae are well studied [40-43]. The occurrence of these larvae is greatly varied season to season and species to species. In general, the maximum infection of trematode larvae occurs in the post monsoon and pre-winter seasons. But some species of trematode larvae in snails are found throughout the year. In some cases, some species of trematode larvae infect more than one host snail species. It has also been reported that one snail species can harbour more than one species of trematode larvae. However, most of the trematode larvae are host-specific and restricted to specific host snail species [43].
Parasitic Castration
The term “castration” is generally used in medical and veterinary practices for the sterilization or removing of testicles or male gonad of man and animals, respectively. Sometime this term also used for removing the female gonad or ovary. Castration process may be a surgical or chemical but ultimately aim of castration is the sterilization which greatly reduces the fecundity of reproduction or production of sex hormones, testosterone and estrogen. Its purpose is only control of overpopulation. But it can also lead to population imbalance. During the evolution, a unique kind of biological castration process is evolved in many species of invertebrate animals which is natural and performed by a small organism, parasite (castrator). Due to physical presence of a parasite causes partial or complete removal of gonads from the host animal or inhibition of gametogenesis and output of reproduction [44]. This biological process is generally known as “parasitic castration”. In fact, parasitic castration is an infectious strategy that requires the eventual intensity-independent elimination of host reproduction as the primary means of acquiring energy [45]. In other words, a single individual parasitic castrator will prevent or block host reproduction when the parasite matures. Such type of parasitic castration is commonly found in most of the fast breeder aquatic animals such as gastropod molluscs or snails [46-54], bivalves [55-57] and number of crustaceans and other invertebrate animals [58-60]. Interesting, besides the sterilization of snail host, parasitic castration also induces the growth of young snail host called gigantism [58,61], sex reversal [62] and potential to alter the gene expression in brain of the snail host also [63].
Mode of Parasitic Castration
Most of the studies on parasitic castration have been performed in freshwater prosobranch gastropod snails in which sexes are separated. But in hermaphrodite pulmonate snails such studies are still scanty [54]. In the freshwater pulmonate hermaphrodite snail species only single gonad is found, called “ovotestis”, which contains both testicular and ovarian tissues. In general, hermaphroditism can convey a selective advantage to an individual by increasing its reproductive potential relative to non-transforming members of the population. This is because age-specific fecundity in many populations is not distributed in the same way for males and females [64]. But evolutionary and origin point of view the significance of formation of ovotestis gonad in these monoecious snail species is not yet clear. However, it may be a specific adaptation and requirement for their better survival and the conservation of species as well.
Interesting, the basic histological architecture of both ovotestis and hepatopancreas (digestive gland) of snails is almost similar whose basic units are acini and digestive gland tubules (Figure 2a), respectively. Both organs are glandular and contain ample amount of reserve food nutrients. Hence, these organs are generally infected by trematode larvae for their development and asexual multiplication. According to the recent study [54] conducted in mature and healthy freshwater snail, Melanoides tuberculatus (correct name is Lymnaea acuminata), ovotestis is found to be closely associated with posterior region of hepatopancreas. Based on histological observations, ovotestis basically consists of numerous oval or pear-shaped acini embedded in the stroma of loose fine inter-acinar connective tissues. Each acinus composed of two distinct unequal regions, the larger testicular region comprising of various stages of spermatogenesis and the smaller one with the different stages of oogenesis or stages of female germ cells and having a single prominent oocyte (Figures 2a and b). However, in general, both the regions are lined by germinal epithelium. Oocytes are mostly found towards the periphery of ovotestis enclosed by thin membrane called tunica propria. This basic histological structure of ovotesis has been observed and studied by using of simple compound microscope. In this study, nutritive cells or sertoli cells have been traced out as these are important cells to continuous supply of nutrients or food energy to germ cells for their growth and differentiation. These cells have also been detected in the testis of dioecious freshwater prosobranch gastropod snail (Bithynia tentaculut) [46]. In other study, conducted in a hermaphrodite terrestrial pulmonate snail, Macrochlamys indica, sertoli cells have also been detected and reported only in young snails [65]. However, to know the exact cellular or histological architecture and details of gametogenesis in ovotestis, ultrastructural studies in different pulmonate snail species are highly suggestive.
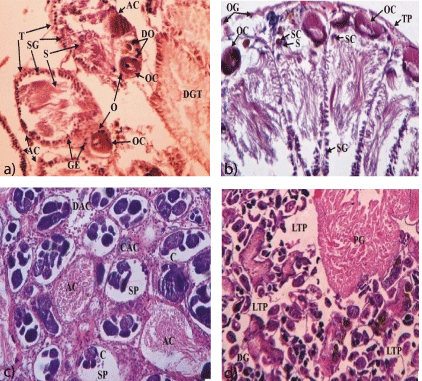
Figure 2: Junction of ovotestis with acini and digestive gland lobes/tubules
of mature and healthy aquatic monoecious pulmonate snail, M. Tuberculata
(correct name is L. acuminata) (Figure a, H&E x 100). Enlarged portion of
acinus showing male germ and female germ cells (Figure b, H&E x 100).
Moderate infection of trematode larvae and degenerative changes in acini
(Figure c, H&E x 100). Section showing autolytic necrosis and almost all the
acini completely replaced by trematode larvae (severe infection) or complete
parasitic castration of ovotestis (Figure d, H&E x 100).
In snails, both hepatopancreas and ovotestis contain reserve foods in the form of glycogen, lipids and proteins [66]. Therefore, both the organs are ideal niche for larval trematode parasites (castrators). In fact, these organs are good sources of continuous supply of energy for trematode larvae required in their growth and multiplication. That is why these organs are invaded by larval tremarode parthanitea. However, hepatopancreas is a common niche for wide range larval trematode species [5]. The growth and multiplication of trematode larvae generally occurred in inter-acinar connective tissues or spaces. But these larvae never invade the acini of ovotestis (Figure 2c). This similar pattern is also found in the hepatopancreas where these trematode larvae occupied the spaces of inter-digestive gland tubules.
Parasitic castration by parasitic castrators (trematode larvae) in aquatic snails has been studied by several workers [46-54] and reported that degenerative changes in the gonads are the result of pressure effects from the trematode larvae combined with the toxic effects of parasitic excreta. But other workers suggest that mechanical damages in ovotestis such as rapture of tunica properia and destruction or disorganization of acini and inter-acinar connective tissues are the resultant of cumulative effect due to migration, feeding and multiplication (parasitemea) of castrator trematode larvae. But the size and types of trematode larvae are also important factors to create a mechanical pressure for damaging of tissues of ovotestis. Besides these histopathological changes or damages, another type of damages such as starvation autolysis and necrosis of acini and inter-acinar connective tissues (Figure 2d) are also found only in the severely infected ovotestis with trematode larvae. In fact, these are physiological damages in the gonads which are overlooked by researchers. However, these physiological changes are well studied histologically and histochemically in heapatopancreas of freshwater snails infected with diverse trematode larval parasites [66- 68]. These studies revealed that starvation autolysis and necrosis are the resultant of release of proteolytic enzymes from the ruptured epithelial cells of digestive gland tubules and enzymatic secretion and metabolic excretion from larval trematode parasites as well [14]. Starvation autolysis and necrosis are possible in the germinal tissues of ovotestis due to squeezing of acini (Figure 2d) at different points and consequently no food can pass into these acini. Nevertheless, the magnitude of these pathological changes in gonadial tissues is much more depending on the degree of parasitaemia. However, these histopathological changes are found more severe in gonad infected with rediae rather than with sporocysts. Because of these larvae have relatively a large sized body and locomotary orgons (lappets). Another reason, redia larva has well developed mouth, pharynx, pharyngeal glands and elongated blind gut. It has also been observed that rediae engulf the host’s cells and utilize the hydrolases [39, 66] for extra cellular digestion. Thus it can be conjectured that redial stages are more destructive than other stages for the host tissues. Besides this, other contributory factors can be parasitic secretions and excretory products that produce toxic effects [14]. Such histopathological changes or damages, mechanical and physiological in hepatopancreas of snails infected with larval trematode parasites are also well studied [69]. However, to know more about the physiological or histopathological changes in parasitized ovotestis or gonads of snails, histochemical and ultrastructural studies are more useful.
Contribution of Parasitic Castration
In humans and domestic animals, castration or sterilization is the most effective way for the control of their overpopulation. But in this way there is a high risk of population imbalance. The parasitic castration in invertebrate animals is a perfect and the most ideal natural way for the control of their overpopulation, such as in diverse fast breeding vector snail species. The various species of snails found in any aquatic habitat are not infected by trematode larvae all together, but they are infected by these larvae of different species of digenean trematode parasites at different seasons [6,40]. It is also not necessary that the parasitic trematode larvae infect only the reproductive organs, gonads. These parasites also infect the hepatopancrease of snails. Most of the snails often lose their reproductive capacity due to parasitic castrations, which have a profound effect on their population and begin to decline. This keeps the population of these different species of vector snails of any aquatic ecosystem under control. This not only reduces the conflict or competition between different species of snails for food and space or shelter, but also maintains the ecological balance in aquatic ecosystem. With the increase in the population of snails, there is also the possibility of threatening the existence of other fauna and flora present in the water. Water quality is also maintained by having a balanced population of snails. Therefore, the parasitic castration of snails proves beneficial to the aquatic ecosystem and its fauna and flora in many ways.
It is well documented the snails are intermediate hosts of wide range of pathogenic digenean trematode parasites of man and animals. These snails harbour their various larval stages. These trematode larvae are also host-specific. Therefore, snails harbour only larvae of specific species of trematode parasites. Therefore, these vector snails spread a specific trematodiasis in man and animals. Nevertheless, the prevalence and spreading of trematodiasis are much more depending on the population of vector snail species. It is evidently clear that parasitic castration keeps the overpopulation and density of snails under control, which also reduces the risk of spreading trematodiases. In any case, the generation of parasitic castration is helpful or beneficial not only in controlling the population of vector snails and trematodiases, but also in keeping the aquatic ecosystem healthy and strong.
Conclusions
Aquatic snails are intermediate hosts of digenean trematode parasites and complete their life cycle. In snails, various larvae (sporocyst, redia, cercaria and rarely metacercaria) of these parasites developed and multiplied in the nutritionally rich reproductive organs, gonads. These larvae also act as castrators and block the reproduction by mechanical and physiological damages in gonads of aquatic snails. This is also known as parasitic castration. This phenomenon is an infectious strategy that requires the eventual intensity-independent elimination of host reproduction as the primary means of acquiring energy. Parasitic castration is also induces gigantism, alteration the gene expression in brain and sex reversion in snail hosts. Parasitic castration has significant contributions in controlling of density and overpopulation of diverse vector snail species. Due to which the risk of spreading trematodiases in humans and animals automatically decreases, while inter and intra-specific competition for food and space is also reduced in aquatic ecosystem. Thus parasitic castration maintains the balance and strength of the ecosystem of aquatic habitats. To know the exact mechanism involve in physiological damages of ovotestis, histochemical and ultrastructure studies are ideal. To determine the effect of parasitic castration on host snail population dynamics, reduction in spreading of trematodiases and competition between inter and intra-specific for food and space and ecological balance of aquatic habitats considerable field and experimental research studies are suggestive.
References
- Rao NVS. Handbook freshwater molluscs of India. Zoological Survey of India, Calcutta, 1989.
- Yusa Y, Sugiura, N, Wada T. Predatory potential of freshwater animals on an invasive agricultural pest, the Apple Snail Pomacea canaliculata (Gastropoda: Ampullariidae), in Southern Japan. Biol Invasions. 2006; 8: 137-147.
- Atwal AS, Dhaliwal GS. Birds, mammals, snails and slugs. Agricultural pests of South Asia and their management. Kalyani Publishers, 2010.
- Baker P, Zimmanck F, Baker SM. Feeding rates of an introduced freshwater gastropod Pomacea insularum on native and nonindigenous aquatic plants in Florida. J Mollusc Stud. 2010; 76: 138-143.
- Choubisa SL. The biology of certain larval trematodes infecting freshwater snails of lakes of Udaipur. Ph.D. thesis, Mohanlal Sukhadia University, Udaipur, Rajasthan, India, 1984.
- Choubisa SL, Sharma PN. Incidence of larval trematodes infection and their seasonal variation in the fresh water molluscs of southern Rajasthan, Records Zool Surv India. 1986; 83: 69-80.
- Choubisa SL. Snail hosts of larval trematodes in Southern Rajasthan. Indian J Parasit. 1991; 15: 49-51.
- Choubisa SL. Study of larval trematode parasites infecting freshwater snails of southern Rajasthan (Technical report), University Grants Commission, New Delhi, 2011.
- Choubisa SL, Sheikh Z. A new variety of freshwater snail, Thiara scabra var. choubisai from Rajasthan, India, Cibtech J Zool. 2013; 3: 44-46.
- Sil M, Basak R, Karanth KP, Aravind NA. A new species of Pila (Gastropoda: Ampullariidae) from Mizoram, India. Mollusc Res. 2021; 41: 204-213.
- Choubisa SL. Mollusc as bio-indicators for the trophic stages of lakes and lotic environments. Bull Pure Appli Sci. 1992; 11A: 35-40.
- Choubisa SL. Snails as bio-indicators for dreaded trematodiasis diseases. The Journal of communicable diseases. 2010; 42: 223-6.
- Choubisa SL, Sheikh Z. Freshwater snails (Mollusca: Gastropoda) as bioindicators for diverse ecological aquatic habitats. Cibtech J Zool. 2013; 2: 22-26.
- Erasmus DA. The Biology of Trematodes, Printed in Great Britain at the Universities Press. Belfast. 1972.
- Cheng TC. General Parasitology, Academic Press, New York and London, 1973.
- Murty AS. Life cycle of Pseudodiplodiscoides pilai (Trematoda: Diplodiscidae) from the gut of the apple snail, Pila globosa (Swainson). J Parasitol. 1973; 59: 323-332.
- Mohandas A. Studies of the freshwater cercariae of Kerala I. Incidence of infection and seasonal variation. Folia parasitologica. 1974; 21: 311-7.
- Jain SP. Studies on amphistomes II. A survey of incidence and nature of amphistome infection in aquatic snail. Agra Univ J Res (Sci). 1976; 25: 81-89.
- Pandey KC, Agrawal N. Studies on cercarial fauna of Kathauta Tal. Lucknow. Indian J Zool. 1977; 18: 1-50.
- Pandey KC, Agrawal N. Larval trematodes and seasonal variations in snails of Kathauta Tal. Lucknow. Indian J Parasit. 1978; 2: 139-143.
- Sharma PN, Choubisa SL. Cercaria udaipuriensis n. sp. from fresh water snails, Vivipara bengalensis from Fateh Sagar Lake. Indian J Parasitol. 1983; 7: 209-212.
- Choubisa SL. A gymnocephalous cercaria, Cercaria Johrii n. sp. from fresh water snail, Melanoides tuberculatus (Muller) of Fateh Sagar Lake, Udaipur (Rajasthan). Indian J Parasitol. 1985; 9: 245-247.
- Choubisa SL. Cercaria gurayai, n. sp. (furcocercaria) from the fresh water snail Faunus atter (Linnaeus). Records Zool Surv India. 1990; 87: 267-271.
- Choubisa SL. On a rare cercaria, Cercaria udaipuriensis II n. sp. From the fresh water snail, Melanoides tuberculatus (Muller). Bio-Sci Res Bull. 1992; 8: 13-16.
- Choubisa SL. Focus on pathogenic trematode cercariae infecting fresh water snails (Mollusca: Gastropoda) of tribal region of southern Rajasthan (India). J Parasit Dis. 2008; 32: 47-55.
- Choubisa SL, Sharma SL. Cercaria tewarii n. sp. (Echinostomatid cercaria) from fresh water snail, Indoplanorbis exustus (Deshayes). Bio-Sci Res Bull 1985; 1: 50-53.
- Sanil NK, Janardanan KP. Two new species of xiphidiocercariae from the apple snail Pila virens in Malabar, Kerala. Journal of Parasitic Diseases. 2016; 40: 1614-1619.
- Rajendran KV, Janardanan KP. Studies on the life cycle of Tremiorchis ranarum Mehra and Negi, 1926. J Helminth. 1993; 67: 95-101.
- Choubisa SL, Sheikh Z. A rare trematode sporocyst from freshwater snail, Malanoides tuberculatus (Miller 1722). Cibtech J Zool. 2013; 2: 6-9.
- Sanil NK, Janardanan KP. Two new species of xiphidiocercariae from the apple snail Pila virens in Malabar, Kerala. Journal of Parasitic Diseases. 2016; 40: 1614-1619.
- Choubisa SL, Jaroli VJ, Sheikh Z. First record of a rare transversotrematid cercaria larva (Trematoda: Digenea) from Rajasthan, India: focus on seasonal occurrence and host-specificity of diverse cercariae. Journal of Parasitic Diseases. 2016; 41: 496-502.
- Choubisa SL, Jaroli VJ. Freshwater larval digenetic trematode parasites in India: an epitomised review. Res J Chem Environ 2020; 24: 146-156.
- Choubisa SL, Sharma PN. Histochemical demonstration of cholinesterase in the nervous system of stregeoid metacercaria, Tetracotyle lymnaei. Indian J Parasit. 1983; 7: 217-219.
- Sharma PN, Choubisa SL. Histochemical demonstration of hydrolytic enzymes in two species of cercariae and in radia. Indian J Parasit. 1985; 9: 153-154.
- Choubisa SL. Histochemical demonstration of esterase in certain freshwater larval trematodes with a note on neuroanatomy. Proceedings: Anim Sci. 1986; 95: 623-628.
- Choubisa SL. Neuroanatomy of furcocercous, Cercaria milleri. Curr Sci. 1988; 57: 402-404.
- Choubisa SL. In-vitro culture of echinostome cercaria Cercaria tewarii (Choubisa and Sharma 1985) from the metacercaria to vitellogenous stage. Indian J Parasit. 1988; 12: 123-128.
- Choubisa SL. Distribution of non-specific esterase in certain larval digeneans with a note on morphology of nervous system. Indian J Exp Biol. 1989; 27: 32-57.
- Choubisa SL. Mode of nutrition in pathogenic trematode larvae (redia and cercaria) which infect hepatopancreas of fresh water snails (Mollusca: Gastropoda). J Parasit Dis. 2008; 32: 68-73.
- Choubisa SL, Sharma PN. Seasonal variations of cercarial infection in snails of Fateh Sagar Lake of Udaipur. Indian J Parasit. 1983; 7: 111-113.
- Choubisa SL. Comparative study on cercarial behaviours and their host specificity. Indian J Parasit. 1991; 15: 125-128.
- Choubisa SL. Seasonal variation of amphistome cercarial infection in snails of Dungarpur district (Rajasthan). J Parasit Dis. 1997; 21: 197-198.
- Choubisa SL. Focus on seasonal occurrence of larval trematode (cercarial) parasites and their host specificity. J Parasit Dis. 2002; 26: 72-74.
- Cheng TC, Howland KH, Moran HJ, Sullivan JT. Studies on parasitic castration: Aminopeptidase activity levels and protein concentrations in Ilyanassa obsoleta (mollusca) parasitized by larval trematodes. Journal of Invertebrate Pathology. 1983; 42: 42-50.
- Lafferty KD, Kuris AM. Trophic strategies, animal diversity and body size. Trends Ecol Evol. 2002; 17: 507-513.
- Reader TAJ. Histological and ultrastructural studies on the testis of Bithynia tentaculata (Mollusca: Gastropoda), and on the effects of Cercaria helvetica XII (Trematoda: Digenea) on this host organ*. Journal of Zoology. 2009; 171: 541-561.
- Sullivan J, Cheng TC, Howland K. Studies on parasitic castration: castration of Ilyanassa obsoleta (Mollusca: Gastropoda) by several marine trematodes. Transact American Microscop Soc. 1985; 104: 154-171.
- Oliva M. Parasitic castration in Fissurella crassa (Archaeogastropoda) due to an adult digenea, Proctoeces lintoni (Fellodistomidae). Memorias do Instituto Oswaldo Cruz. 1992; 87: 37-42.
- Lafferty KD. Effects of parasitic castration on growth reproduction and population dynamics of the marine snail Cerithidea californica. Marine Ecology Progress Series. 1993; 96: 229-237.
- Oliva M, Olivares A, Diaz C, Pasten M. Parasitic castration in Concholepas concholepas (Gastropoda: Muricidae) due to a larval digenean in northern Chile. Dis Aquat Organ. 1999; 36: 61-65.
- Tétreault F, Himmelman JH, Measures L. Impact of a castrating trematode, Neophasis sp., on the common whelk, Buccinum undatum, in the northern Gulf of St. Lawrence. The Biological bulletin. 2000; 198: 261-271.
- Fredensborg BL, Mouritsen KN, Poulin R. Impact of trematodes on host survival and population density in the intertidal gastropod Zeacumantus subcarinatus. Mari Ecol Progr Seri. 2005; 290: 109-117.
- Averbuj A, Cremonte F. Parasitic castration of Buccinanops cochlidium (Gastropoda: Nassariidae) caused by a lepocreadiid digenean in San José Gulf, Argentina. Journal of Helminthology. 2010; 84: 381-389.
- Choubisa SL, Sheikh Z. Parasitic Castration in Freshwater Snail Melanoides tuberculatus (Mollusca: Gastropoda). Proceedings of the National Academy of Sciences, India Section B: Biological Sciences. 2012; 83: 193-197.
- Valderrama K, Oliva M, Campos B, Brown D. Parasitic castration of Eurhomalea lenticularis (Bivalvia: Veneridae) by a digenetic trematode: quantitative histological analysis. Diseases of Aquatic Organisms. 2004; 59: 151-158.
- Lajtner J, Lucic A, Marušic M, Erben R. The effects of the trematode Bucephalus polymorphus on the reproductive cycle of the zebra mussel Dreissena polymorpha in the Drava River. Acta Parasitol. 2008; 53: 85-92.
- Yee-Duarte JA, Ceballos-Vázquez BP, Shumilin E, Kidd KA, Arellano- Martínez M. Parasitic castration of chocolate clam Megapitaria squalida (Sowerby, 1835) caused by trematode larvae. J Shellf Res. 2017; 36: 593- 599.
- Hall SR, Becker C, Cáceres CE. Parasitic castration: a perspective from a model of dynamic energy budgets. Integrative and comparative biology. 2007; 47: 295-309.
- Lafferty KD, Kuris AM. Parasitic castration: the evolution and ecology of body snatchers. Trends in parasitology. 2009; 25: 564-572.
- Sivajothi S, Reddy BS. A review on parasitic castration in veterinary parasitology. J Entomol Zool Stud. 2018; 6: 635-639.
- Wilson RA, Denison J. The parasitic castration and gigantism of Lymnaea truncatula infected with the larval stages of Fasciola hepatica. Zeitschrift für Parasitenkunde. 2004; 61: 109-119.
- Reader TAJ. The biology of larval trematodes in Bithynia tentacufata (Mollusca: Gastropoda). M.Sc. Thesis, University of London, 1970.
- Rice T, McGraw E, O’Brien EK, Reverter A, Jackson DJ, Degnan BM. Parasitic castration by digenian Allopodocotyle sp. alters gene expression in brain of the host mollusc Haliotis asinine. FEBS Letters. 2006; 580: 3769- 3774.
- Warner RR. The adaptive significance of sequential hermaphroditism in animals. American Natural. 1975; 109: 61-82.
- Roy S, Chaki KK, Nag TC, Misra KK. Ultrastructure of ovotestis of young and adult pulmonate mollusk, Macrochlamys indica Benson, 1832. Journal of Microscopy and Ultrastructure. 2016; 4:184.
- Choubisa SL. Histological and histochemical observations on the digestive gland of Melanoides tuberculatus (Gastropoda) infected with certain larval trematodes and focus on their mode of nutrition. Proceedings: Animal Sciences. 1988; 97: 251-262.
- Choubisa SL. Histopathological observations on the digestive gland of Lymnaea auricularia infected with larval trematodes. Proceedings: Animal Sciences. 1990; 99: 363-368.
- Choubisa SL. Focus on histopathogenesis of trematode larvae. J Parasit Dis. 1998; 22: 57-59.
- Choubisa SL, Sheikh Z, Jaroli VJ. Histopathological effects of larval trematodes on the digestive gland of freshwater snail species, Vivipara bengalensis and Lymnaea acuminata. Journal of Parasitic Diseases. 2012; 36: 283-286.