
Short Communication
Austin J Nutr Metab. 2015;2(1): 1013.
Dietary Sorghum Prevents the Elevation of Blood Pressure in KK-Ay Mice
Watanabe N1*, Sakamoto Y2, Fujimoto M3, Takumi M3 and Takamura Y4
1Graduate School of Human Life Sciences, Showa Women’s University, Japan
2Department of Nutrition and Food Science, Ochanomizu University, Japan
3Koyo Sangyo Co., Ltd, Japan
4Mottainai Biomass Research Corporation, Japan
*Corresponding author: Nakamichi Watanabe, Graduate School of Human Life Sciences, Showa Women’s University, Japan
Received: April 10, 2015; Accepted: May 16, 2015; Published: June 01, 2015
Abstract
We validated the effect of sorghum wax on disease progression in KK-Ay/ TaJcl mice. We revealed that dietary sorghum wax prevented the elevation of systolic blood pressure accompanied by a decrease of plasma angiotensin-2. This beneficial effect might be induced by the decrease of white adipose tissue weight, reduced secretion of angiotensinogen, and increase of brown adipose tissue weight.
Keywords: Blood pressure; KK-Ay; Octacosanol; Policosanol; Sorghum; Wax
Introduction
Octacosanol is a 28-carbon aliphatic alcohol that exists in/on rice bran, sugarcane, wheat, and maize. Research associated with octacosanol was started in the 1960s by Cureton. He suggested that dietary octacosanol, derived from wheat germ, increased physical endurance and improved skeletal muscle function [1]. Octacosanol has been used as a medicine for dyslipidemia to decrease plasma cholesterol in Cuba since the 1990s. Moreover, intake of octacosanol has been suggested to have beneficial effects on hypercholesterolemia and type-2 diabetes in humans [2,3].
Long-chain aliphatic alcohols, aldehydes, and fatty acids with a high melting point are considered to be wax. Much wax is present on the epidermis of the stalk of sorghum, an annual plant of the Gramineae family. The wax is comprised of policosanol (-OH) and policosanal (-CHO), such as octacosanol (C28-OH), octacosanol (C28-CHO), triacontanol (C30-OH), and triacontanal (C30-CHO) [4]. Therefore, the intake of sorghum wax is expected to be effective in preventing dyslipidemia.
Because dyslipidemia is induced by obesity, we selected KKAy/ TaJcl mice for this investigation. These mice are generally used as a model of type-2 diabetes and exhibit hyperphagia, obesity, hypertension, and hyperlipidemia [5,6]. The aim of this study was to validate the effect of sorghum wax on disease progression in KK-Ay/ TaJcl mice.
Materials and Methods
Animals and experimental protocol
Four-week-old male KK-Ay/TaJcl mice were purchased from CLEA Japan, Inc. (Tokyo, Japan). The mice were kept in individual plastic cages at 23 ± 2°C with a 12-hour light-dark cycle (light from 8 a.m. to 8 p.m.). After mice were acclimated for one week on commercial non-purified chow diet (CRF-1: Charles River Laboratories Japan Inc.), they were divided into control and sorghum groups (n = 8 / group) to allow the HbA1c levels of each group to be equal at the outset. The experimental diets of each group were prepared based on an AIN-93G diet. The diet of the sorghum group contained 0.5 w/w% sorghum waxes, whereas that of the control group contained 0.5 w/w% olive oil instead of sorghum wax. The wax consisted of 1.7 w/w% octacosanol, 3.8 w/w% triacontanol, w/ w10.8 % octacosanol, and 36.8 w/w% triacontanal. The mice were provided with the food and water ad libitum. Body weight, food intake, water intake, and blood pressure were measured once 7 days. After feeding with the experimental diets for 6 weeks, the mice were euthanized by decapitation and samples of blood and organs were collected. The blood samples were used to measure HbA1c, and the remainder of each sample was centrifuged at 1,900 × g for 10 min to obtain plasma. Plasma samples were used to measure glucose, total cholesterol, triglyceride, insulin, adiponectin, resistin, leptin, and angiotensin-2. This study was approved by the experimental animal ethics committee of Showa Women’s University and was performed according to the experimental animal guidelines.
Measurement of blood parameters
HbA1c was measured with DCA Vantage (Siemens Japan K.K., Tokyo, Japan). Plasma levels of glucose, total cholesterol, and triglyceride were measured using commercial kits, i.e. the Glucose C-test, Cholesterol E-test, and Triglyceride E-test, respectively (Wako Pure Chem., Osaka, Japan). Plasma levels of insulin, adiponectin, resistin, leptin, and angiotensin-2 were measured using commercial ELISA kits, i.e. Ultra Sensitive Mouse Insulin ELISA kit (Morinaga Institute of Biological Science, Kanagawa, Japan), Adiponectin Mouse ELISA kit (R&D Systems Inc., Minnesota, USA), Resistin Mouse ELISA kit (R&D Systems Inc., Minnesota, USA), Leptin Mouse ELISA kit (R&D Systems Inc., Minnesota, USA), and Angiotensin-2 EIA kit (Phoenix Pharmaceuticals Inc., California, USA), respectively.
Measurement of systolic blood pressure
We measured blood pressure using a non-invasive tail-cuff approach with a BP-98A-L (Softron, Tokyo, Japan). Blood pressure was measured in triplicate, and then calculated mean value.
Statistical analysis
All data are expressed as the mean ± standard error. We used student’s unpaired t-test to evaluate significant differences between the control and sorghum groups. Significant difference was achieved when the P-value was under 0.05.
Results
Body weight, food intake, water intake, and some organs weights
As shown in Table 1, the epididymis white adipose tissue (WAT) weight of the sorghum group was significantly lower than that of the control group, whereas the interspacular brown adipose tissue (BAT) weight of the sorghum group was significantly higher than that of the control group. On the other hand, there were no significant differences between the two experimental groups in body weight, food intake, water intake, or the weights of the heart, liver, spleen, kidney, and pancreas and calf skeletal muscles.
Control
Sorghum
Body weight (g)
40.5±1.7
39.5±1.3
Food intake (g/day)
13.5±0.4
13.5±2.5
Water intake (g/day)
27.7±3.1
27.2±2.5
Some organs weights (g)
Heart
0.175±0.006
0.191±0.013
Liver
2.054±0.087
2.023±0.115
Spleen
0.106±0.008
0.113±0.016
Kidney
0.303±0.014
0.283±0.152
Pancreas
0.280±0.018
0.278±0.014
Epididymis white adipose tissue
1.907±0.047
1.759±0.071*
Interspacular brown adipose tissue
0.328±0.003
0.391±0.124*
Calf skeletal muscle
0.245±0.007
0.246±0.009
Data are mean ± SE in 8 mice.
Data are mean ± SE in 8 mice.
Table 1: Body weight, food intake, water intake, and some organs weights.
Blood and plasma analysis
As shown in Table 2, plasma angiotensin-2 levels of the sorghum group were significantly lower than those of the control group. On the other hand, the blood levels of fasted glucose, HbA1c did not exhibit significant differences. The plasma levels of cholesterol, triglyceride, insulin, adiponectin, resistin, and leptin also did not exhibit significant differences.
Control
Sorghum
Blood analysis
Fasted glucose (mg/dL)
93.8±7.6
81.5±9.8
HbA1c (%)
10.8±0.4
10.9±0.5
Serum analysis
Cholesterol (mg/dL)
133.3±4.5
132.4±9.3
Triglyceride (mg/dL)
179.7±18.9
197.3±21.9
Insulin (ng/mL)
15.0±0.9
16.0±0.9
Adiponectin (µg/mL)
4.2±0.4
4.1±0.2
Resistin (ng/mL)
6.5±0.5
7.7±0.4
Leptin (ng/mL)
51.9±4.3
57.3±4.9
Angiotensin-2 (ng/mL)
26.8±3.5
17.9±2.6*
Data are mean ± SE in 8 mice.
Significantly different from the control group: *p < 0.05.
Table 2: Blood and serum analysis.
Changes of systolic blood pressure
The changes of systolic blood pressure during the experimental period are shown in Figure 1. The systolic blood pressures of the sorghum group were significantly lower than those of the control group at the 5 and 6 week.
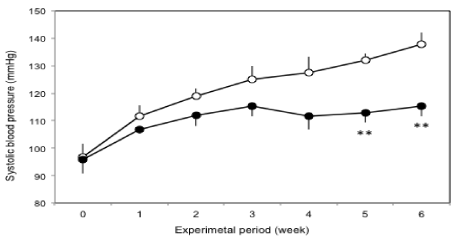
Figure 1: Changes of systolic blood pressure during the experimental period
in KK-Ay/TaJcl mice.
Symbols: O: Control group; ●: Sorghum group. Each data are expressed
as the mean ± SE of 8 mice. Significantly different from the control group:
**p <; 0.01.
Discussion
The prevention of the elevation of systolic blood pressure in the KK-Ay/TaJcl mice that were fed sorghum wax could be induced by the decrease of plasma angiotensin-2, which, in turn, might be induced by the decrease of the WAT weight. WAT has been reported to be the organ that secretes angiotensinogen. Angiotensinogen is well known as the precursor of angiotensin-2, which has been suggested to change in parallel with systolic blood pressure [7]. Moreover, gene transcription of angiotensinogen in intra-abdominal fat has also been suggested to be induced by obesity [8].
The antihypertensive action of sorghum wax might be induced by the increase of BAT weight, because BAT has also been suggested to be associated with blood pressure. The activation of BAT by arotinolol hydrochloride in the hypertensive patients with obesity has been suggested to lead to a decrease of blood pressure [9].
Incidentally, Kabir et al. suggested that oral administration of octacosanol to rats would be distributed in adipose tissue, in particular BAT, although there was very little absorption from the intestine [10]. Because octacosanol might have an effect on BAT and WAT, this report could support our findings that the weights of BAT and WAT in mice fed sorghum wax were higher and lower than those of the control group, respectively.
One issue that we were not able to resolve in this study is what the active ingredients were. Most studies associated with wax have been performed using octacosanol. By contrast, only 1.7% octacosanol was present in the sorghum wax, whereas signif cant proportions of the other policosanol and policosanal were present. Since there have been no reports comparing the number of carbons or the functional groups, additional studies are needed to resolve this issue.
In this study, we revealed that dietary sorghum wax prevented the elevation of systolic blood pressure accompanied with the decrease of plasma angiotensin-2 level. This beneficial effect might be induced by a decrease weight of the WAT, the organ that secretes angiotensinogen, and an increase of BAT weight.
References
- Cureton TK. The physiological effects of wheat germ oil on humans in exercise. Springfield, Illinois, USA. 1972; 22-96.
- Castaño G, Mas R, Fernández L, Illnait J, Mesa M, Alvarez E, et al. Comparison of the efficacy and tolerability of policosanol with atorvastatin in elderly patients with type II hypercholesterolaemia. Drugs Aging. 2003; 20: 153-163.
- Crespo N, Illnait J, Más R, Fernández L, Fernández J, Castaño G. Comparative study of the efficacy and tolerability of policosanol and lovastatin in patients with hypercholesterolemia and noninsulin dependent diabetes mellitus. Int J Clin Pharmacol Res. 1999; 19: 117-127.
- Hwang KT, Weller CL, Cuppett SL, Hanna MA. Policosanol Contents and Composition of Grain Sorghum Kernels and Dried Distillers Grains. Cereal Chem. 2004; 81: 345-349.
- Kennedy AJ, Ellacott KL, King VL, Hasty AH. Mouse models of the metabolic syndrome. Dis Model Mech. 2010; 3: 156-166.
- Ohashi K, Ishikawa H, Ohta Y. Octacosanol ameliorates hyperlipidemia and oxidative stress in KKAy mice with type 2 diabetes. J Anal Bio-Sci. 2011; 34: 223-233.
- Iwai M, Horiuchi M. Role of renin-angiotenin system in adipocyte function. Adiposcience. 2005; 2: 249-253 (in Japanese).
- Rahmouni K, Mark AL, Haynes WG, Sigmund CD. Adipose depot-specific modulation of angiotensinogen gene expression in diet-induced obesity. Am J Physiol Endocrinol Metab. 2004; 286: E891-895.
- Yoshida T. Brown adipose tissue and obesity. Therapeutic Research. 1993; 14: 2399-2415 (in Japanese).
- Kabir Y, Kimura S. Biodistribution and metabolism of orally administered octacosanol in rats. Ann Nutr Metab. 1993; 37: 33-38.