
Research Article
Austin J Nutr Metab. 2015;2(1): 1011.
A Randomized Study of the Effect of Fish Oil on n-3 Fatty Acid Incorporation and Nutritional Status in Lung Cancer Patients
Andersen JR1,3*, Dannerfjord S1, Nørgaard M1, Lauritzen L1, Lange P2, Jensen N Aa2, Boisen LW2, Jensen RG2, Andersen MJ2 and Sørensen JB2
1Department of Nutrition, Exercise and Sports, University of Copenhagen, Denmark
2Clinic for Oncology 5111, Rigshospitalet, Denmark
3Nutrition Unit 5711, Rigshospitalet, Denmark
*Corresponding author: Andersen JR, Associate Professor, Department of Nutrition, Exercise and Sports, University of Copenhagen, Denmark
Received: January 04, 2015; Accepted: March 20, 2015; Published: March 31, 2015
Abstract
Long-chain n-3 polyunsaturated fatty acids (n-3 LCPUFA) have been proposed to have beneficial effect on cancer cachexia. The aims of the present study were to a) determine the incorporation of n-3 LCPUFA in erythrocytes (RBC) as a measurement of compliance to fish oil (FO)-supplement in lung cancer patients undergoing anti-neoplastic therapy; and b) evaluate the effect of the FO-supplement on weight-loss, mid arm muscle circumference, energy and protein intake, hand grip strength and quality of life. Forty-two patients with advanced lung cancer were randomized immediately after referral to ingest either 20 ml of FO or 20 ml of rapeseed oil (RO) daily. Patients were evaluated every three weeks. Twenty-five patients participated in the study for more than 21 days. The RBC content in FO-group increased with 35%, 137% and 44%, respectively (p < 0.001, p < 0.001 and p < 0.001), but did not change in the ROgroup. Neither intention-to-treat analysis nor per-protocol-analysis revealed any statistically significant differences between the groups with respect to clinical outcomes.
Keywords: Lung cancer; Cachexia; Fish oil; n-3 Fatty acids
Abbreviations
ALA: Alpha-Linolenic Acid; DHA: Docosahexaenoic Acid; EPA: EicosaPentaenoic Acid; RBC: Erythrocyte; FO: Fish Oil; n-3 LCPUFA: Long-Chain n-3 Polyunsaturated Fatty Acids; RO: Rapeseed Oil; QoL: Quality of Life
Introduction
Progressive nutritional deterioration with insufficient intake of macro- and micro-nutrients is common in cancer patients and is associated with changes in carbohydrate, lipid and protein metabolism [1,2]. Malnutrition occurs in 60% of all patients with small cell- and non-small cell lung cancer [2], and this is partly a consequence of cachexia, which is a multi-factorial syndrome including anorexia, severe weight loss, muscular atrophy and weakness [1,3,4,5]. Previous investigations have shown that cachexia deteriorates the patients quality of life (QoL) [6], shortens survival time, and reduces the response to chemotherapy [1,7,8,9]. Supplementations with energy and protein has not been proven efficient in inhibiting the deterioration [6,10,11], and the potential weight gain, if any, consists primarily of fat and water, and not lean body mass [6,11,12,13,14].
Cachexia is partly due to an increase in inflammatory cytokines, and as long-chain n-3 polyunsaturated fatty acids (n-3 LCPUFA) have been shown to have anti-inflammatory and cytokine reducing effects [15], they may be beneficial in cancer patients with a high inflammatory response. n-3 LCPUFA has been shown to normalize some of the metabolic abnormalities in cachexia such as hypermetabolism and insulin-resistance in patients with pancreatic cancer [16,17,18]. Furthermore, good clinical results primarily on QoL have been achieved in studies with palliative pancreatic cancer patients, if patients are compliant to the treatment with fish-oil (FO) containing supplements [19].
For these reasons we wanted to test if supplements with n-3 LCPUFA lead to incorporation of these fatty acids into the cell membranes during cytotoxic treatment and if that has beneficial effects on weight loss in patients with lung cancer.
Materials and Methods
The investigation was approved by the regional ethical committee.
Patients with small cell- or non-small cell lung cancer referred to the Clinic of Oncology, Rigshospitalet, Copenhagen, were eligible for the study. All patients received platinum-based chemotherapy (Carboplatin or Cisplatin) in combination with Vinorelbine or Etoposid. Patients were consecutively included disregarding previous weight loss. Patients were excluded, if they had ingested daily supplements of n-3 LCPUFA within 60 days prior to the time of randomization, suffered from spontaneous bleeding tendency or were in anti-coagulant therapy. Overall 44 patients were randomized by concealed allocation to receive either FO (N=21) or rapeseed oil (RO, N=23). However, one patient in the FO-group were excluded from the study before baseline due to clinical deterioration and one in the RO-group due to concomitant prostate cancer, leaving 20 patients in the FO-group and 22 patients in the RO-group. The baseline characteristics of the included patient in the two groups are shown in Table 1.
Fish oil group
Rapeseed oil group
p
Sex (male/female)
13 / 7
9 / 13
0.211
Ages (y)
64.5 (59.0-73.3) (20)
69 (60.5-71.3) (22)
0.801
Body weight (kg)
76.6 (64.3-84.1) (19)
69.5 (57.1-72.8) (22)
0.100
Height (m)
1.74 ±0.1 (18)
1.67 ±0.1 (20)
0.029
BMI (kg/m2)
24.9 (21.6-25.9) (17)
22.9 (21.6-27.6) (20)
0.855
Weight change during 3 month prior to inclusion (%).
0.0 (-4.2-0.0) (19)
– 6.7 (-14.8- -2.1) (20)
0.032
Energy intake (MJ/d)
8.5 (6.6-10.2) (16)
6.8 (5.7-9.0) (20)
0.098
Protein intake (g/d)
75 (64-90) (16)
70 (57-86) (20)
0.484
QoL: Fatigue score
33.3 (16.7-50.5) (19)
44.4 (22.2-66.7) (22)
0.316
QoL: Global health score
66.7 (50-83.3) (19)
58.3 (50-77) (22)
0.812
MS (kg)
33.0 (21.8-38.8) (20)
22.5 (18.8-28.4) (22)
0.195
MaC (cm)
29.4 (27.8-32.6) (20)
28.2 (25.9-31.3) (22)
0.378
Data are given as mean ± SD-value for normal distributed data (student t-test) and median (1. quartile - 3. quartile) for non-parametric data (Mann-Whitney).
Abbrevations: BMI: Body Mass Index; QoL: Quality of Life measured by EORTC-C30 and LC13. Scores are sums in the main sections of the questionaires, MS: Muscular Strength, MaC: Mid-arm Circumference.
Table 1: Baseline characteristics of the patients with lung cancer referred to anti-neoplastic treatment in the two intervention groups.
Patients were randomly assigned to the two treatment groups in a double-blinded way by sealed envelopes, and had their oils delivered in dark bottles packed by the producer to contain either FO or RO. Patients were instructed to ingest a total of 20 ml of the given oil every day with their meals. Both oils supplied 681 kJ pr. 20 ml. The FO was produced by FF-Denmark and contained 0.235 g of n-3 LCPUFA pr. ml (0.1 g/ml of eicosapentaenoic acid (EPA) and 0.12 g/ml of docosahexaenoic acid (DHA)). The RO was supplied by Aarhus Karlshamn A/S and contained 0.078 g of ALA pr. ml and no n-3 LCPUFA. If disclosed by taste, patients were asked not to reveal the nature of the oil to the investigators. Total oil consumption was registered by daily records. Patients had to participate for at least 21 days to allow for incorporation of n-3 LCPUFA into the cell membranes.
The known side effects of FO are regurgitation, nausea and transient diarrhoea [20-25]. In case of suspected side effects of the oils, these were discontinued for three days to evaluate if they were responsible for the inconvenience. If a subject experienced serious side effects that could be potentially attributed to the oils, the code was broken by the doctor in charge of the patient. Ten patients withdrew from the study because of side effects; diarrhea (2), vomiting (3), increased illness (5). Furthermore, seven subjects dropped out of the study before the minimum period of 21 days due to death (2), reason not specified (4) and loss of contact (1). The reasons for drop-out were evenly distributed among the two oil groups (Figure 1).
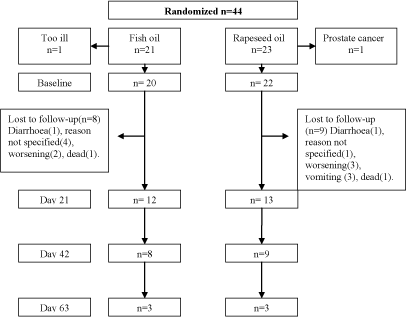
Figure 1: CONSORT flow-chart of 44 patients with lung cancer randomized
to supplementary treatment with either 20 ml/d of fish oil or rapeseed oil.
All patients were blindly evaluated and interviewed by the same person at three-week intervals. Outcome measures at these clinical control visits were:
Weight: Muscular strength was measured by a dynanometer (Jamar, Sammons Prestons Inc). Mid-arm circumference was measured on the right arm. Energy and protein intake was estimated by two different techniques: initially by a three days dietary registration at home (two weekdays and one week-end-day), and after 12 days by 24-hour dietary recall interview as patients were not able to perform any more registrations. A special designed photomap of portion sizes was used for quantification during the interviews.
Patients completed the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-C30 and LC13, which is specific for lung cancer patients. The scores related to fatigue and overall health state was used to reflect the patient’s quality of life (QoL).
Five ml of blood was collected in ice-cold EDTA-conditioned tubes. The blood samples were centrifuged (2300 g for 5 min at 4 °C) to isolate erythrocytes (RBC), which was washed three times in isotonic NaCl solution. Isolated RBC were reconstituted 1:1 in physiological saline with 1mM EDTA and 0,005% BHT and kept at -80°C until they were analyzed. Lipids were extracted from RBC by the Folch procedure [26], Trans esterified by BF3 and the resulting fatty acid methyl esters were separated using a gas chromatography (Hewlett-Packard - HP6890 with a Supelco SP2380 capillary column (30 m, i.d. 0.25 mm, and film thickness of 0.2 mm)). The levels of the individual fatty acids are presented as percent of the total gas chromatogram area (area %, equivalent to percent of all fatty acids from C14:0 to DHA). Successful blood sampling and RBC fatty acid analysis was obtained from 40 patients at baseline and only 25 patients at the end of the intervention. Compliance was evaluated blindly by comparing the patients` daily supplement consumption records with the measured n-3 LCPUFA levels in RBC.
All data were analyzed with Analyse-it® Standard statistical Software (Analyse-it Software Ltd. Leeds, United Kingdom) and significance was set to p < 0.05. Data for height and duration of participation were tested for normality using the Shapiro-Wilk normality test. Group comparisons were performed using Student t-test for normal distributed data, and results are given as mean ± SD. Nonparametric distributed data were analyzed using Mann-Whitney test for unpaired data and Wilcoxon test for paired data, and results are given as median (1. and 3. quartile). Chi-square test (Χ²- test) or Fishers exact test were used for binary data and the Spearman’s rank test for correlation.
Results and Discussion
Patients in RO-group had prior to inclusion lost significantly more weight than patients in FO-group (Table 1). This is a kind of “bad luck randomization”, and as no significant weight loss was observed in either group after the intervention, on could speculate if the rape-seed oil did better that the fish oil, as a progressive weightloss was stopped. However, the information about weight-loss prior to the entrance in the study was given by the patient and not calculated by measurements, and we know that weight is varying due chemotherapy due to edema. As a consequence we do not find it justified to conclude anything about the influence on the oils on the weight changes. According to the supplement consumption records, the patients in the FO-group consumed an average of 17.2 (12.9-18.0) ml oil pr. day for an average period of 48 days, whereas those in the RO-group ingested an average of 15.9 (11.6-18.0) ml/d in an average period of 49 days (p= NS). RBC fatty acid analysis was performed at baseline in 19 patients in the FO- and in 21 patients in RO-group and revealed no significant difference between the two groups (Table 2). Similarly, there was no difference in the intervention periods in the patients showing up for blood samples (Table 2). Twenty-five patients (60%) were available for analysis after the intervention. Drop-out rates were the same in the two groups. The reasons werealso similar, and it is doubtful whether the reasons for drop-out had anything to do with the oils. At the same time as the patients started the oil treatment, they also started chemotherapy, so we are reluctant to relate the symptoms to the oil treatment. About 1/3 of the patients dropped out because of rapid worsening of the disease or death. FO supplementation augmented n-3 LCPUFA and EPA in RBC significant more than RO supplement (Table 2). RBC-EPA was most pronouncedly affected by the FO supplement and increased by an average of 137 %. As shown in Figure 2, the relative increase in the EPA content of the RBC was closely associated with duration of the supplementation period.
Before n=19
After n=21
Fatty acids in RBC
Fish oil group
Rapeseed oil group
p value
Fish oil group
Rapeseed oil group
p value
SFA
40.1 ±1.3
39.8 ±2.1
0.652 NS
38.7 ±2.0
38.6 ±2.3
0.917 NS
MUFA
20.0 ±1.5
20.7 ±1.8
0.202 NS
19.1 ±1.9
20.2 ±1.7
0.070 NS
PUFA
37.6 ±2.4
37.5 ±3.1
0.894 NS
40.6 ±4.3
39.3 ±3.6
0.300 NS
n-6 PUFA
27.8 ±2.3
27.4 ±2.0
0.610 NS
27.5 ±3.6
28.3 ±2.4
0.388 NS
n-3 PUFA
9.8 ±2.2
10.1 ±2.7
0.771 NS
13.1 ±4.0
11.0 ±2.4
0.044
ALA
0.1 ±0.1
0.2 ±0.1
0.466 NS
0.1 ±0.1
0.2 ±0.1
0.035
EPA
0.8 ±0.3
0.9 ±0.4
0.116 NS
1.9 ±1.5
1.0 ±0.4
0.009
DHA
4.1 ±1.1
4.6 ±1.6
0.286 NS
5.9 ±1.9
5.1 ±1.4
0.156 NS
Data are expressed as % of fatty acids and given as mean ± SD. Group comparisons were performed by Student t-test. Abbrevations: SFA: Saturated Fatty Acids, MUFA: Mono Unsaturated Fatty Acids, PUFA: Poly Unsaturated Fatty Acids, ALA: a-Linolenic Acid, EPA: Eicosapentaenoic Acid, DHA: Docosahexaenoic Acid. The duration of the intervention was (mean ± SD) 37.0 ± 23.3 in the fish oil group, and 39.8 ± 19.7 in the rapeseed group (p=0.700).
Table 2: Erythrocyte (RBC) fatty acid content before and after intervention with fish oil or rapeseed oil.
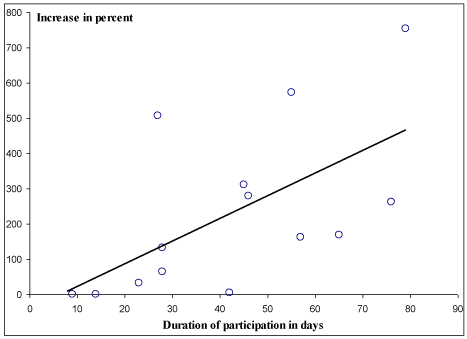
Figure 2: Correlation between duration of intervention in days and total
percent increase in EPA content in RBC, in the fish oil group. r=0.77, p=0.001
(n=14 due to missing data).
FO supplementation had no effect on weight-loss when analyzing the data as intention to treat or per pre-protocol (Table 3). Furthermore, no significant correlations were observed between EPA-RBC-incorporation and changes in any of the clinical outcomes (data not shown).
Fish oil group
Rapeseed oil group
p value
Weight; kg
0.0 (-1.1-0.8) (n=19)
0.0 (-0.5-0.0) (n=21)
0.957NS
Energy intake; kJ/day
0.0 (-2113-3035) (n=16)
0.0 (-120-827) (n=20)
0.706 NS
Energy intake; percent of needed/day
0.0 (-22.8-31.5) (n=16)
0.0 (-1.8-9.4) (n=20)
0.909 NS
Protein intake; gram/day
0.0 (-6-29) (n=16)
0.0 (-4-12) (n=20)
0.444 NS
Protein intake; percent/day
0.0 (-5.8-38.3) (n=16)
0.0 (-5.2-21.1) (n=20)
0.896 NS
QoL – fatigue
0.0 (0.0-11.1) (n=19)
0.0 (-11.1-0.0) (n=22)
0.461 NS
QoL – global health
0.0 (-8.4-0.0) (n=19)
0.0 (0.0-0.0) (n=22)
0.495 NS
MS; percent
0.0 (0.0-3.1) (n=20)
0.0 (-5.7-0.0) (n=22)
0.245 NS
MaC; percent
0.0 (0.0-4.4) (n=20)
0.0 (0.0-2.5) (n=22)
0.763 NS
Data is calculated on individual differences relative to baseline in the two groups and are given as median (1. quartile- 3. quartile). Group comparisons were performed by Mann-Whitney rank sum test. Abbrevations: QoL: Quality of Life, MS: Muscular Strength, MaC; Mid-arm Circumference.
Table 3: Changes in the clinical outcomes during the intervention in patients with lung cancer randomized to supplementary treatment with either 20 ml/d of fish oil or rapeseed oil.
Contrary to most studies, the FO was administered relatively early in the course of disease with the objective to prevent weight loss. However, because of the high dropout rate, it is difficult to conclude with certainty whether or not the n-3 LCPUFA supplement may have had a clinically relevant effect. Within this field there has been a general focus on EPA as the main anti-cachectic agent in fish oil [19]. However, the role of DHA separated from EPA has not been clearly ascertained as most trials have supplies both fatty acids in combination [27]. One should refrain from concluding that DHA possesses no anti-cachectic features, as DHA has been shown to suppress some of the same pro-cachectic agents as EPA [28].
The FO-supplement gave rise to a significant increase in RBC n-3 LCPUFA, most pronouncedly in EPA, which correlated significantly with the duration of the FO-supplementation period. The effects of FO on RBC fatty acid composition in these cancer patients were comparable to the changes that was observed in two previous trials with healthy subjects [29,30].
Based on the self-reported consumption records, the subjects in the FO-group consumed approximately 17 ml of fish oil per day of the intended 20 ml/d and compliance in this study was thus actually superior to some of the previous trials in cancer patients [31,32]. The ingested dose of FO supplied on average 4.0 g/d n-3 LCPUFA (1.7 g EPA and 2.0 g DHA), which is within the range of doses recommended in a recent systematic review [33]. Others have suggested a minimum of 2 g/d of EPA [34,35], as some studies with lower doses have failed to show any beneficial effects [31,32,35].
There was some variation in absolute increase in RBC EPAcontent observed within the individual subjects in the FO-group; one patient had an increase of a factor of five in just 27 days, whereas another patient only had an increase of a factor of three during a period 76 days. This could indicate differences in metabolism, interfering differences in diet, or differences in body composition, but is most likely due to differences in compliance. The difference in achieved tissue level emphasizes the importance of a measurement of the immediate effect on fatty acid composition in membranes. Many of the previous cancer trials has assessed compliance only by self-reported consumption records and some have examined the n-3 LCPUFA content of plasma lipids [3,16,18,32,36,37]. The plasma n-3 LCPUFA content can however only be considered as an indicator of fatty acids ingestion during a short period of time, whereas RBC fatty acid content reflects the intake over a period of weeks or months [38]. As RBC has a mean survival of about 120 days in healthy persons, it is expected, that steady state is achieved in the incorporation after four months. Our results indicated that determination of the content of n-3 LCPUFA in RBC would be a good choice to monitor compliance in cancer patients. The present study shows that n-3LCPUFA is incorporated in RBC in a time dependent manner. A minimal period of supplementation must therefore be expected before any beneficial effect can be expected, and this might explain why two large clinical interventions studies found no advantageous outcome on cancer cachexia of n-3 LCPUFA supplementation for two weeks [31,39].
Conclusion
A daily intake of 17 ml/d FO for an average of 48 days did increase RBC membranes levels of EPA and DHA in lung cancer patients to the same extent as in healthy subjects and that RBC fatty acid measurement can be used as an indicator of compliance during FO treatment. However, FO supplement did not affect weight loss in these patients. As this study included few patients and had a high dropout rate, it is difficult to conclude whether n-3 LCPUFA supplementation have clinically relevant effects on cachexia in lung cancer patients when administered early in the course of their disease.
Acknowledgements
We are grateful to laboratory technician Pia Madsen for her valuable efforts. The study received financial support from FF Denmark.
References
- Argilés JM1. Cancer-associated malnutrition. Eur J Oncol Nurs. 2005; 9 Suppl 2: S39-50.
- Bozzetti F. Nutrition support in patients with cancer. Payne-James J, Grimble G, Silk DBA, editors. In: Artificial nutrition support in clinical practice. Greenwich, 2001; 639-680.
- Fearon KC, Barber MD, Moses AG, Ahmedzai SH, Taylor GS, Tisdale MJ, et al. Double-blind, placebo-controlled, randomized study of eicosapentaenoic acid diester in patients with cancer cachexia. J Clin Oncol. 2006; 24: 3401-3407.
- Fearon KC, Moses AG. Cancer cachexia. Int J Cardiol. 2002; 85: 73-81.
- Tisdale MJ. Cancer anorexia and cachexia. Nutrition. 2001; 17: 438-442.
- Ovesen L, Allingstrup L, Hannibal J, Mortensen EL, Hansen OP. Effect of dietary counseling on food intake, body weight, response rate, survival, and quality of life in cancer patients undergoing chemotherapy: A prospective, randomized study. J Clin Oncol. 1993; 11: 2043-2049.
- Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980; 69: 491-497.
- van Eys J. Effect of Nutritional Status on Response to Therapy. Cancer Res. 1982; 42: 7747-7753.
- Warren S. The immediate causes of death in cancer. Am J Med Sci. 1932; 184: 610-619.
- Evans WK, Nixon DW, Daly JM, Ellenberg SS, Gardner L, Wolfe E, et al. A randomized study of oral nutritional support versus ad lib nutritional intake during chemotherapy for advanced colorectal and non-small-cell lung cancer. J Clin Oncol. 1987; 5: 113-124.
- Lundholm K, Daneryd P, Bosaeus I, Körner U, Lindholm E. Palliative nutritional intervention in addition to cyclooxygenase and erythropoietin treatment for patients with malignant disease: Effects on survival, metabolism, and function. Cancer. 2004; 100: 1967-1977.
- Bauer J, Capra S, Battistutta D, Davidson W, Ash S; Cancer Cachexia Study Group. Compliance with nutrition prescription improves outcomes in patients with unresectable pancreatic cancer. Clin Nutr. 2005; 24: 998-1004.
- Evans WK, Makuch R, Clamon GH, Feld R, Weiner RS, Moran E, et al. Limited impact of total parenteral nutrition on nutritional status during treatment for small cell lung cancer. Cancer Res. 1985; 45: 3347-3353.
- Nixon DW, Lawson DH, Kutner M, Ansley J, Schwarz M, Heymsfield S, et al. Hyperalimentation of the cancer patient with protein-calorie undernutrition. Cancer Res. 1981; 41: 2038-2045.
- Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004; 79: 935-945.
- Barber MD, Ross JA, Voss AC, Tisdale MJ, Fearon KC. The effect of an oral nutritional supplement enriched with fish oil on weight-loss in patients with pancreatic cancer. Br J Cancer. 1999; 81: 80-86.
- Barber MD, Preston T, McMillan DC, Slater C, Ross JA, Fearon KCH. Modulation of the liver export protein synthetic response to feeding by an n-3 fatty-acid-enriched nutritional supplement is associated with anabolism in cachectic cancer patients. Clin Sci 2004; 106: 359-364.
- Wigmore SJ, Ross JA, Falconer JS, Plester CE, Tisdale MJ, Carter DC, et al. The effect of polyunsaturated fatty acids on the progress of cachexia in patients with pancreatic cancer. Nutrition. 1996; 12: S27-30.
- Barber MD, Fearon KC, Tisdale MJ, McMillan DC, Ross JA. Effect of a fish oil-enriched nutritional supplement on metabolic mediators in patients with pancreatic cancer cachexia. Nutr Cancer. 2001; 40: 118-124.
- Harvie MN. The influence of the acute - phase response on energy balance in advanced cancer patients. Proceedings of the nutrition society. 1998; 57: 103A.
- Selby P, Hobbs S, Viner C, Jackson E, Jones A, Newell D, et al. Tumour necrosis factor in man: clinical and biological observations. Br J Cancer. 1987; 56: 803-808.
- Siddiqui RA, Harvey KA, Zaloga GP, Stillwell W. Modulation of lipid rafts by Omega-3 fatty acids in inflammation and cancer: implications for use of lipids during nutrition support. Nutr Clin Pract. 2007; 22: 74-88.
- Sobrado J, Moldawer LL, Bistrian BR, Dinarello CA, Blackburn GL. Effect of ibuprofen on fever and metabolic changes induced by continuous infusion of leukocytic pyrogen (interleukin 1) or endotoxin. Infect Immun. 1983; 42: 997-1005.
- Tisdale MJ. Wasting in cancer. J Nutr. 1999; 129: 243S-246S.
- Tisdale MJ. Metabolic abnormalities in cachexia and anorexia. Nutrition. 2000; 16: 1013-1014.
- Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226: 497-509.
- Colomer R, Moreno-Nogueira JM, García-Luna PP, García-Peris P, García-de-Lorenzo A, Zarazaga A, et al. N-3 fatty acids, cancer and cachexia: a systematic review of the literature. Br J Nutr. 2007; 97: 823-831.
- Shaikh IA, Brown I, Schofield AC, Wahle KW, Heys SD. Docosahexaenoic acid enhances the efficacy of docetaxel in prostate cancer cells by modulation of apoptosis: the role of genes associated with the NF-kappaB pathway. Prostate. 2008; 68: 1635-1646.
- Cao J, Schwichtenberg KA, Hanson NQ, Tsai MY. Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin Chem. 2006; 52: 2265-2272.
- Wilkinson P, Leach C, Ah-Sing EE, Hussain N, Miller GJ, Millward DJ, et al. Influence of alpha-linolenic acid and fish-oil on markers of cardiovascular risk in subjects with an atherogenic lipoprotein phenotype. Atherosclerosis. 2005; 181: 115-124.
- Bruera E, Strasser F, Palmer JL, Willey J, Calder K, Amyotte G, et al. Effect of Fish Oil on Appetite and Other Symptoms in Patients With Advanced Cancer and Anorexia/Cachexia: A Double-Blind, Placebo-Controlled Study. J Clin Oncol. 2003; 21: 129-134.
- Fearon KC, Von Meyenfeldt MF, Moses AG, Van Geenen R, Roy A, Gouma DJ, et al. Effect of a protein and energy dense N-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomised double blind trial. Gut. 2003; 52: 1479-1486.
- Burns CP, Halabi S, Clamon G, Kaplan E, Hohl RJ, Atkins JN, et al. Phase II study of high-dose fish oil capsules for patients with cancer-related cachexia. Cancer. 2004; 101: 370-378.
- Fearon KC. The anticancer and anticachectic effects of n-3 fatty acids. Clin Nutr. 2002; 21: 73-77.
- Fearon KCH, von Meyerfeldt M, Moses AGW, van Geenen R, Roy A, Gouma D, et al. An energy and protein dense, high n-3 fatty acid oral supplement promotes weight gain in cancer cachexia. Eur J Cancer. 2001; 37: S27-S28.
- Moses AWG, Slater C, Preston T, Barber MD, Fearon KCH. Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancer can be modulated by an energy and protein dense oral supplement enriched with n-3 fatty acids. Brit J Cancer. 2004; 90: 996-1002.
- Wigmore SJ, Barber MD, Ross JA, Tisdale MJ, Fearon KC. Effect of oral eicosapentaenoic acid on weight loss in patients with pancreatic cancer. Nutr Cancer. 2000; 36: 177-184.
- Shannon J, King IB, Moshofsky R, Lampe JW, Gao DL, Ray RM, et al. Erythrocyte fatty acids and breast cancer risk: a case-control study in Shanghai, China. Am J Clin Nutr. 2007; 85: 1090-1097.
- Jatoi A, Rowland K, Loprinzi CL, Sloan JA, Dakhil SR, MacDonald N, et al. An eicosapentaenoic acid supplement versus megestrol acetate versus both for patients with cancer associated wasting: a north central cancer treatment group and national cancer institute of canada collaborative effort. J Clin Oncol. 2004; 22: 2469-2476.