
Research Article
Austin J Mol & Cell Biol. 2015; 2(1): 1006.
Nitrate Enhances Microcystin-LR Induced Toxicity in Mice
Lone Y, Koiri RK* and Bhide M
Department of Zoology, Dr. Harisingh Gour Central University, India
*Corresponding author: Koiri RK, Department of Zoology, Dr. Harisingh Gour Central University, Sagar, Madhya Pradesh – 470003, India
Received: October 31, 2015; Accepted: November 16, 2015; Published: November 18, 2015
Abstract
The toxic effects of cyanotoxins and minerals have been explored in different species, but still there is lack of information regarding their combined toxicological effects. The present investigation reports glycolytic-oxidative-nitrosative status induced by intraperitoneal exposure of Microcystin-LR (10 mg/kg bw) alone and in combination with oral exposure of sodium nitrate (50 mg/kg bw) for 14 days in mice. Microcystin-LR (MC–LR) exposure produced significant (p<0.005) elevation in Malondialdehyde (MDA), Lactate Dehydrogenase (LDH) and Nitric Oxide (NO) in heart, kidney and spleen of mice. Combined exposure to MC-LR and sodium nitrate produced severe effects with an appreciably more prominent elevation in extent of LPO, LDH and NO in the tissues of mice. The study clearly indicates that a cocktail of MC-LR and sodium nitrate induce a cascade of reaction in the exposed animals, thereby augmenting the toxicological damage. Therefore, this is an indication that the interaction of these toxicants in nature could be responsible for aggravating their toxic potential in livestock.
Keywords: Microcystins; Microcystin-LR (MC-LR); Lactate dehydrogenase; Lipid peroxidation
Abbreviations
EDTA: Ethylene Diamine Tetraacetic Acid; H2O2: Hydrogen Peroxide; LDH: Lactate Dehydrogenase; LPO: Lipid Peroxidation; MC-LR: Microcystin-LR; MDA: Malondialdehyde; ROS: Reactive Oxygen Species
Introduction
Toxic cyanobacteria represent a serious public health and ecological problem in drinking and recreational waters worldwide [1]. Cyanobacteria produce a range of bioactive and toxic metabolites, among them microcystins are most widely studied [2]. Special attention has been drawn to MC-LR because of its ability to cause acute poisoning and having cancer promoting potential in humans exposed to low concentrations of MC-LR in drinking water. Therefore, the World Health Organization has set that the provisional guideline value of MC-LR and nitrate in drinking water as 1 μg/l and 10 mg/l respectively [3,4]. The concentration of MCs in many water bodies is far beyond that guideline, e.g., in Sagar lake water (India) MC-LR and nitrate was found to be 0.67 μg/ml and 560 mg/L respectively [5,6]. Liver is the primary target organ of MC-LR toxicity as a result of Protein Phosphatase 1 (PP1) and 2A (PP2A) inhibition and recent evidence points to an alternative mechanism of toxicity involving oxidative damage. Several studies have found that Lipid Peroxidation (LPO) levels and Reactive Oxygen Species (ROS) increases with exposure to microcystins in different species [7-10].
Problem of nitrate has been given considerable attention in recent years due to intensive use of nitrates in agricultural fertilizers which reach to humans and animals by various routes [11,12]. Nitrate is found in soil, air, water, vegetables, food and is produced within the human body [13]. Nitrate, nitrite and MC-LR are linked environmentally through the excess of agricultural fertilizers that raises nitrogen concentrations in surface water that could contribute to cyanobacterial growth in surface water. Excessive nitrate could be a major threat to the environment and pose a potential health risk for humans and animals [14]. In fact large quantity of nitrates are known to accumulate in certain foodstuffs such as guinea corn, maize, potatoes, carrots, pumpkins, sunflower and cabbage even due to normal application of fertilizer at the rate of 150 kg/ha [11,15]. It is also used as a food preservative and antimicrobial agent [16]. The main source of nitrate intake in the human body is through food and water [17]. More than 70% of the nitrates are present in vegetables and drinking water and accounts up to 21% of total nitrates intake in a typical human diet [11,18]. Ingested nitrate is changed into nitrite, which binds to hemoglobin to form methemoglobin particularly in infants, who are susceptible to developing methemoglobinemia [19]. Nitrate is a precursor in the production of N-Nitroso Compounds (NOC), a class of genotoxic compounds, most of which are animal carcinogens [20]. Humans and animals are exposed simultaneously to more than one chemical in the environment. Such interactions may be harmful as both the kinetics and dynamics of the environmental chemicals can be modified by their co-occurrence [21].
The significance of our study is identification that the nitrite and MC-LR may have synergistic toxic effects as they co-occur in the environment. The combined effects of two or more toxins in living creatures can report a complex picture of synergistic, additive, synergistic or even antagonistic effects [22]. Clearly, given that these two toxins will co-occur in fresh water, it is important to assess the degree to which their interaction is concerned.
Here, we concentrate on a condition that may have serious impact on fresh waters [23], the concurrent effects of two main toxins that arise as end products of Microcystis blooms: dissolved nitrite, as a result of cellular degradation, and dissolved MC-LR released during cell lysis [24]. In animals and humans, concurrent exposure to MCLR and nitrate can lead to serious health problems and production of free radicals by these chemicals might be one of the contributing factors toward toxicity. Although, appropriate data are available on individual toxic effects of MC-LR and nitrate but there is a lack of information regarding their combined toxicological effects. The present research investigation was therefore conducted with the primary aim to explain the interactive effect of MC-LR and nitrate on lipid peroxidation, lactate dehydrogenase and nitric oxide in heart, kidney and spleen of mice.
Materials and Methods
Chemicals
MC-LR was purchased from Sigma-Aldrich Co., USA. Thiobarbituric acid, β-NADH (β-Nicotinamide Adeninedinucleotide, reduced), Tris, methanol, butanol, pyridine, Tris-Maleate, sodium pyruvate, sulfanilamide and naphthylethylene diamine dihydrochloride were obtained from Himedia, Mumbai, India. Pyridine and sodium nitrate were purchased from Central Drug House (P) Ltd, New Delhi. Phosphoric acid was purchased from Ranbaxy Laboratories Ltd, India.
Sampling of cyanobacteria
The cyanobacterial material used in this experiment was collected from surface blooms (phytoplankton cells) of Sagar Lake, India during May and June, 2014. Microcystis aeruginosa was predominant in the water blooms (microscopic examination) and were lyophilized for extraction of toxins.
Extraction of toxin
Lyophilized algae cells were extracted three times with 75% (v/v) methanol. The methanol extract was centrifuged, and the supernatant was applied to a C18-reversed phase cartridge, which had been preconditioned by washing with methanol and then distilled water. The extract was analyzed for MC-LR content via a reverse-phase highperformance liquid chromatography (HPLC, Waters 515, Waters Corporation, Milford, MA, USA). Crude extract concentrations were determined by comparing the peak areas of the test samples with those of the MC-LR standards. The MC-LR content in the extract was found to be 88.45 μg/ml. Microcystin extracts were finally suspended in salt solution (0.9% NaCl).
Animals
12-14 weeks old male Park mice weighing from 22 to 26 g, housed in the animal facility of Sagar Institute of Pharmaceutical Sciences, Sagar, India with a 12h:12h light–dark cycle were used for experiments. Mice were maintained at standard laboratory conditions with the supply of food and water ad libitum. This work was approved by Ethics Review Committee of Sagar Institute of Pharmaceutical Sciences, Sagar, India (ethics approval no. SIPS/EC/2015/64).
Experimental design
The mice were randomly divided into 3 groups with 4 mice in each. The second group of mice was designated as MC-LR and they were treated with MC-LR (10 μg/kg bw/day, ip) for 14 days and the third group were designated as nitrate group and were co-administered with MC-LR (10 μg/kg bw/day, ip) and sodium nitrate (50 mg/kg bw/ day, orally) for 14 days. The normal control group was also treated simultaneously with the same volume of 0.9% saline solution.
Preparation of samples for biochemical studies
For biochemical studies, 3-4 mice from each group were sacrificed; heart, kidney and spleen were dissected out, washed in ice cold physiological saline and stored at -70°C. The cell lysates were centrifuged at 20,000×g for 30 min and supernatant obtained were used for biochemical studies. Protein concentrations in the extracts were measured following the method of Lowry et al. [25].
Enzymatic analysis
LDH activity was measured spectrophotometrically following the method of Kornberg [26] and as described in our earlier report [27]. Briefly the reaction mixture (3 ml) consisted of 20 mM Tris-Cl (pH 7.4), 6 mM NADH, suitably diluted tissue extract and 1 mM sodium pyruvate. The decrease in absorbance at 340 nm was recorded up to 10 min. The oxidation of 1 μmole of NADH per min at 25oC was defined as 1 unit of the enzyme and values were presented as unit/ mg protein.
Measurement of lipid peroxidation
The level of lipid peroxidation was determined by measuring the amount of Malondialdehyde (MDA), the product of lipid peroxidation, following the method described in an earlier report [28]. Briefly, 0.5 ml of the extract was incubated with 1 ml of 0.2 M Tris-Maleate buffer (pH 5.9) in a water bath at 37oC for 30 min. After adding 1.5 ml of Thiobarbituric Acid (TBA), the mixture was incubated in boiling water bath for 10 min using tight condensers. After the mixture was cooled down, 3 ml pyridine: n-butanol mix (3:1 v/v) and 1 ml of 1 N NaOH was added and allowed to stand for 10 min. The absorbance at 548 nm was noted and the levels of lipid peroxidation were expressed as nmol MDA/mg protein.
Determination of nitric oxide
Direct measurement of nitric oxide is difficult because of its short half-life (3-5 s) and the Griess reaction is one of the most widely used indirect methods of nitric oxide detection which relies on a diazotization reaction originally described by Griess [29]. This detects nitrite (NO2–), one of the stable and non volatile breakdown products of NO. Griess reagent system uses sulfanilamide and N-1-Napthylethylenediamine Dihydrochloride (NED) under acidic (phosphoric acid) conditions. Briefly, 100 μl of cell lysate were incubated with an equal volume of Griess reagent (one part of 1% sulphanilamide in 2.5% H3PO4 plus one part of 0.1% naphthylethelenediamine dihydrochloride) in distilled water at room temperature for 10 min and OD was recorded at 550 nm. Nitrite concentration was determined by using a standard plot constructed against sodium nitrite.
Statistical analysis
Experimental data were expressed as mean ± SD and Student’s t test was applied for determining the level of significance between the control and the experimental groups and p<0.05 was considered significant.
Results
Nitrate enhances the increase of MC-LR induced lipid peroxidation
The effects of repeated administration of MC-LR and coadministration of MC-LR and nitrate on MDA level of heart, kidney and spleen are depicted in Figure 1a, 1b and 1c. MC-LR exposure produced significant rise in the extent of lipid peroxidation with maximum increase of ~1-2 folds in all tissues. However, maximum increase of ~3-4 folds in MDA level was observed in all tissues of MCLR and sodium nitrate co-exposure group (p<0.01).
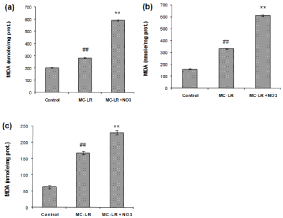
Figure 1: Effect of MC-LR and nitrate treatment on the level of MDA in heart (a), kidney (b) and spleen (c) of mice. The values represent mean ± SD where n=3.
#p<0.05 (normal control versus MC-LR treated group), *p<0.05 (MC-LR treated group versus nitrate treated group).
Nitrate enhances the MC-LR induced nitrosative damage in heart, kidney and spleen
Over expression of iNOS and constitutive NOS (eNOS & nNOS) is associated with increased NO production leading to protein nitration and apoptosis. Treatment with MC-LR caused significant increase in the production of NO in heart, kidney and spleen (Figure 2a, 2b and 2c; p<0.05). Co-treatment of mice with MC-LR and nitrate led to a significant increase in the NO production (p<0.05).
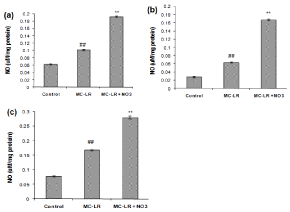
Figure 2: Effect of MC-LR and nitrate treatment on the level of NO in heart (a), kidney (b) and spleen (c) of mice. The values represent mean ± SD where n=3.
#p<0.05 (normal control versus MC-LR treated group), *p<0.05 (MC-LR treated group versus nitrate treated group).
Nitrate increases LDH activity of heart, kidney and spleen induced by MC-LR
Treatment of normal mice with MC-LR (10 μg/kg bw/day, ip) for 14 days caused a significant increase in LDH activity of heart, kidney and spleen (~0.5-1.7 folds) as compared to control (p<0.01) indicating tissue injury. As illustrated in Figure 3a, 3b and 3c, the level of LDH increased significantly in MC-LR mice treated with nitrate (50 mg/kg bw/day, orally) for 14 days (~2-3.2 folds, p<0.01).
Discussion
Oxidative stress occurs due to imbalance between pro-oxidant and antioxidant equilibrium, leading to characteristic changes in all biomolecules ultimately resulting in tissue damage [30]. Previous studies had documented the induction of oxidative stress with MCLR [31] in mice or nitrate [32] in rat. However, studies contributing to the effects of MC-LR and nitrate in combination have not been documented. A significant increase (p<0.05) in LPO was observed in all tissues of MC-LR treated mice while co-exposure of nitrate and MC-LR displayed the highest level of LPO (Figure 1a, 1b and 1c). Increased levels of Malondialdehyde (MDA), reflects the level to which free radicals attacks cellular lipids [33]. Elevated levels of MDA in all tissues of mice could be due to formation of free radicals induced by MC-LR, sodium nitrate and their co exposure. Greater extent of lipid peroxidation in co-exposed group indicates a higher degree of free radical damage to the cellular membranes possibly due to a synergistic or additive effect of the two toxins on reactive oxygen species. Our results are in agreement with Dubey et al., who reported that co-administration of deltamethrin and fluoride in rats causes oxidative stress as evident by elevated level of MDA in liver [21].
Nitrates and nitrites are ready source of nitric oxide and induce free-radical generation in vivo which overcomes the host antioxidant defense system [34]. In the present investigation, nitric oxide level increased in all tissues of MC-LR treated mice, thus revealing that NO is induced soon after MC-LR treatment due to the action of organelle-specific NOSs isoforms [35,36]. In the present study, ~2-3 fold increase of nitric oxide in heart, spleen and kidney of nitrate and MC-LR co-treated treated mice (Figure 2a, 2b and 2c) might be due to conversion of nitrate and nitrite into nitric oxide and also can be speculated to be due to the excessive synthesis of NO from L- arginine which reacts with superoxide to form peroxynitrite ultimately causing oxidative stress [37]. Nitrates have been reported in the formation of methemoglobin and carcinogenic nitrosamine in human [38,39]. Information available shows that nitrates are ready sources of NO, that may increase Lipid Peroxidation (LPO) which can be harmful to different organs including kidney [32,40]. Our results are in agreement with Anwar et al., which showed that administration of sodium nitrate in drinking water resulted in significant increase in TBARS of renal tissue of rat as compared to the control [41]. Previous reports have shown that fenvalerate and sodium nitrate exposure produced significant rise in LPO level with maximum increase of 46.1% and 43.1% respectively as compared to control, however, maximum increase of 69.6% in LPO was observed in fenvalerate and sodium nitrate co-exposure in blood of Bubalus bubalis [42].
In animals and humans, simultaneous exposure to MC-LR and nitrate can lead to organ damage which can be evaluated by measuring levels of various biochemical enzymes. LDH has been used as an indicator for cellular damage and cytotoxicity of toxic agents [43]. In the present investigation, LDH activity was measured to evaluate heart, kidney and spleen function. LDH activity increased in all tissues of MC-LR treated mice, which was further elevated when MC-LR treated mice were co-administered nitrate (~2-3 folds, Figure 3a, 3b and 3c). This was probably due to increase in the activity as the result of de novo synthesis or due to anaerobic metabolism needed to encounter the metabolic demands of the tissue [44]. High level of LDH observed in heart, kidney and spleen of mice co-administered with MC-LR and nitrate, is speculated to be due to inhibition of complexes I–III and cytochrome C-oxidase of the mitochondrial electron transport chain by NO, inhibiting cellular respiration and ATP production [45]. It has been reported that co-administration of fenvalerate and nitrate in Bubalus bubalis led to a significant increase of LDH in serum as compared to individual exposure [42].
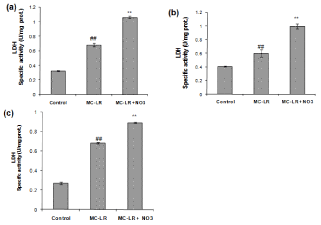
Figure 3: Effect of MC-LR and nitrate treatment on the level of LDH in heart (a), kidney (b) and spleen (c) of mice. The values represent mean ± SD where n=3.
#p<0.05 (normal control versus MC-LR treated group), *p<0.05 (MC-LR treated group versus nitrate treated group).
Conclusion
Exposure to MC-LR and sodium nitrate alone and in combination, causes damage to heart, kidney and spleen in mice by producing significant changes in lipid peroxidation, LDH and NO in mice indicating their ability to alter antioxidant defence in mice.
Co-exposure to both the toxicants produced comparatively higher degree of oxidative injury as compared to their individual exposures. The study clearly indicates that a cocktail of MC-LR and sodium nitrate induces a cascade of reaction in the exposed animals, thereby augmenting the toxicological damage. Therefore, this is a sign that the interaction of these toxicants in nature could be responsible for aggravating their toxic potential in livestock.
Acknowledgement
Lone Y thanks Dr. Harisingh Gour Central University, Sagar for fellowship. This work was financially supported by a project from UGC-Faculty Research Promotion Scheme (FRPS) & SERB, Govt. of India, sanctioned to RKK. The authors are thankful to Department of Zoology, Dr. Harisingh Gour Central University, Sagar, for providing infrastructural facilities and financial support.
References
- Kuiper-Goodman T, Falconer IR, Fitzgerald DJ. Human health aspects. Chorus I, Bartram J, editors. In: Toxic Cyanobacteria in Water: a Guide to Public Health Significance, Monitoring and Management. E&FN Spon. London. 1999; 113-153.
- Welker M, von Döhren H. Cyanobacterial peptides - nature’s own combinatorial biosynthesis. FEMS Microbiol Rev. 2006; 30: 530-563.
- Chilvers C, Inskip H, Caygill C, Bartholomew B, Fraser P, Hill M. A survey of dietary nitrate in well-water users. Int J Epidemiol. 1984; 13: 324-331.
- Chorus I, Bartram J. Toxic cyanobacteria in water: A guide to their public health consequences, monitoring and management. E&FN Spon. London. 1999; 416.
- Jhariya DC, Shandilya AK, Dewangan R. Nitrate Pollution in the Groundwater Around Sagar Town. Madhya Pradesh, India International Conference on Chemical, Ecology and Environmental Sciences (ICEES’2012). 2012; 17-18.
- Lone Y, Koiri RK, Bhide M. An overview of the toxic effect of potential human carcinogen Microcystin-LR on testis. Toxicol Rep. 2015; 2: 289-296.
- Ding WX, Shen HM, Zhu HG, Ong CN. Studies on oxidative damage induced by cyanobacteria extract in primary cultured rat hepatocytes. Environ Res. 1998; 78: 12-18.
- Li X, Liu Y, Song L, Liu J. Responses of antioxidant systems in the hepatocytes of common carp (Cyprinus carpio L.) to the toxicity of microcystin-LR. Toxicon. 2003; 42: 85-89.
- Pinho GL, da-Rosa CM, Maciel FE, Biachini A, Yunes JS, Proenca LA, et al. Antioxidant responses and oxidative stress after microcystin exposure in the hepatopancreas of an estuarine crab species. Ecotoxicol Environ Saf. 2005; 61: 353-360.
- Yin L, Huang J, Huang W, Li D, Liu Y. Responses of antioxidant system in Arabidopsis thaliana suspension cells to the toxicity of microcystin-RR. Toxicon. 2005; 46: 859-864.
- Manassaram DM, Backer LC, Moll DM. A review of nitrates in drinking water: Maternal exposure and adverse reproductive and developmental outcomes. Environ Health Perspect. 2006; 114: 320-327.
- Mande SA, Liu M, Djaneye-Boundjou G, Liu F, Bawa ML, Chen H. Nitrate in drinking water: a major polluting component of groundwater in gulf region aquifers, south of Togo. Int J Phy Sci. 2012; 7: 144-152.
- Ogur R, Coskun O, Korkmaz A, Oter S, Yaren H, Hasde M. High nitrate intake impairs liver functions and morphology in rats; protective effects of α-tocopherol. Environ Toxicol Pharmacol. 2005; 20: 161-166.
- Kaya S, Akar F. Inorganik Maddeler. Kaya S, Pirincci I, Bilgili A, editors. In: Veteriner Hekimliginde Toksikoloji, IkinciBaski. Ankara MedisanYay in Evi. 2002; 240-245.
- Awodi S, Ayo JO, Nwude CI, Dzenda T. Effects of sodium nitrite and ascorbic acid on the erythrocyte osmotic fragility in red Sokoto goats In Proceedings of 10th Annual Conference of the Animal Science Association of Nigeria (ASAN). University of Ado-Ekiti, Nigeria. 2005; 65-68.
- Speijers GJA, van-den BPA. Nitrate (and potential endogenous formation of N- nitroso compounds). Online. In WHO food additives series 50. 2003.
- IPCS (International Programme on Chemical Safety). Nitrates and nitrites. Ruse M, editor. In: Poisons, information Monograph (Group Monograph) Go16. Chemical. 1999; 35.
- ATSDR (Agency for Toxic Substances and Disease Registry). Nitrate/nitrite toxicity. Wigington PS, editor. In: Case studies in environmental medicine. USA: Department of Health and Human Services, DTEM. 2001.
- Manassaram DM, Backer LC, Moll DM. A review of nitrates in drinking water: maternal exposure and adverse reproductive and developmental outcomes. Cien Saude Colet. 2007; 12: 153-163.
- Bogovski P, Bogovski S. Animal Species in which N-nitroso compounds induce cancer. Int J Cancer. 1981; 27: 471-474.
- Dubey N, Raina R, Khan AM. Sub-acute deltamethr in and fluoride toxicity induced hepatic oxidative stress and biochemical alterations in rats. Bull Environ Conta Toxicol. 2013; 9: 334-338.
- Folt CL, Chen CY, Moore MV, Burnaford J. Synergism and antagonism among multiple stressors. Limnol Oceanogr. 1999; 44: 864-877.
- Berg K, Skulberg OM, Skulberg R. Effects of decaying toxic blue-green algae on water quality –a laboratory study. Arch Hydrobiol. 1987; 108: 549-563.
- Jones GJ, Orr PT. Release and degradation of microcystin following algicide treatment of a Microcystisaeruginosa bloom in a recreational lake, as determined by HPLC and protein phosphatase inhibition assay. Water Res. 1994; 28: 871-876.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193: 265-275.
- Kornberg A. Lactate dehydrogenase of muscle. Colowick SP, Kaplan NO, editors. In: Methods in enzymology. Academic press, New York. 1955; 1: 441-443.
- Koiri RK, Trigun SK, Mishra L, Pandey K, Dixit D, Dubey SK. Regression of dalton’s lymphoma in vivo via decline in lactate dehydrogenase and induction of apoptosis by a ruthenium (II)-complex containing 4-carboxy N-ethylbenzamide as ligand. Invest New Drugs. 2009; 27: 503-516.
- Mehrotra A, Trigun SK. Moderate grade hyper ammonemia induced concordant activation of antioxidant enzymes is associated with prevention of oxidative stress in the brain slices. Neurochem Res. 2012; 37: 171-181.
- Greiss JP. Benerkungen zu der Abhandlung der HH. Wesley and Benedikt ueber einige Azoverbindungen. Ber Dtsch Chem Ges. 1879; 12: 426-428.
- Mircescu G. Oxidative stress of chronic kidney disease. Acta Endocrinol. 2008; 5: 433-446.
- Jayaraj R, Anand T, Rao PV. Activity and gene expression profile of certain antioxidant enzymes to microcystin-LR induced oxidative stress in mice. Toxicology. 2006; 220: 136-146.
- Hassan HA, El-Agmy SM, Gaur RL, Fernando A, Raj MH, Ouhtit A. In vivo evidence of hepato- and reno-protective effect of garlic oil against sodium nitrite-induced oxidative stress. Int J Biol Sci. 2009; 5: 249-255.
- Rehman H, Ali M, Atif F, Kaur M, Bhatia K, Raisuddin S. The modulatory effect of deltamethrin on antioxidants in mice. Clin Chim Acta. 2006; 369: 61-65.
- Kashko MF, Khokha AM, Antsulevich SN, Doroshkevich NA, Voronov PP. [Influence of ethanol and ethanol-induced lipid peroxidation on the steroidogenic activity of testicles]. Ukr Biokhim Zh (1978). 1993; 65: 89-94.
- Chen T, Shen P, Zhang J, Hua Z. Effects of microcystin-LR on patterns of iNOS and cytokine mRNA expression in macrophages in vitro. Environ Toxicol. 2005; 20: 85-91.
- Ji Y, Lu G, Chen G, Huang B, Zhang X, Shen K, et al. Microcystin-LR induces apoptosis via NF-κB/iNOS pathway in INS-1 cells. Int J Mol Sci. 2011; 12: 4722-4734.
- Avci G, Kucukkur TI, Birdane YO, Eryavuz A, Ozdemir M. Influence of high dietary nitrate intake and sulphur supplementation on oxidant/antioxidant balance and on some haematological parameters in Angora goats. Rev Med Vet. 2012; 163: 79-84.
- Chan TY. Vegetable-borne nitrate and nitrite and the risk of methaemoglobinaemia. Toxicol Lett. 2011; 200: 107-108.
- Kapor A. Nitrite removal from drinking water, review. J Envir Engi Asso. 2004; 124: 378-384.
- Rocha BS, Gago B, Barbosa RM, Lundberg JO, Radi R, Laranjinha J. Intragastric nitration by dietary nitrite: implications for modulation of protein and lipid signaling. Free Radic Biol Med. 2012; 52: 693-698.
- Anwar MM, Mohamed NE. Amelioration of liver and kidney functions disorders induced by sodium nitrate in rats using wheat germ oil. J Rad Res App Sci. 2015; 8: 77-83.
- Gill KK, Sandhu HS, Kaur R. Evaluation of biochemical alterations produced by combined exposure of fenvalerate and nitrate in Bubalus bubalis. Veterinary World. 2014; 7: 146-151.
- Bagchi D, Bagchi M, Hassoun EA, Stohs SJ. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology. 1995; 104: 129-140.
- Hanan M, Amani AG, Khalifa E. Quercetin, coenzyme q10, and l-canavanine as protective agents against lipid peroxidation and nitric oxide generation in endotoxin-induced shock in rat brain. Pharmacol Res. 2001; 43: 3.
- Lizasoain I, Moro MA, Knowles RG, Darley-Usmar V, Moncada S. Nitric oxide and peroxynitrite exert distinct effects on mitochondrial respiration which are differentially blocked by glutathione or glucose. Biochem J. 1996; 314: 877- 880.