
Mini Review
J Plant Chem and Ecophysiol. 2017; 2(1): 1015.
Bacterial Wetwood Disease
Alizadeh M*
Department of Plant Protection, University of Tabriz, Iran
*Corresponding author: Mehrdad Alizadeh, Department of Plant Protection, University of Tabriz, Tabriz, 29 bahman Blvd, 51368, Iran
Received: April 19, 2017; Accepted: September 18, 2017; Published: September 25, 2017
Abstract
Wetwood, slime flux, oozing slime, or alcoholic flux all are different names of one bacterial disease in which the infected tissues (woods) are frequently discolored or water-soaked. Different from gases are produced by bacteria with fermentation action. These gases produce pressure within the tree and this pressure force saps from the trunks and branches through cracks, pruning or lawn mower wounds, and other injured parts. Thereby, slime flux is oozing of sap on the surface of tree. Despite reported different causal agents for this disease, there isn’t specific pathogen for pathogenicity and the information is incomplete. Furthermore, there isn’t any method to control wetwood disease. The main objective of this mini review is brief illustration about wetwood disease characteristics.
Keywords: Wetwood; Slime flux; Water-soaked; Soap; Gas
Introduction
Among the diseases of ornamental trees, wetwood is the one of the most severe diseases, epidemic all over the world. It is a widespread disease that causes disease in many trees. This disease can be seen in wide range of shady and forest trees including apple, elm, plane tree, spruce, berry, London plane, acacia, aspen, dogwood magnolia, Russian olive, beech, fir, maple, sour gum, birch, hemlock, mountain ash, sycamore, box elder, hickory, mulberry, sweet gum, butternut, horsechestnut, oak, tulip tree, cottonwood, linden, pine, walnut, crabapple, locust, poplar, willow and gymnosperms [1,2]. The symptoms are observed in different parts of trees including trunks, branches, roots and leaves. This disease is most easily diagnosed by the existence of a liquid or sap that oozes or bleeds from cracks, frost cracks, wounds, crotches, pruning and lawn mower wounds, branch stubs and other injured parts or other weak points in the wood and bark of trunks and branches (Figure 1 & Figure 2).
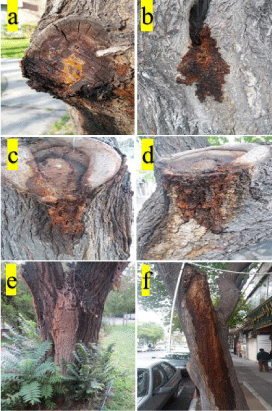
Figure 1: Oozing or bleeding of wetwood; a-f: on elm trees.
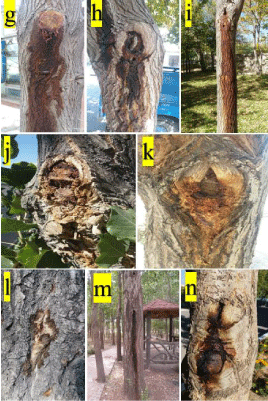
Figure 2: Oozing or bleeding of wetwood; g-i: on not fruit berry trees; j-m: on
poplar trees, n: on ash tree.
As the liquids or saps flow down the surface of the bark, some vertical dark or light or yellowish or brownish streaks remain. The symptoms on leaves are forms of chlorosis that change over time to necrotic areas. Symptoms in the inner part of the trunk, big branches, and roots appear as dark brown to black areas (Figure 3). These symptoms can be seen in complete or incomplete circles in crosssection [3].

Figure 3: o-p: Dark brown to black discoloration of the wood in the central
core of the branch and trunk on elm trees.
Description of Wetwood Disease
Most symptoms of wetwood is limited to the central parts and associated with color changing, but in the contaminated branches it can develop even to cambium. On 15-year-old to 30-year-old elms, symptoms are just observed on the top of the crown [4]. Wetwood discharges, most of the time accompanying gas producing. The gases and liquids produced by bacteria fermentation lead to increasing inner pressure of sap which in turn pours on the bark through pores and wound. The bacteria grow within the infected tree using the sap as a main nutrient source with sufficient elements, such as species belonging to genus Clostridium, Bacillus, Enterobacter, Klebsiella, and Pseudomonas. Using the sap, heartwood is depleted from oxygen (making anaerobic conditions), methane is produced, the pH of the sap is increased (pH 7 to 8 in infected trees vs. pH 6 in healthy trees), and a high pressure is progressed in the infected-wood (60 psi in infected trees vs. 5-10 psi in healthy trees) [3]. Methane production by anaerobic bacteria in the red oaks is the best indicator for wetwood disease [5]. Sap leaking and symptoms emerging revealed in spring, autumn and cool days and stopped in hot days during the summer. However, the temperature range of symptom appearance may vary in the different areas of the world. The bacterial (facultative anaerobes) of wetwood disease are soil-inhabiting and they are absorbed by roots in tree via the water in xylem vessels (transpiration stream or xylem elements) where these bacteria in sapwood and heartwood (central cores of tree) by production and releasing pectolytic enzymes (pectolases) assail to the middle lamellae between wood cells and fibers in the parts of main branch or trunk [6]. The structural integrity of the wood in the main trunk and brunch damage by these pectolases and so this process by enzymes cause radial and lateral separations of the parts of wood fibers [6]. Thereby, wetwood is a serious obstacle in the production of industrial products in forests and it causes drying and cracking, so the timbers lose their values [7].
The causal agents of bacteria that cause wetwood, penetrate through wounds, pores, and crack on trunk and branches. Common causative agents of wounds are lawn mower and the equipment & apparatus of Pruning. Studied have explained that trees contamination by bacteria are more acceptable through root [8]. Since the most of bacteria live in aquatic and soil habitats, this can be concluded that contamination occurs through root. However, initial bacterial infection occurs in young branches which age less than 10 years. Bacterial infection on root tissue is not observed in initial phases [9].
The bacteria associated with wetwood is commonly found in aquatic and soil habitats. Possibly, infection occurs in anaerobic condition, which this condition is developed in soil sphere and elm hurt tissue [3]. These bacteria destroy the internal tissues of trees by fermentation action, thereby the movement paths of nutrients is blocked, making it susceptible to attack by saprophytes and opportunist pathogens. The color of the exudates leaking out from the tree changes under the effect of saprophytes [10].
History of Wetwood
First report of wetwood is related to Carter (1945) from United States according which the causal agent of disease belongs to Erwinia or Lellittia and claimed that this disease is caused by just one bacterium that is Lelliottianimipressuralis species [11], furthermore in 1961 and 1977, some authors claimed that bacteria are the main causal agents of wetwood. These studies about wetwood have determined that isolated bacteria are airborne [8,12]. On 1971 and 1972, genus clostridium, which forms endospore, isolated from wetwood [7,13]. In 1977, different group of bacteria including Xanthomonas, Agrobacterium, Acinetobacter, Corynebacterium, and Erwinia were isolated from tissues infected by wetwood [14]. Several main bacteria are found as the causal agents in wetwood disease, such as Enterobactercloacae, Lelliottianimipressuralis, Bacillus metaterium and Pseudomonasfluorescence. Enterobacter cloacae are primarily associated with wetwood [15]. In 1981 Murdock explained that bacterial agents of wetwood can be opportunist bacteria of humans [9]. On 1981, research showed different population of bacteria observed in wetwood on spruce, willow and elm [16].
In 1983 presence of high population of bacteria has been reported on wetwood, however, it was believed that the infectious source is not clear [3]. Furthermore, different species of bacteria have been founded associated with wetwood disease, such as Enterobacter cloacae, Enterobacter agglomerans, Klebsiellaoxytoca, Serratiafonticola, Bacillus megaterium, Pseudomonas fluorescens, Streptococcus mitis, Pseudomonas spp., Staphylococcus spp. and Acinetobacter spp. [3]. Furthermore, based on these results, it was realized that the species of B. megaterium were the most prevalent isolated bacterium from near area of wetwood disease. These results had proved that don’t support Carter’s result, and only one bacterium cannot cause wetwood, but a population of bacteria and yeasts may cause wetwood symptoms in elms [3]. In 1986 Jonson and Jerry expressed that, genera Agrobacterium, Acinetobacter, Bacillus, Clostridia, Pseudomonas, and Xanthomonas are isolated from infected tissues and may play a role in wetwood disease [10]. To more accurate identification of wetwood causative agent, the DNA of some of isolated bacteria by alkalinelysis method replicated partial of 16S rDNA gene by general primers 63F and 1387R, which showed these primers are really useful in the identification of those bacteria [17]. Lelliottianimipressuralis that claimed to be the main causal agent for wetwood, was isolated from infected tissues in wetwood disease from elm trees in Iran [18].
In some cases larva and adults of insects, such as various flies and beetles, commonly visit on the oozing slime and feed on this sap or liquid. Larval stages may develop and complete your life cycles within this saps or liquids. In the 2016 year, the existence of wetwood disease has been reported with associated nematodes including Panagrellusulmi and Panagrolaimusrigidus from Tabriz, Iran [19]. Recently, the epidemiological assessment of wetwood disease has been carried out with Geographic Information System (GIS) in the different regions of Tabriz. Thereby, the results showed that the wetwood disease has out broken in the most of elm trees and it was prevalent in 80 percent of Tabriz city areas [20].
Disease Management
There are not control method(s) or chemical treatments for wetwood disease. Infected trees will usually remain living for many years, but these trees may grow weak and we might need to remove them [21]. Avoiding damage and stress to the roots and stem of trees is the best method to prevent serious wetwood problems and infections. Drought conditions can increase wetwood damages, especially during spring and summer seasons, so it is essential that these trees receive adequate and appropriate water [22]. So, watering during the growing and winter season is properly critical [23]. When these trees showed nutrient deficiencies, fertilizing of infected trees is recommended [4]. Proper pruning techniques help to prevent disease spread to healthy trees [20].
Conclusion
Despite the existence of this disease in different trees, there isn’t perfect information about causal agents, dispersal of disease and pathogens, precise host ranges, transmission modes or vectors, relation with other organisms, isolation methods, culture media, bacterial isolation methods and etc. According to different information and reports from universities, wetwood disease is increasing in ornamental trees and other trees. To the best of our knowledge, identification and diagnosis of pathogen(s) are very essential and significant in stopping wetwood. The copper based sprayers may be effective in the reduction of bacteria population and control this disease.
References
- Mohan SK, Colt WM, Barney DL. Bacterial wetwood and slime flux of trees. Technical Sheet of University of Idaho. 1990.
- Nancy RP. Bacterial wetwood and slime flux of landscape trees. Unvesity of Illinois. 1999.
- Murdoch CW, Campan RG. Bacterial species associated with wetwwod of elm. Phytopathology. 1983; 73: 1270-1273.
- Hamilton WD. Wetwood and slime flux in landscape trees. Journal of Arboriculture. 1980; 6: 247-249.
- Xu Z, Leininger TD, Lee AW, Tainter FH. Chemical properties associated with bacterial wetwood in red oaks. Wood and fiber science. 2007; 33: 76-83.
- Wilson AD. Bacterial wetwood detection in Fagus grandifolia and Prunus serotina sapwood using a conducting polymer electronic-nose device. The fifth international conference on sensor device technologies and applications. 2014.
- Ward JC. Anaerobic bacteria associated with honeycomb and ring failure in red and black oak lumber. Phytopathology. 1972; 62: 796-796.
- Hillis WE. Secondary changes in wood. In The structure, biosynthesis, and degradation of wood. 1977; 247-309.
- Murdoch C. Bacterial wetwood in elm (Doctoral dissertation, University of Maine). 1981.
- Johnson DW, Jerry WR. Wetwood (Slime Flux) of Elm, Cottonwood, and Mulberry. Diseases of Trees in the Great Plains. 1986; 2: 64.
- Brady C, Cleenwerck I, Venter S, Coutinho T, De Vos P. Taxonomic evaluation of the genus Enterobacter based on multilocus sequence analysis (MLSA): proposal to reclassify E. nimipressuralis and E. amnigenus into Lelliottia gen. nov. as Lelliottia nimipressuralis comb. nov. and Lelliottia amnigena comb. nov., respectively, E. gergoviae and E. pyrinus into Pluralibacter gen. nov. as Pluralibacter gergoviae comb. nov. and Pluralibacter pyrinus comb. nov., respectively, E. cowanii, E. radicincitans, E. oryzae and E. arachidis into Kosakonia gen. nov.as ....Systematic and applied microbiology. 2013; 36: 309-319.
- Hartley CCP, Davidson RW, Crandall BS. Wetwood, bacteria, and increased pH in trees. 1961.
- Shigo AL, Stankewi J, Cosenza BJ. Clostridium sp. associated with discolored tissues in living oaks. Phytopathology. 1971; 61: 122.
- Tiedemann G, Bauch J, Bock E. Occurrence and significance of bacteria in living trees of Populusnigra L. European Journal of Forest Pathology. 1977; 7: 364-374.
- Stipes RJ, Campana RJ. Compendium of elm diseases. American Phytopathological Society. 1981; 96.
- Schink B, Ward JC, Zeikus JG. Microbiology of wetwood: role of anaerobic bacterial populations in living trees. Microbiology. 1981; 123: 313-322.
- Marchesi JR, Sato T, Weightman AJ, Martin TA, Fry JC, Hiom SJ, et al. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Applied and environmental microbiology. 1998; 64: 795-799.
- Khodaygan P, Sedaghati E, Sherafati F. Isolation of Enterobacter nimipressuralis associated with bacterial wetwood from elm (Ulmus spp.) in Rafsanjan. Iranian Journal of Plant Pathology. 2012; 47: 163.
- Abolafia J, Alizadeh M, Khakvar R. Description of Panagrellus ulmi sp. n. (Rhabditida, Panagrolaimidae) from Iran, and comments on the species of the genus and its relatives. Zootaxa. 2016; 4162: 245-267.
- Alizadeh M, Moharrami M, Rasouli AA. Geographic Information System (GIS) as a tool in the epidemiological assessment of wetwood disease on elm trees in Tabriz city, Iran. Cercetări Agronomice în Moldova. 2017; 50: 91-100.
- Olsen MW. Diseases of urban plants in Arizona. 1999.
- Fance G. Bacterial wetwood slime flux. University of Wyoming. 1994.
- Henn A. The Plant Doctor: Bacterial wetwood and alcoholic flux. Mississippi State University. 2007.