
Research Article
Austin J Nutr Metab. 2024; 11(1): 1134.
Comparative Analysis of Bee Pollen Antioxidant Properties from Western Oromia, Ethiopia
Ofijan Tesfaye*
Oromia Agricultural Research Institute, Haro Sebu Agricultural Research Center, Haro Sebu, Kellem Wollega, Oromia, Ethiopia
*Corresponding author: Ofijan Tesfaye, Oromia Agricultural Research Institute, Haro Sebu Agricultural Research Center, Haro Sebu, Kellem Wollega, Oromia, Ethiopia. Tel: +251912858750 Email: apistesfaye@gmail.com
Received: October 14, 2024; Accepted: October 31, 2024 Published: November 07, 2024
Abstract
Bee pollen has a rich history of use in traditional medicine and as a dietary supplement, owing to its diverse nutrient composition. The biological makeup of bee pollen can differ significantly depending on factors such as the plant species it’s collected from, where it’s harvested geographically, and how it’s handled after collection. These variations can influence its potential health benefits and nutritional profile. The study was conducted to see the pollen yield and compare the antioxidant properties of methanol extract of bee pollen based on botanical origin. The botanical origin was identified using the harmonized method of melissopalynological analysis by observing microscope slides. Phenol, flavonoid, and DPPH scavenging activity was done by standard method. The minimum and maximum mean pollen yield was cropped in August (4.2 ± 1.2 g/hive) and November (265.41 ± 40.66 g/hive) respectively. However, the month of March, April, and July was a time when no pollen was released by plants and were said to be dearth periods in the study area. Five major plants namely Bidens spp., Guizotia scabra, Croton macrostychus, Eucalyptus spp., and Zea mays whose samples are enough for antioxidant analysis were selected. The results indicated that pollen released from Bidens spp. exhibited minimum total phenolic compound contents (TPCC; 27.5 ± 0.8 mg GAE (Gallic acid equivalent)/100g) and total flavonoid compound content (TFCC; 18.8 ± 0.7 mg (QE (Quercetin equivalent)/100g) while Eucalyptus spp. recorded maximum TFCC (62.4 ± 0.5 mg (Gallic acid equivalent)/100g) and TFCC (49.6 ± 0.2 mg QE (Quercetin equivalent)/100g) by methanol extraction. Based on the EC50 values, the current finding showed antioxidant activity with EC50 values ranging from 0.036 ± 0.005 (Eucalyptus spp.) to 0.233 ± 0.057 (Bidens spp.) mg/mL, which were lower than that of the positive ascorbic acid control (EC50, 0.023 ± 0.005 mg/mL). Therefore, Ethiopian bee pollen extracted with methanol could be considered a nutritional addition to food to prevent various diseases related to free radicals.
Keywords: Antioxidant activity; Flavonoid; Melissopalynological analysis; Phenol; Pollen
Introduction
Honey bees (Apis mellifera L.) collect pollen from plant blossoms, enriching it with salivary enzymes and nectar in the process, to create bee pollen [1]. These tiny, granular-looking grains are then transported back to the apiary by the bees [2]. Bee pollen serves as a plentiful and essential food source for the colony, meeting their protein requirements [3]. The color of each pollen bead reflects its specific botanical source, which can vary from a single bloom or a variety of flower species [4]. Various factors such as climatic conditions, botanical and geographic differences, and commercial production procedures contribute to the differences in pollen grains' shapes and the levels of bioactive and nutritious components they contain [5].
The antioxidant capacity of pollen is indeed one of its key features, playing a crucial role in protecting cells from damage caused by oxidative agents like free radicals [6]. Antioxidants, a group of chemicals, can inhibit or slow down the oxidation process of other molecules, thus preventing changes and mutations that could lead to illnesses [7]. Interestingly, studies comparing the antioxidant activity of organic pollen and honey have shown that organic pollen exhibits stronger antioxidant activity than honey. This finding underscores the potential health benefits associated with consuming bee pollen, particularly in terms of its antioxidant properties [8].
The antioxidant qualities of bee pollen are primarily responsible for its various biological activities, thanks to its potent ability to neutralize free radicals and its high polyphenolic content [9]. Polyphenols, including flavonoids found in bee pollen, play a key role in scavenging free radicals and electrophiles, thereby deactivating reactive oxygen species (ROS) and preventing them from becoming mutagens [10]. While there's generally a positive correlation between the concentration of polyphenols and the antioxidant capacity of bee pollen samples, some research suggests that high concentrations of phenolic compounds don't always correlate directly with antioxidant activity in bee pollen extracts [9]. This indicates that antioxidant activity extends beyond phenolic substances alone. Other molecules present in bee pollen, such as carotenoids, glutathione, phytoalexins, and vitamins C and E, also contribute to its antioxidant properties [10,11]. Consequently, the antioxidant capacity of bee pollen is heavily influenced by its specific composition, which can vary between samples based on their origin [11,12].
Numerous studies have highlighted significant variations in the chemical composition, types, and antioxidant activity of pollen grains derived from different plant species and geographical regions [13,14]. Factors such as the plant species producing the pollen, the temperature and soil conditions of their growth environment, and the timing of harvest all influence the composition and properties of bee pollen [12]. In regions with rich plant diversity like the study area in Western Oromia, Ethiopia, there is considerable potential for pollen production. However, limited available data describing the pollen yield throughout the year, along with its antioxidant properties. To address this gap, our study aims to compare the antioxidant properties of pollen collected by bees from various botanical origins in Western Oromia, Ethiopia. This research will contribute valuable insights into the antioxidant potential of bee pollen in this region and its significance in promoting health and well-being.
Material and Methods
Pollen Sample Collection and Botanical Origin Analysis
The necessary data for this work was gathered from the Haro Sebu Apiculture Research team's apiary in Western Oromia, Ethiopia. Pollen traps with 16% trapping efficiency was installed at the entrance of beehives, pollen loads were gathered from September 2021 to August 2022. Every week, the pollen pellets were taken out of the tray, color-sorted, weighed, and then individually placed in a clean paper bag (Figure 1A). They were then allowed to dry at room temperature. After that, the color-based identification was kept for further examination in freezers at 13oC.

Figure 1: An original figure created by the authors of the manuscript; A)
Unsorted (color mixed pollen pellet) and weighing, B) Sorting based on color
and slide preparation for each color separately, C) Microscopically identifying
pollen grain morphology and taking pictures on a desktop connected with a
microscope.
For floral source identification, each color-based bee pollen pellets were subjected to established methods (melissopalynological examination) [15, 16]. Representative pellets of each color were used to collect pollen grain samples, which were then dissolved in water droplets, put on glass slides, examined under a light microscope (400x magnification) [Figure 1B-C], and identified at the genus or species level using a pollen atlas made from native Ethiopian flora [17].
Antioxidant Properties of Bee Pollen
Methanolic pollen extraction (MPE): The pollen samples were extracted using methanol solvent (99.9% methanol) individually [18]. To achieve this, two grams of dried pollen powder underwent extraction by stirring with 25 mL of methanol, followed by maceration at 25°C for 60 minutes using a temperature-controlled shaker incubator (ZHWY-103B). The resulting mixture was then filtered through Whatman N°4 paper.
The residue underwent two additional extractions with 25 mL portions of methanol using the same procedure. The combined methanolic extracts were evaporated at 40°C to dryness using a rotary evaporator (Stuart R3300). The dried extracts were re-dissolved in methanol at a concentration of 50 mg mL-1 and stored at 4°C for further use.
Total Phenolic Compound Content (TPCC): The TPCCt was determined using the Folin-Ciocalteau colorimetric method [19]. To perform this, 0.5 mL of the MPE was mixed with 2.5 mL of Folin- Ciocalteau reagent, diluted to a ratio of 1:10, and 2.0 mL of 4% sodium carbonate solution. After incubating the mixture in the dark at room temperature for two hours, the absorbance of the MPE was measured at 740 nm. Gallic acid, ranging from 0 to 200 mg mL-1, was used as a standard chemical to generate a calibration curve. Finally, the TPCC was expressed in milligrams of Gallic acid equivalent (GE) per gram of bee pollen dry weight based on the mean value of triplicate data. The calibration formula (y = 17.941x + 0.2778; R2 = 0.9948) was derived from the calibration curve (Figure 2).

Figure 2: Calibration curve for total phenol compound content.
Total Flavonoid Compound Content (TFCC): The TFCC was determined following the method outlined by Zou et al. [19]. A 0.5 mL aliquot of the MPE diluted at a ratio of 1:10 was mixed with 4.3 mL of 99.9% methanol, 0.1 mL of 10% aluminum nitrate (Al(NO3)3), and 0.1 mL of 1 M potassium acetate.
After incubating the mixture for 40 minutes at room temperature, the absorbance was measured at 415 nm using a spectrophotometer (UV-Vis Mini 1240, Shimadzu Co.). Quercetin, ranging from 0 to 200 mg mL-1, was utilized as a standard chemical to generate a calibration curve. The total flavonoid content was then reported as the mean value of triplicate assays and expressed in milligrams of quercetin equivalent (QE) per gram of bee pollen dry weight based on the mean value of triplicate data. The calibration formula (y = 9.4688x + 0.2578; R2 = 0.9962) was derived from the calibration curve (Figure 3).
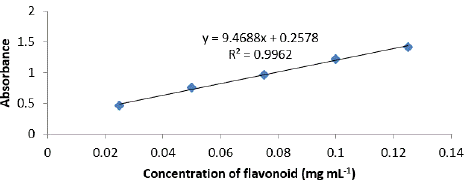
Figure 3: Calibration curve for total flavonoid compound content.
Determination of free radical scavenging activity: The antioxidant activity of the MPE was evaluated using the 2,2-diphenyl- 1-picrylhydrazyl (DPPH) radical scavenging assay. First, a 0.004% solution of DPPH radical in methanol was prepared. Subsequently, 2 mL of this solution was mixed with 1 mL of various concentrations (ranging from 0.02 to 50 mg mL-1) of the pollen extracts dissolved in methanol. The resulting mixtures were then incubated for 30 minutes in the dark at room temperature. The scavenging capacity was measured spectrophotometrically by monitoring the decrease in absorbance at 517 nm. Ascorbic acid was used as a standard and a mixture without extract served as the control. The ability of the samples to scavenge DPPH radicals was determined by comparing the reduction in sample color with that of the control using the following equation, and the results were expressed as percentage values:
Where: = absorbance of the control; = absorbance of the sample. The extract concentration providing 50% of radical scavenging activity IC50 (minimum concentration required to inhibit to 50% of DPPH initial concentration) was calculated from the graph of radical scavenging activity percentage against extract concentration.
Data Analysis
SAS version 9.1.3 (SAS Institute, 2003) computer package was used for analyzing all the data. Means and standard deviations of the recorded data were calculated using SAS Software (SAS Inc., 2003). Determination of the significant differences between bee pollen samples was done using one-way ANOVA. TPCC, TFCC, and IC50 concentration for DPPH radical scavenging activity were used for mean separation.
Result and Discussion
Pollen Yield and Major Bee Plant Identification
The mean monthly pollen trapped from the hive (n = 3) throughout the year (September 2021 up to August 2022) is depicted in Table 1. Pollen yield started in August (4.2 ± 1.2g/colony) and increased till November (265.41 ± 40.66 g/colony) and started to decline in December (171.13 ± 7.89 g/colony) to February (11.56 ± 3.1 g/colony). However, no pollen was harvested in the dry seasons (March and April) and rainy seasons (July to mid-August). The current finding forwarded a message for beekeepers of the study area to the super hive and control swarm from October to December whilst March, April, July, and August could be used as dearth periods and therefore hive reduction and supplementary feeding should be provided to maintain their colonies within the hive.
Five major bee plants were identified from the harvested pollen pellets and their %age was indicated in Figure 4. Out of them, the maximum and minimum %age was by Bidens spp. (35.2 %) and Z. mays (7.7%) respectively. Each of their pollen grain morphology is illustrated in Figure 5. They were selected based on the amount of data collected for an antioxidant compound analysis. The sample should be enough for a laboratory analysis. The Bidens spp., G. scabra and Eucalyptus spp. plant releases their flowering in November to December, C. macrostychus flowered from May to June, and Z. mays flowered from August to September (Table 2).
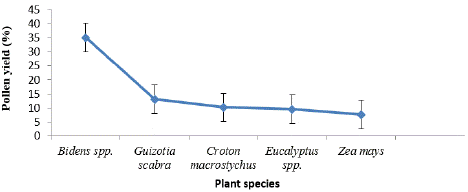
Figure 4: Percentage pollen yield among pollen types.
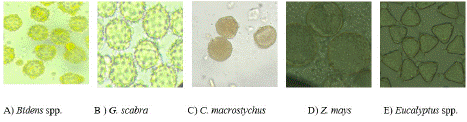
Figure 5: Microscopic pollen grain morphology of the selected pollen types.
Antioxidant Properties
Total Phenol Compound Content (TPCC): The TPCC of 5 different plant-origin bee pollen samples were indicated in Table 3. Statistically significant variation was demonstrated among the tested pollen types. The TPCC ranged from 27.5 ± 0.8 (Bidens spp.) to 62.4 ± 0.5 (Eucalyptus spp.) mg of Gallic acid equivalents per 100 gram of MPE. The disparity that occurred might be due to the botanical origin and season of the sample collected. The samples were collected in different months of the year which could be affected by soil nutrient composition and moisture availability.
The TPCC of bee pollen from different plant species in Ethiopia ranged from 19.52 to 39.84 mg of Gallic acid equivalent(mgGAE) per 100 gram of pollen [19]. Different findings were observed in other regions, with values ranging from 10.5 to 213.2 mgGAE g-1 in Brazil [21], 30.46 mgGAE g-1 in Northwest Algeria [22], 24.1 to 45.5 mgGAE g-1 in the Baltic region [23], and 10.5 to 16.8 mgGAE g-1 in Portugal [24]. Interestingly, the total phenolic content of the current study fell within the range reported in previous studies (10.5 to 213.2 mgGAE g-1).
The disparities in TPCC could be attributed to several factors, including the botanical and geographical origin of the pollen, soil type on which the plants are grown, the season of sample collection, the species of bees involved in pollen collection, post-harvest handling of the sample, and the methods employed for compound extraction [1,6,7]. These findings underscore the influence of botanical origin on the phenolic composition of bee pollen and highlight the importance of considering these factors in future research endeavors.
Total Flavonoid Compound Content (TFCC): Table 3 presents the TFCC of the pollen samples. The results varied from 18.8 ± 0.7 mg of Quercetin equivalent per 100 gram of pollen (mgQE 100g-1) in pollen from Bidens spp. to 49.6 ± 0.2 mgQE 100g-1 in pollen from Eucalyptus spp. Notably, all tested pollen samples from different plant species exhibited a significant difference among them (p<0.05). Although there is limited information available in the journal about the flavonoid content of bee pollen in Ethiopia, TFCC in bee pollen has been determined in various previous studies. For example, TFCC in bee pollen from the southern region of Brazil ranged from 2.10 to 28.33 mgQE g-1 [24], from Northwest Algeria it was reported as 8.92 mgQE g-1 [21], from the Baltic region it ranged from 6.1 to 11.6 mgQE g-1 [22], and from South Korea it ranged from 1.84 to 6.66 mgQE g-1 [19]. These findings highlight the variability in TFCC among different floral sources and underscore the importance of considering floral differences in future research on bee pollen.
In the current study, the range of TFCC observed in the five major MPE samples aligns with the TFCC range reported in previous studies (1.84 to 28.33 mg QE g-1). However, pollen samples from Eucalyptus spp. (49.6 ± 0.2 mgQE g-1) and G. scabra (31.3 ± 0.7 mgQE g-1) exhibited notably higher TFCC compared to previous studies. Several factors could account for this discrepancy in results. Firstly, differences in botanical and geographical origins of the pollen samples could lead to variations in TFCC. Additionally, the species of bees involved in pollen collection (entomological origin) might contribute to differences in flavonoid composition. Furthermore, variations in the analytical methods used for compound analysis, including procedures and reagents, could influence the observed TFCC values. Overall, the higher TFCC observed in pollen samples from Eucalyptus spp. and G. scabra in the current study underscores the potential significance of these plant species as rich sources of flavonoids in bee pollen. Further research exploring the factors influencing TFCC variations in bee pollen would provide valuable insights into maximizing its nutritional and health benefits.
DPPH free radical scavenging activity: Table 4 presents the scavenging activity of the free DPPH radical in terms of IC50 values (the effective concentration at which 50% of the DPPH radical is scavenged) for each botanical source-based MPE. The results indicate that all five MPE samples exhibited antioxidant activity, with IC50 values ranging from 0.036 ± 0.005 to 0.233 ± 0.057 mg mL-1. These values were lower than that of the control (Ascorbic acid), which had an IC50 value of 0.023 ± 0.005 mg mL-1. Among the tested pollen samples, the highest antioxidant activity was observed in the pollen collected by bees from Eucalyptus spp., with an IC50 value of 0.036 ± 0.005 mg mL-1, followed by a pollen collected from G. scabra plant, with an EC50 value of 0.076 ± 0.005 mg mL-1. In contrast, pollen collected from Bidens spp. plants demonstrated relatively lower antioxidant activity, with an IC50 value of 0.233 ± 0.057 mg mL-1. These findings highlight the varying antioxidant capacities of bee pollen samples collected from different botanical sources. The higher antioxidant activity observed in pollen from Eucalyptus spp. and G. scabra underscores their potential as valuable sources of antioxidants, which could contribute to the health-promoting properties of bee pollen. The IC50 values obtained in the current study fall within a range similar to that of pollen samples from New Zealand and Portugal (0.04 to over 0.5 mg mL-1) [25]. However, they notably differ from IC50 values reported in other regions. For instance, pollen samples from the southern region of Brazil exhibited EC50 values ranging from 0.81 to 4.69 mg mL-1 [24], while samples from Portugal had IC50 values ranging from 2.16 to 5.87 mg mL-1 [23]. Additionally, pollen samples from South Korea showed IC50 values ranging from 0.292 to over 1 mg mL-1 [19]. Notably, no similar studies have been conducted in Ethiopia until now. When comparing the current results with data from other regions, the findings suggest that pollen collected from Ethiopia exhibited high antioxidant activity, as indicated by the low IC50 values. Lower IC50 values signify a higher radical scavenging activity of the samples, highlighting the potential of Ethiopian bee pollen as a rich source of antioxidants. These findings underscore the importance of further research to explore the antioxidant properties of bee pollen in Ethiopia and its potential health benefits. Indeed, various studies have highlighted significant differences in antioxidant activity, chemical component concentration, and types among pollen grains derived from different plant species and geographical locations [13,14]. The type of plant from which bee pollen originates plays a crucial role in determining its composition and properties. Additionally, the growing conditions of the plants, including factors such as soil quality, climate, and altitude, can significantly influence the chemical composition of the pollen. Moreover, the timing of pollen harvest is also a critical factor, as the stage of flowering and environmental conditions during pollen collection can impact its nutritional profile and biological activity. Overall, these factors collectively contribute to the intrinsic biological composition and characteristics of bee pollen, emphasizing the need to consider both botanical and environmental factors in understanding its potential health benefits [12].
Conclusion
The current study revealed a variation in pollen yield throughout different months, with the lowest mean yield recorded in August (4.2 ± 1.2 g/hive) and the highest in November (265.41 ± 40.66 g/ hive). Interestingly, March, April, and July were identified as dearth periods in the study area, during which no pollen was released by plants. Pollen released from Bidens spp. exhibited the lowest TPCC (27.5 ± 0.8 mgGAE/100g) and TFCC (18.8 ± 0.7 mgQE/100g), while Eucalyptus spp. recorded the highest TFCC (62.4 ± 0.5 mgGAE/100g) and TFCC (49.6 ± 0.2 mgQE/100g) through methanol extraction. In terms of antioxidant activity, the current findings demonstrated that all tested plant source pollens exhibited good antioxidant activity, with IC50 values ranging from 0.036 ± 0.005 (Eucalyptus spp.) to 0.233 ± 0.057 (Bidens spp.) mg mL-1. These values were lower than that of the positive ascorbic acid control (IC50, 0.023 ± 0.005 mg mL-1). Particularly noteworthy was the very strong antioxidant activity exhibited by Eucalyptus spp. and G. scabra. Based on these results, Ethiopian bee pollen extracted with methanol could indeed be considered a valuable nutritional addition to food, offering potential benefits in preventing various diseases related to free radicals. The rich antioxidant properties of bee pollen, particularly from sources such as Eucalyptus spp., highlight its potential as a natural healthpromoting supplement.
Author Statements
Data Availability
The datasets used and/or analyzed during the current study are available upon reasonable request from the relevant author.
Funding Statement
No special grant was provided for this project.
Conflicts of Interest
No potential conflict of interest was reported by the author.
Acknowledgments
I would like to thank the Oromia Agricultural Research Institute for financial support and Addis Ababa University, College of Natural and Computational Sciences, Department of Food Science, for antioxidant analysis. I also thank the Holota Apiculture Research Center for its assistance in the melissopalynological analysis. This research was carried out at Addis Ababa University, Department of Food Science, for antioxidant analysis and Holota Apiculture Research Center for melissopalynological analysis.
References
- Ares AM, Valverde S, Bernalm JL, Nozal MJ, Bernal J. Extraction and determination of bioactive compounds from bee pollen. J Pharm Biomed Anal. 2018; 147: 110–124.
- Campos MG, Webby RF, Markham KR, Mitchell KA, Da Cunha AP. Age- Induced Diminution of free radical scavening capacity in bee pollens and the contribution of Consistent flavonoids. J Agric Food Chem. 2003; 51: 742–745.
- Lopes AJO, Vasconcelos CC, Garcia JBS, Pinheiro MSD, Pereira FAN, de Sousa Camelo D, et al. Anti-Inflammatory and Antioxidant Activity of Pollen Extract Collected by Scaptotrigona affinis postica: In silico, in vitro, and in vivo Studies. Antioxidants. 2020; 9: 103.
- Thakur M, Nanda V. Composition and functionality of bee pollen: A review. Trends Food Sci Technol. 2020; 98: 82–106.
- Keskin M, Özkök A. Effects of drying techniques on chemical composition and volatile constituents of bee pollen. Czech J Food Sci. 2020; 38: 203–208.
- Rodríguez-Pólit C, Gonzalez-Pastor R, Heredia-Moya J, Carrera-Pacheco SE, Castillo-Solis F, Vallejo-Imbaquingo R, et al. Chemical Properties and Biological Activity of Bee Pollen. Molecules. 2023; 28: 7768.
- Martinello M, Mutinelli F. Antioxidant activity in bee products: A review. Antioxidants. 2021; 10: 71.
- Feás X, Vázquez-Tato MP, Estevinho L, Seijas JA, Iglesias A. Organic bee pollen: Botanical origin, nutritional value, bioactive compounds, antioxidant activity and microbiological quality. Molecules. 2012; 17: 8359–8377.
- Saraiva LC, Cunha FV, Léllis DR, Nunes LC. Composición, actividad biológica y toxicidad del polen apícola: Estado-del-arte. Boletín Latinoam. Caribe Plantas Med. Aromáticas. 2018; 17: 426–440.
- Denisow B, Denisow-Pietrzyk M. Biological and therapeutic properties of bee pollen: A review. J Sci Food Agric. 2016; 96: 4303–4309.
- Leja M, Mareczek A, Wyzgolik G, Klepacz-Baniak J, Czekonska K. Antioxidative properties of bee pollen in selected plant species. Food Chem. 2007; 100: 237–240.
- Kocot J, Kielczykowska M, Luchowska-Kocot D, Kurzepa J, Musik I. Antioxidant potential of propolis, bee pollen, and royal jelly: Possible medical application. Oxidative Med Cell Longev. 2018; 2018: 7074209.
- Alici´c D, Šubari´c D, Jaši´c M, Pašali´c H, Ackar Ð. Antioxidant properties of pollen. Hrana Zdr. Boles. Znan. Strucni Casopis Za Nutr. Dijetetiku. 2014: 3: 6–12.
- Fatrcová-šramková K, Nôžková J, Kacániová M, Máriássyová M, Rovná K, Stricík M. Antioxidant and antimicrobial properties of monofloral bee pollen. J. Environ. Sci. Health Part B. 2013; 48: 133–138.
- Louveaux J, Maurizio A, Vorwohl G. Methods of melissopalynology. Bee World. 1978; 59: 139–157.
- Von Der Ohe W, Oddo LP, Piana ML, Morlot M, Martin P. Harmonized methods of melissopalynology. Apidologie. 2004: 18-25.
- N Adgaba. Atlas of Pollen Grains of Major Honey Bee Flora of Ethiopia. Holeta Bee Research Centre, Commercial Printing Enterprise, Addis Ababa, Ethiopia. 2007: 152.
- Addi A, Ensermu K, Teshome S. Proximate composition and antioxidant power of bee collected pollen from moist Afromontan forests in southwest Ethiopia. Agr Sci Res J. 2017; 7: 83-95.
- Zou Y, Hu J, Huang W, Zhu L, Shao M, Dordoe C, et al. The botanical origin and antioxidant, anti-BACE1 and antiproliferative properties of bee pollen from different regions of South Korea. BMC Complementary Medicine and Therapies. 2020; 20: 1-4.
- Freire KRL, Lins ACS, Dórea MC, Santos FAR, Camara CA, Silva TMS. Palynological origin, phenolic content, and antioxidant properties of honeybee-collected pollen from Bahia, Brazil. Molecules. 2012; 17: 1652–64.
- Rebiai A, Lanez T. Chemical composition and antioxidant activity of Apis mellifera bee pollen from Northwest Algeria. J Fundam Appl Sci. 2012; 4: 155 –63.
- Kaškoniene V, Ruočkuviene G, Kaškonas P, Akuneca L, Maruška A. Chemometric Analysis of Bee Pollen Based on Volatile and Phenolic Compound Compositions and Antioxidant Properties. Food Anal Methods. 2015; 8: 1150 –63.
- Morais M, Moreiraa L, Feás X, Estevinho LM. Honeybee-collected pollen from five Portuguese natural parks: Palynological origin, phenolic content, antioxidant properties and antimicrobial activity. Food Chem Toxicol. 2011; 49: 1096 –101.
- Carpes S, Mourão GB, Alencar SM, Masson ML. Chemical composition and free radical scavenging activity of Apis mellifera bee pollen from southern Brazil. Braz J Food Technol. 2009; 12: 220 –9.
- Campos MG, Webby RF, Markham KR, Mitchell KA, Da Cunha AP. Ageinduced diminution of free radical scavenging capacity in bee pollens and the contribution of constituent flavonoids. J Agric Food Chem. 2003; 51: 742 –5.