
Research Article
Austin J Nutri Food Sci. 2020; 8(3): 1148.
Oral Kefir Grains Supplementation Improves Metabolism and Liver Antioxidant Enzymes Expression in Malnourished Mice
Fabio Ribeiro SANTOS¹, Amanda Souto MACHADO², Deborah Farias LELIS², André Luiz Sena GUIMARÃES², Alfredo Mauricio B. DE PAULA², Renato Sobral MONTEIROJUNIOR ²,Theles de Oliveira COSTA¹, Igor Viana BRANDI¹, Junio COTA¹, Sergio Henrique Sousa SANTOS¹,²*
1Institute of Agricultural Science, Food Engineering, Universidade Federal de Minas Gerais (UFMG), Brazil
2Laboratory of Health Science, Postgraduate Program in Health Sciences, Universidade Estadual de Montes Claros (Unimontes), Montes Claros, MG, Brazil
*Corresponding author: Sergio H S Santos. Institute of Agricultural Sciences. Food Engineering, Universidade Federal de Minas Gerais (UFMG); Avenida Universitaria, Brazil
Received: November 12, 2020; Accepted: November 17, 2020; Published: November 24, 2020
Abstract
Scope: This study has a novel approach to investigate the effects of oral supplementation of kefir grains on metabolic improvement and the expression of the antioxidant enzymes Glutathione Peroxidase (GPx) and Catalase (CAT) of the liver in malnourished mice.
Method and Results: Swiss mice were divided into four groups and subjected to two treatment phases: the food restriction phase of 20% in relation to the control group was maintained until animals reached a weight deficit of about 20% in relation to their original weight and the renutrition phase, the animals received diets every day for 30 days. Diets (chow powder plus kefir grains) were administered orally. Thereafter, during throughout the experiment measurements of body weight and energy consumption were obtained. After the end of treatment, fasting glucose tolerance tests were performed at night and insulin sensitivity with fed mice. Soon in then, the mice were euthanized by beheading in guillotine and the blood and liver were collected for evaluation of biochemical parameters, Histopathological assessments and Reverse transcriptase (RT-PCR).
Conclusion: The present study demonstrated the kefir grains ability to modulate inflammation and hepatic oxidative-stress under malnourished-state.
Keywords: Food Restriction; Malnutrition; Hepatic; Oxidative Stress; Nutrition
Introduction
Malnutrition is characterized by proteins and/or other nutrients intake deficiency or imbalance. In this sense, this disorder can manifest itself in several ways: due to a primary factor, resulting from insufficient food intake; or secondary factors, resulting from its complications or disease [1,2]. It is a prevalent condition that affects one in three people worldwide [3]. Malnutrition alters body composition and is associated with a systemic inflammatory state [4]. Besides, malnutrition that can lead to an increase in genes related to oxidative stress [5] and affects the expression of enzymes with antioxidant activity, such as Glutathione Peroxidase (GPx) and catalase in several organs including the liver [6].
The liver plays an important role in malnutrition. This organ is responsible for a plethora of biochemical pathways in the production, modification, and use of nutrients and other metabolically important substances. It is also one of the vital organs of our body as it is important in the detoxification of toxic chemical substances and drugs [7,8]. Hepatic function may be modulated by several nutrients and/or food supplementation, including kefir.
Kefir grains are described as a symbiotic association of yeasts, lactic acid bacteria, and acetic acid bacteria surrounded by a matrix of polysaccharides referred to as kefiran. Kefir is rich in lactic, acetic and gluconic acid, ethyl alcohol, carbon dioxide, vitamin B12 and polysaccharides that give the product unique sensory characteristics. The lactic acid formed from lactose fermentation acts as a natural preservative, making kefir a biologically safe product, combining it with nutrients, calcium and iron, facilitating their absorption. The product also has high digestibility, which is attributed to the nature of the curd, whose proteins undergo, during fermentation, denaturation in various degrees, thus obtaining a curd of finely divided particles, easily penetrated by gastric juice [9,10]. Hence, this work aimed to evaluate the effect of kefir grains supplementation on the metabolism and liver inflammatory and antioxidant markers in malnourished mice.
Materials and Methods
Animals and Diet
The experiment was carried out with 32 male Swiss mice, aged 6 weeks, divided into 4 groups (n = 8 each). The animals were kept in an initial adaptation phase (10 days), with free access to water and the standard chow (Presence) for rats and mice containing 67.5% carbohydrates, 22.5% proteins, and 10% lipids. All procedures performed involving animals were in accordance with the institution’s ethical standards (CEEBEA - State University of Montes Claros - Annex 1). The animals were kept under controlled conditions of light and temperature. (Protocol number 189/2019).
Kefir Preparation
Kefir grains were donated from the biotechnology laboratory of the Institute of Agricultural Sciences of UFMG, at the Montes Claros Campus, and rehydrated three weeks before the experiments. The grains were inoculated (5%, weight/volume) and propagated in sterilized milk at 20 °C for 20 h for activation. After the filtration of the grains, the fermented milk was discarded and the grains were separated and refrigerated at 20 °C [11]. Regarding the nutritional composition, kefir can vary widely and is influenced by the composition of milk, the origin and composition of grains, time, temperature of fermentation, and storage conditions. However, the nutritional composition of kefir is still not well described in the literature. Thus, the nutritional composition of kefir is described in Table 1 [12].
Nutritional attributes
Nutritional
components
Concentration
100g
Vitamins (mg)
Vitamin B1
<1
Vitamin B2
<0.5
Vitamin B3
0.3
Amino acids (g)
Threonine
0.18
Lysine
0.38
Valine
0.22
Isoleucine
0.26
Methionine
0.14
Phenylalanine
0.23
Tryptophan
0.07
Minerals (g)
Potassium
1.65
Calcium
0.86
Magnesium
1.45
Phosphor
0.3
Microelements (mg)
Copper
0.73
Zinc
9.27
Iron
2.03
Manganese
1.3
Cobalt
0.02
Molybdenum
0.03
Table 1: Nutritional composition of kefir.
Malnutrition Protocols and Renutrition Diets
Immediately after the adaptation period, the animals were submitted to two treatment phases: the caloric restriction phase to lead to malnutrition [13] and the renutrition phase.
The caloric restriction of 20% in relation to the control group was maintained until animals reached a weight deficit of about 20% in relation to their original weight. Subsequently, during the renutrition phase, the animals received diets every day for 30 days. Diets (chow powder plus kefir grains) were administered orally. Groups with their respective renutrition diets are shown in Table 2.
Group identification
Renutrition diet
Estimated caloric consumption per animal/day (kcal)
Control – Nourished
Standard chow* (7.5g)
25.67
Control + kefirgrains**
Standard chow (7.30g) + kefirgrains (0.20g/mL)
25.67
Malnourished
Standard chow (6g)
19.75
Malnourished + kefirgrains **
Standard chow (5.80g) + kefirgrains (0.20g/mL)
19.75
*Presence Chow: 3.95 kcal/1g; **kefir grains: 4.272 kcal/g.
Table 2: Groups of animals according to the type of diet.
Glucose tolerance and insulin sensitivity tests (GTT and IST)
For the glucose tolerance test, 2 mg glucose/g of body weight was injected intraperitoneally into mice after an overnight fast. Glucose levels were monitored at 0, 15, 30, 60, and 120 min after injection, using blood samples taken from the animal’s tail. Insulin sensitivity tests were performed with the animals in the fed state, after intraperitoneal injection of insulin (0.75 U/kg of body weight), where tail blood samples were collected at times 0, 15, 30, and 60 min after injection for measuring blood glucose levels.
Biochemical Analyses
The serum was obtained after centrifugation (3200 rpm for 10 minutes at 4 °C). Total cholesterol, triglycerides, high-density lipoprotein (HDL), Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST) and alkaline phosphatase were evaluated using enzymatic kits (Wiener®, Argentina). Measurements were performed on a Wiener BT-3000 plus Chemistry Analyzer (Wiener®, Argentina).
Histopathological Assessments
For microscopic evaluation, the liver samples were fixed in 10% buffered formalin at 40 °C overnight, dehydrated by increasing degrees of alcohol, xylene and paraffin, and then embedded in paraffin, sectioned at 5 μm and subsequently stained with hematoxylin and eosin to assess the liver tissue architecture and the infiltration of inflammatory cells [14]. Slides were analyzed using an inverted microscope FSX100 (Sao Paulo, Brazil). Inflammatory cell counts were obtained using Image J software (Wayne Rasband, National Institutes of Health, Bethesda, MD) [15,16].
Reverse Transcriptase (RT-PCR)
The total RNA extracted from the liver was prepared using the TRIzol reagent (Invitrogen Corp.®, San Diego, California, USA), treated with DNAse and reverse transcribed with M-MLV (Invitrogen Corp.®). Endogenous glyceraldehyde 3-phosphate dehydrogenase (GAPDH), free radical deactivating enzymes such as glutathione peroxidase (GPx) and Catalase (CAT) were evaluated using specific primers and SYBR green reagent (Applied Biosystems®, USA) on a plus-one platform (Applied Biosystems®). The relative comparative CT method was applied to compare the levels of gene expression between the groups, using the equation 2-ΔΔCT [17]. Primer sequences are described in Table 3 [18].
Gene
Forward
Reverse
GAPDH
AGGTCGGTGTGAACGGATTTG
GGGGTCGTTGATGGCAACA
CAT
GGAGGCGGGAACCCAATAG
GTGTGCCATCTCGTCAGTGAA
GPx
CCACCGTGTATGCCTTCTCC
AGAGAGACGCGACATTCTCAAT
*GAPDH: Endogenous glyceraldehyde 3-phosphate dehydrogenase, CAT: Catalase and GPx: Glutathione peroxidase.
Table 3: RT-PCR primers used in this study.
Table 3 - RT-PCR primers used in this study.
Statistical Analysis
All data were analyzed using the GraphPad Prism software (Version 5.0®, San Diego, California, USA), with target confidence level of 95% (p <0.05). Data are expressed as the mean ± SEM. Statistical significance between groups of mice was assessed by oneway ANOVA, followed by Tukey’s post-test and Student’s t-test. Statistical significance was established at p <0.05.
Results
Food and Energy Consumption, Body Weight, and Adiposity
Food (ST, 0.0750 ± 0.015; ST + GK, 0.0750 ± 0.015; FR, 0.0660 ± 0.015; FR + GK, 0.0660 ± 0.015) and energy (ST, 0.2420 ± 0.050; ST + GK, 0.2420 ± 0.050; FR, 0. 2530 ± 0.051; FR + GK, 0.2530 ± 0.051) consumption did not differ significantly between the groups that received kerfir and the control group (Figures 1a and 1b). In the analysis of body weight, we observed an expected reduction in body weight in the group of malnourished mice when compared to the control, not malnourished, group (ST, 58.63 ± 3,154 vs. FR, 45.15 ± 0.328) (Figure 1c).
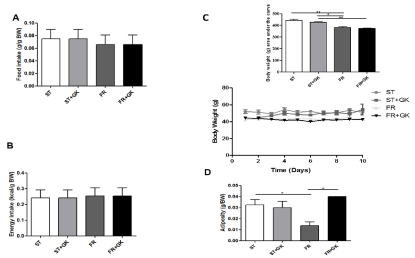
Figure 1: Food and energy consumption, body weight, and adiposity.
Body weight, food and energy consumption of mice fed a standard diet and renourished. Food consumption (A), energy consumption (B), daily body weight and
area on the curve (C), and adiposity (D). Data are presented as mean ± SEM. Statistically significant differences between groups are indicated as *p <0.05, **p
<0.01, ***p <0.001 compared to the Standard Diet (ST) and renutrition groups (ST, ST + GK, FR and FR + GK).
In the analysis of the area on the bodyweight curve, no significant difference was observed between the malnourished group and the one treated with kefir grains (FR, 381.1 ± 9.429 vs. FR + GK, 378.7 ± 4.060) (Figure 1c). Regarding adiposity, a significant increase was observed in malnourished mice that received kefir in relation to the malnourished control group (FR, 0.014 ± 0.003 vs. FR+GK, 0.040 ± 0.0) (Figure 1d).
Tolerance tests, Insulin Sensitivity, and Biochemical Analyses
The analysis of the area under the glucose tolerance test curve did not show statistically significant differences between the malnourished and the malnourished group that received kefir (FR, 22433 ± 1506 vs. FR + GK, 21255 ± 1054) (Figure 2a). Similar results were found in the analysis of the area under the curve of the insulin sensitivity test (FR, 6465 ± 926.9 vs. FR + GK 6063 ± 1164) (Figure 2b). No statistically significant differences were found in serum glucose levels while fasting (RF, 156.3 mg / dL ± 10.31 vs. FR + GK, 150.7 mg / dL ± 3,528) and triglycerides (RF, 294.4 mg / dL ± 19.36 vs. FR + GK, 348.0 mg / dL ± 24.68) among the mice in the treated malnourished group and their respective malnourished control (Figure 2c).
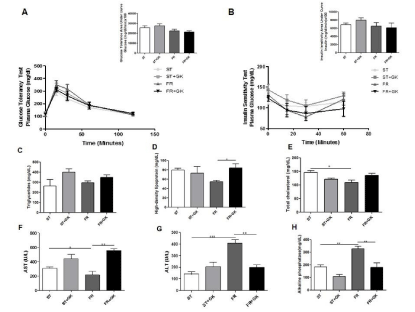
Figure 2: Tolerance tests, insulin sensitivity, and biochemical analyses.
The glycemic and biochemical profile of mice fed a standard diet and renourished. Glucose tolerance test and area on the curve (a), insulin sensitivity test and
area on the curve (b), triglycerides (c), HDL (d), total cholesterol (e), AST (f), ALT (g), alkaline phosphatase (h). Data are presented as mean ± SEM. Statistically
significant differences between groups are indicated as *p <0.05, **p <0.01, ***p <0.001 in comparison with the standard diet groups (ST) and renutrition groups (ST,
ST + GK, FR, and FR + GK).
Regarding the lipid profile of the animals, an increase in HDL levels was observed in the malnourished group treated with kefir compared to the control (FR, 54.57 mg/dL ± 2.88 vs. FR + GK, 84.20 mg/dL ± 9,035) (Figure 2d). Total cholesterol levels were statistically lower in malnourished animals when compared to the nonmalnourished control (ST, 145.8 mg/dL ± 7,351 vs. FR, 135.3 mg/ dL ± 7,860) (Figure 2e). Interestingly, increased levels of TGO were observed in mice subjected to caloric restriction and treated with kefir (FR, 215.3 mg/dL ± 51.97 vs. FR + GK, 556.0 mg/dL ± 20.00) (Figure 2f). An improvement in the profile of liver enzymes was also observed in the group of treated malnourished mice when compared to the malnourished control, with reduced levels of TGP (FR, 406.7 mg/dL ± 33.19 vs. FR + GK, 198.5 mg/dL ± 21.95) and alkaline phosphatase (FR, 327.3 mg/dL ± 20.54 vs. FR + GK, 181.3 mg/dL ± 34.26) (Figure 2g-h).
Liver Weight, Histological Analysis, and Real-Time PCR
Malnourished mice that received kefir showed increased liver weight when compared to the malnourished control group (FR, 215.3 g/BW ± 0.004 vs. FR + GK, 0.050 g/BW vs. 0.0) (Figure 3a). A statistically significant reduction in the inflammatory infiltrate was observed in the group of animals treated with kefir when compared to their respective control (ST, 53.63 ± 4.136; ST + GK, 41.13 ± 1.469; FR, 45.38 ± 2.611; FR + GK, 33.00 ± 3.343) (Figure 3b). RT-PCR analyses showed a significant increase in the expression of the antioxidant enzymes GPx (ST, 1.70 ± 0.858; ST + GK, 77.97 ± 19.50; FR, 2.475 ± 0.9; FR + GK, 173.7 ± 38.66) (Figure 3c) and CAT (ST, 1.146 ± 0.407; ST + GK, 21.35 ± 2.823; FR, 1.408 ± 0.674; FR + GK, 25.38 ± 5.165) (Figure 3d) in groups of animals fed a diet supplemented with kefir grains.
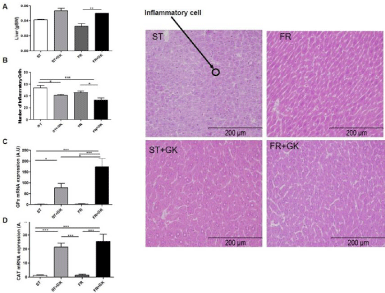
Figure 3: Liver weight, histological analysis, and Real-time PCR.
Kefir grains reduced inflammatory infiltrates and modulated mRNA expression of free radical deactivating enzymes, such as Glutathione Peroxidase (GPx) and
Catalase (CAT) in malnourished mice. Liver (a), number of inflammatory cells (b), mRNA expression of GPx (c) and mRNA expression of CAT (d). Data are
presented as mean ± SEM. Statistically significant differences between groups are indicated as *p <0.05, **p <0.01, ***p <0.001 compared to the standard diet (ST)
and renutrition groups (ST, ST + GK, FR and FR + Gk).
Discussion
Liver damage caused by malnutrition due to food restriction has already been described by other studies [19], mainly because the liver is a central organ in metabolism [20]. In the present study, we demonstrated that malnutrition generates liver damage. An increase in the serum levels of the enzymes TGP and alkaline phosphatase was observed, as well as a reduction in liver weight, inflammation and in the expression of the antioxidant enzymes GPx and CAT. These results validate the findings of other authors who also showed a decrease in body and liver weight [18,19] in animals submitted to food restriction.
The arguments to confirm some of these changes related to malnutrition are well described in the literature. According to Guzman-Silva et al. (2004) [21], malnutrition is related to the loss of liver mass in order to provide energy to important organs, such as brain and heart [21,22], which justifies the analyzed difference.
The damages caused by oxidative stress have been related to malnutrition, which could alter the antioxidant protection mechanisms [23]. Normally, organisms are equipped with mechanisms to eliminate these reactive species, involving both nonenzymatic and enzymatic pathways. Enzymatic pathways include some free-radical-deactivating enzymes such as catalase, Glutathione Peroxidase (GPx), and Superoxide Dismutase (SOD) [24,25].
In this context, we analyzed the effect of malnutrition on enzymes related to oxidative stress. We observed a decrease in mRNA expression of the catalase and glutathione peroxidase genes in the malnourished group (FR). This suggests that the modulation of the gene expression of these enzymes can be affected by malnutrition.
Similar results were found in another study conducted on the thymus of malnourished lactating rats [23], in which decreased expression of the catalase and GPx genes in malnourished animals was observed [23]. In addition, it has been shown that protein-energy malnutrition results in a reduction in the expression of the antioxidant enzyme glutathione S-transferase in the rat liver, which can increase radicals and oxidative stress in the organ [26]. Experimental studies have suggested that protein malnutrition causes inflammatory changes in the liver, including an increase in interleukin-6 production [27].
Supplementation with low cost and convenient antioxidants such as vitamin E may be useful in preventing the progression of liver damage [24,28,29]. In this study, we describe for the first time that supplementation of malnourished animals with kefir grains was able to reverse liver damage as well as increase adiposity and expression of GPx and CAT.
Kefiran, an important component of kefir, inhibited pulmonary inflammation induced by ovalbumin in a murine model of asthma, suppressing the release of eosinophils and other inflammatory cells in Bronchoalveolar Lavage Fluid (BAL) and lung tissue, as well as levels of proinflammatory cytokines interleukin-4 (IL-4) and interleukin-5 (IL-5) [30]. Another study described the antioxidant effect of kefir in rats with kidney damage [31]. Similar results were found in a study with rats exposed to lead, in which case kefir also induced antioxidant activity [32].
Conclusion
In summary, we show that oral supplementation with kefir grains improved the metabolic profile of malnourished animals, increasing adiposity, HDL, decreasing serum levels of TGP and alkaline phosphatase, liver inflammation and increasing the expression of antioxidant enzymes GPx and catalase. The results of the present study suggest, for the first time, the modulation of inflammation and hepatic oxidative stress by kefir grains in malnourished animals. These findings may contribute to better understand the metabolic effects mediated by kefir grains in the context of malnutrition. However, the mechanisms by which kefir grains activate the enzymes GPx and CAT in the liver need to be further investigated.
Acknowledgment
This work was partially supported by the Coordenadoria de Aperfeicoamento do Pessoal de Nivel Superior (CAPES), Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq), and Fundacao de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG).
Author Contributions
FRS and ASM: study design, data analysis and drafting of the article; DFL and ASM: drafting of the article; DFL and FRS: data acquisition; AMBP, IVB and JCS: interpretation of data, SHSS and ALSG: critically revising the manuscript for important intellectual content. All authors have approved the final version of the manuscript for submission.
Availability of Data and Materials
The data that support the findings of this study are available from the corresponding author, [SHSS], upon reasonable request.
Ethics Approval and Consent to Participate
The Montes Claros State University (Unimontes), Brazil, Ethics Committee approved the study #189/2019.
Human and Animal Rights
No humans were used in the study. All reported experiments on animals were performed in accordance to the protocol approved by the Animal care and use of Committee for Ethics in Animal Experimentation and Welfare (CEBEA).
References
- Meier R, Stratton R. Basic concepts in nutrition: epidemiology of malnutrition. e-SPEN, the European e-Journal of Clinical Nutrition and Metabolism. 2008; 3: e167-e70.
- Verbrugghe M, Beeckman D, Van Hecke A, Vanderwee K, Van Herck K, Clays E, et al. Malnutrition and associated factors in nursing home residents: A cross-sectional, multi-centre study. Clinical nutrition. 2013; 32: 438-443.
- Institute IFPR. Global Food Policy Report: International Food Policy Research Institute. 2016.
- Soeters P, Bozzetti F, Cynober L, Forbes A, Shenkin A, Sobotka L. Defining malnutrition: a plea to rethink. Clinical nutrition. 2017; 36: 896-901.
- Ghone RA, Suryakar AN, Kulhalli P, Bhagat SS, Padalkar RK, Karnik AC, et al. A study of oxidative stress biomarkers and effect of oral antioxidant supplementation in severe acute malnutrition. Journal of clinical and diagnostic research: JCDR. 2013; 7: 2146.
- Partadiredja G, Worrall S, Simpson R, Bedi K. Pre-weaning under nutrition alters the expression levels of reactive oxygen species enzymes but not their activity levels or lipid peroxidation in the rat brain. Brain research. 2008; 1222: 69-78.
- Nompleggi DJ, Bonkovsky HL. Nutritional supplementation in chronic liver disease: an analytical review. Hepatology. 1994; 19: 518-533.
- Rui L. Energy metabolism in the liver. Compr Physio. 2014; l 4: 177-197.
- Hertzler SR, Clancy SM. Kefir improves lactose digestion and tolerance in adults with lactose maldigestion. Journal of the American Dietetic Association. 2003; 103: 582-587.
- SOUZA G, GARCIA S, VALLE J. Kefir e suatecnologia-aspectosgerais. Boletim Ital. 1984; 21.
- Chen H, Tung Y, Tsai C, Lai C, Lai Z, Tsai H, et al. Kefir improves fatty liver syndrome by inhibiting the lipogenesis pathway in leptin-deficient ob/ob knockout mice. International journal of obesity. 2014; 38: 1172.
- Rosa DD, Dias MM, Grzeskowiak LM, Reis SA, Conceicao LL, Maria do Carmo GP. Milk kefir: nutritional, microbiological and health benefits. Nutrition research reviews. 2017; 30: 82-96.
- Fock RA, Vinolo MAR, Rocha VdMS, de Sa Rocha LC, Borelli P. Proteinenergy malnutrition decreases the expression of TLR-4/MD-2 and CD14 receptors in peritoneal macrophages and reduces the synthesis of TNF-a in response to Lipopolysaccharide (LPS) in mice. Cytokine. 2007; 40: 105-114.
- Schneider DI. An introduction to programming using visual basic 2012: Prentice Hall Press. 2013.
- Aguiar J, Gomes E, Fonseca-Silva T, Velloso N, Vieira L, Fernandes M, et al. Fluoxetine reduces periodontal disease progression in a conditioned fear stress model in rats. Journal of periodontal research. 2013; 48: 632-637.
- Gomes E, Aguiar J, Fonseca-Silva T, Dias L, Moura-Boas K, Roy A, et al. Diazepam reverses the alveolar bone loss and hippocampal interleukin-1beta and interleukin-6 enhanced by conditioned fear stress in ligature-induced periodontal disease in rats. Journal of periodontal research. 2013; 48: 151- 158.
- Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) Method. Methods [Internet]. 2001; 25: 402-408.
- Pan Y, Long X, Yi R, Zhao X. Polyphenols in Liubao tea can prevent CCl4- induced hepatic damage in mice through its antioxidant capacities. Nutrients. 2018; 10: 1280.
- Morris HJ, Carrillo OV, Llaurado G, Alonso ME, Bermudez RC, Lebeque Y, et al. Effect of starvation and refeeding on biochemical and immunological status of Balb/c mice: an experimental model of malnutrition. Immunopharmacology and immunotoxicology. 2011; 33: 438-446.
- Couto JLA, Vieira RCdS, Barbosa JM, Machado SS, Ferreira HdS. Alteracoes da funcaohepatica de camundongosdes nutridos e infectadospelo Schistosomamansoni. Rev Soc Bras Med Trop. 2008; 41: 390-393.
- Guzman-Silva MA, Wanderley AR, Macedo VM, Boaventura GT. Recuperacao da desnutricaoemratos median teraco esadicionadasounao de suplementoalimentar e de vitaminas e mineraisdurante o periodo de crescimento. Revista de Nutricao. 2004.
- de Almeida Pinheiro T, Barcala-Jorge AS, Andrade JMO, de Almeida Pinheiro T, Ferreira ECN, Crespo TS, et al. Obesity and malnutrition similarly alter the renin-angiotensin system and inflammation in mice and human adipose. The Journal of nutritional biochemistry. 2017; 48: 74-82.
- Gavia-García G, González-Martínez H, Miliar-García Á, Bonilla-González E, de los Ángeles Rosas-Trejo M, Königsberg M, et al. Oxidative damage and antioxidant defense in thymus of malnourished lactating rats. Nutrition. 2015; 31: 1408-1415.
- Fardet A, Chardigny J-M. Plant-based foods as a source of lipotropes for human nutrition: a survey of in vivo studies. Critical reviews in food science and nutrition. 2013; 53: 535-590.
- Lykke M, Hother A-L, Hansen CF, Friis H, Mølgaard C, Michaelsen KF, et al. Malnutrition induces gut atrophy and increases hepatic fat infiltration: studies in a pig model of childhood malnutrition. American journal of translational research. 2013; 5: 543.
- Cho MK, Kim YG, Lee MG, Kim SG. The effect of cysteine on the altered expression of class a and μ glutathione S-transferase genes in the rat liver during protein-calorie malnutrition. Biochimicaet Biophysica Acta (BBA)- Molecular Basis of Disease. 2000; 1502: 235-246.
- Ling P-R, Smith RJ, Kie S, Boyce P, Bistrian BR. Effects of protein malnutrition on IL-6-mediated signaling in the liver and the systemic acute-phase response in rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2004; 287: R801-R808.
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012: 2005-2023.
- Hernández-Aquino E, Muriel P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World journal of gastroenterology. 2018; 24: 1679.
- Kwon OK, Ahn KS, Lee MY, Kim SY, Park BY, Kim MK, et al. Inhibitory effect of kefiran on ovalbumin-induced lung inflammation in a murine model of asthma. Archives of pharmacal research. 2008; 31: 1590-1596.
- Punaro GR, Maciel FR, Rodrigues AM, Rogero MM, Bogsan CS, Oliveira MN, et al. Kefir administration reduced progression of renal injury in STZ-diabetic rats by lowering oxidative stress. Nitric Oxide. 2014; 37: 53-60.
- Ozcan A, Kaya N, Atakisi O, Karapehlivan M, Atakisi E, Cenesiz S. Effect of kefir on the oxidative stress due to lead in rats. Journal of Applied Animal Research. 2009; 35: 91-93.