
Research Article
Austin J Nutr Metab. 2015;2(2): 1020.
Integrative Health Check Reveals Suboptimal Levels in a Number of Vital Biomarkers
Travica N*, Ried K, Bujnowski R and Sali A
National Institute of Integrative Medicine (NIIM), Australia
*Corresponding author: Nikolaj Travica, National Institute of Integrative Medicine, Melbourne, Australia
Received: June 01, 2015; Accepted: June 29, 2015; Published: June 30, 2015
Abstract
Background: Health checks are becoming more available in the community, with the intention of disease detection and prevention in asymptomatic people. This article summarises findings in an Australian adult sample from a health check conducted at the National Institute of Integrative Medicine (NIIM). There has been controversy regarding reference ranges and what constitutes optimal and standard reference ranges. Biomarkers were compared with standard reference ranges, and with optimal health reference ranges.
Methods: A total of 139 participants voluntarily undertook the NIIM Health Check across a 4 year period. Participants underwent a full day of medical examinations, including liver and kidney function, thyroid, full blood count, glucose, vitamin and mineral tests. Suboptimal values were evident in a large portion of participants in vitamin D, vitamin B12, homocysteine, and iodine levels. Variables such as age, gender, Body Mass Index (BMI) and season were important covariates.
Conclusion: The sub-optimal levels in vitamin D, vitamin B12, sub-standard levels in iodine, and excessive homocysteine, were consistent with previous population studies and are associated with a number of preventative diseases such as dementia, hypertension, cancer and diabetes. Our analysis highlights the importance to screen for biomarkers prone to deficiencies in Australia.
Keywords: Biomarkers; Health Check; Iodine; Homocysteine; Vitamin D; Vitamin B12
Introduction
The recent National Healthy Survey indicates an all-time high prevalence of chronic diseases among Australians, including cancer, diabetes, cardiovascular disease, long-term mental or behavioural conditions and asthma [1,2]. Almost all Australians (99%) aged 15 and over have at least one risk factor for poorer health such as high blood pressure or vitamin deficiency due to poor nutrition, and about 1 in 7 people have five or more risk factors [3]. Encouraged by these statistics, we have initiated a health check program that evaluates both current and potential matters of health and offers follow-up advice.
Medical screening has a long history [4]. The World Health Organization (WHO) has encouraged a holistic view of health by defining it as ‘a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity’ [5]. Screening tests and examinations, including comprehensive health checks can help with detection and prevention of diseases.
Health checks have become a common part of hospitals, insurance companies, schools and workplaces. Notably, the Victorian government implemented the Work Health program which conducted approximately 800,000 workplace health checks with the intention of promoting a healthier workforce. However, Vitamin D, Vitamin B12, homocysteine and iodine levels are not routinely assessed in general practice, which we investigated in this study [6].
The health check data used for this analysis was acquired from the National Institute of Integrative Medicine, ‘The NIIM Health Check’, collected over a four year period. The analysis examined levels required for optimal health rather than minimal levels to avoid disease. For a number of biomarkers such as vitamin D, standard reference ranges are below the optimal ranges that are needed for optimal health. Many vitamin D experts advocate maintaining 25(OH) D levels at >75 nmol/L (used in the study) up to as high as 80 nmol/ml or 200 nmol/L [7-9] whereas the standard reference range is considered >50nmol/L. Optimal vitamin D levels (>75nmo/L) have been associated with maximum mineral bone density, increased intestinal calcium absorption, decreased risk of osteoporosis and risk of fracture, higher serum phosphorus levels, increased performance speed and proximal muscle strength, and a significant decrease in the likelihood of chronic diseases such as cancers, auto immune disease, osteoarthritis and diabetes [7-9]. The vitamin B12 standard reference range is 200-700 pg/ml whereas the proposed optimal range is 500 - 1,300 pg/ml [10]. Higher vitamin B12 ranges have been associated with increased cognitive function, and reflexes, decreased brain atrophy, confusion, weakness and depression [10]. Additionally, many experts suggest that the optimal health range for homocysteine is <7µmol/L and the standard reference range is 5.0-12µmol/L, with the optimal range showing a significant association with a lower likelihood of stroke, atherosclerosis and improved overall cardiovascular function [11].
The aim of the present study was to determine whether a sample of health check participants would provide results consistent with previous population literature regarding a number of vital biomarkers.
Methods
The data was obtained from a de-identified cohort of asymptomatic individuals in the National Institute of Integrative Medicine’s ‘NIIM Health Check’, between November 2010 and December 2014. The NIIM Health Check includes a broad spectrum of innovative medical testing considered one of the most comprehensive integrative health checks in Australia.
Participants responded to an advertisement on the NIIM website and provided consent for their de-identified data to be used in this study [12]. Participants were considered eligible if they were capable of attending three appointments across one-month duration, consisting of an initial 5-hour screening appointment a 1.5 hour medical imaging appointment, and a 2 hour final reviewing consultation with a GP practicing integrative medicine (the combination of evidencebased complementary and conventional medicine).
Socio-demographic data was obtained from a standardised online health questionnaire routinely administered to participants prior to their initial appointment. The first appointment involved comprehensive pathology testing. The final appointment took place 4 weeks following the first appointment and included a consultation with an integrative GP and an osteopath, where all reports and test results were discussed. Following the NIIM Health Check, treatment strategies for any abnormalities were discussed, which included behavioural factors such as diet, sleep, exercise, sun exposure, as well as supplementation and/or medication. The NIIM Health Check pathology results are presented here. Pathology tests included liver and kidney function, cholesterol, thyroid, full blood count, glucose, vitamin and mineral tests.
Statistical Analyses
Descriptive analyses were undertaken for all blood tests and were compared to population reference ranges (Melbourne Pathology) and optimal ranges [7,8,10,13-15]. Data was sub-grouped by age, gender, BMI and season. An Analysis of Variance (ANOVA) was used to establish significant differences. All analyses were conducted with IBM SPSS version 22.
Results
A total of 139 participants undertook the NIIM Health Check. Not all biomarkers were measured for all participants, depending on patient requests (Table 1). Extremely high outlier values, due to possible supplementation, were excluded. Mean age of the overall study population was 48.8 years (range 28-82 years) with an even gender balance (52.5% males). Mean blood test results at baseline by gender and the proportion of participants that had levels in the normal range are summarised in Tables 1 and 2. Overall, there was a significant difference between genders on a number of blood tests, such as ferritin (p<0.001), transferrin (p=0.001), saturated transferrin (p=0.003), haemoglobin (p<0.001), red blood cell (p<0.001), platelets (p=0.002), erythrocyte sedimentation rate (p=0.009), and creatinine (p<0.001) (data not shown). A majority of participants were in the normal range for each biomarker, but close to half of the female sample was below the red blood cell and creatinine normal range (56.4% and 69.6% respectively). Standard reference ranges are closely linked to optimal health and minimal risk of diseases. However, for a number of biomarkers, standard reference values were below levels considered to achieve optimal health (Table 2).
Biomarkers
Reference range
n
% of total n
Mean
SD
% within reference range
Total=139
BMI
18.5-24.9 kg/m2
119
86
26.9
4.7
36.1
SBP
<140mm Hg
115
83
123.3
15.3
84.3
DBP
<90mm Hg
115
83
74.6
10.1
91.3
Glucose
3.6-6mmol/L
115
83
4.89
1.7
93.9
TSH
0.5-5.5mU/L
81
58
2
2.3
97.5
T4
11.0-21pmol/L
57
41
15.9
6.2
89.5
T3
3.2-6.4pmol/L
57
41
15.9
6.2
91.2
Folate
>800nmol/L
83
60
2013.5
604.2
96.4
CRP
<5mg/L
115
83
1.9
3.1
92.2
Cortisol
170-550nmol/L
58
42
353.6
166.9
91.4
Iron
5-30umol/L
112
81
18.3
6.5
94.6
Ferritin
30-500ng/mL
118
85
163.8
118.0
91.5
Transferrin
2-3.2 g/L
115
83
2.6
0.4
90.4
Transferrin S
10-45%
114
82
28.4
11.4
91.2
Haemoglobin
130-180g/L
115
83
143
12.1
84.3
RBC
4.3-5.8x10ˆ12/L
114
82
4.6
0.7
74.6
MCV
80-100fL
112
81
90.3
9.4
95.5
Platelets
150-450x10ˆ9/L
116
83
227.5
51.5
95.7
White Cells
4.0-11x10ˆ9/L
113
81
6.3
4.2
91.2
Neutrophils
2-7.5x10ˆ9/L
115
83
3.4
1.2
95.7
Lymphocytes
1.0-4x10ˆ9/L
115
83
1.7
0.5
100
Monocytes
0-1.0x10ˆ9/L
115
83
0.3
0.12
100
Eosinophil
0-0.5x10ˆ9/L
113
81
0.1
0.2
98.2
ESR
2.0-14mm/hr
114
82
6.9
6.4
78.1
Sodium
135-145mmol/L
114
82
140.3
2.1
100
Potassium
3.5-5.5mmol/L
114
82
4.2
0.3
100
Chloride
95-110mmol/L
113
81
102.3
2.7
99.1
Bicarbonate
20-32mmol/L
114
82
27.5
2.6
100
Urea
3.5-8.5mmol/L
110
79
5.1
1.7
86.4
Creatinine
60-110umol/L
111
80
76.8
19.6
81.1
eGFR
>60 rate
101
73
84.4
14.9
97
ALP
35-110U/L
109
78
61.7
20.5
91.7
GGT
5-50U/L
110
79
22.6
14.1
94.5
CoQ10
709-1392mmol/L
91
66
998.2
534.0
72.5
Abbreviations: 1. Standard Melbourne Pathology reference ranges used. Abbreviations: SD: Standard Deviation; mm Hg: Millimeter of mercury; mU/L: Milliunits per Litre; pmol/L: Picomole per litre; nmol/L: Nanomole per Litre; mg/L: Milligram per Litre; μmol/L: Micromoles per Litre; ng/L: Nanogram per Litre; mmol/L: Millimoles per Litre; g/L: Gram per Litre; fL: femtolitres ( 10-19); ng/ml: Nanogram per Millilitre; mm/hr: Millilitre per Hour; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure
Table 1: NIIM Health Check serum tests.
Male
Female
Biomarker
Optimal
Subgroup
Total N
% of total N
Mean
SD
% in ref range
Total N
% of total N
Mean
SD
% in ref range
P-value (m/f difference)
Vitamin D
>75 nmol/L6-7
73
55
71.3
25.9
26.7
60
45
65.4
20.1
16.7
Obese
15
11
61
18.4
26.7
12
9
58.3
18.4
16.7
0.07
Iodine
100-199 μg/L24-25
55
55
84.1
57.2
29.1
45
45
61.2
53.4
13.3
0.04
Vitamin B12
500-1300 pg/ml34
45
52
470.7
196.1
24.4
41
48
425.4
232.8
24.4
0.34
Homocysteine
<7 μmol/L39
65
52
9.6
3.2
6.7
59
48
9.18
3.18
15.4
=39years
23
19
10.1
3.6
8.7
17
14
7.94
2.8
23.5
0.05
Abbreviations: m/f: Male/female; nmol/L: Nanomoles per Litre; μg/L: Microgram per Litre; μmol/L: Micromoles per Litre. For reference ranges see Figure1 Legend
Table 2: Serum vitamin D, iodine, vitamin B12, and homocysteine levels.
In our population, mean vitamin D, vitamin B12, homocysteine, and iodine levels were below their optimal levels (Figure 1B) even though a majority of participants were within the standard reference range for each of these biomarkers (Figure 1A). We explored these further by BMI and age categories (Table 2). Mean vitamin D serum level was 68.64 nmol/L, below the optimal level of >75nmol/L. A trend revealed that mean vitamin D levels were higher in males than in females (mean difference =11nmol/L ± 8.9, p=0.16) in the youngest age category (=39 years), compared to a mean difference of 2.59nmol/L (40-59 years, p= 0.68) and 4.9nmol/L (=60 years, p= 0.55). BMI was inversely correlated to vitamin D levels in healthy weight males (80.81nmol/L) and obese males (61nmol/L) (r= -0.25, p= 0.048). Additionally, vitamin D levels were correlated to seasonal changes in winter months in Australia(r= -0.24, p=0.06). There was a borderline significant difference of 11.14μmol/L between males and females during winter months (p=0.056), and a significant difference for females between winter (59.48μmol/L) and summer (70.3 μmol/L) months (p= 0.037).
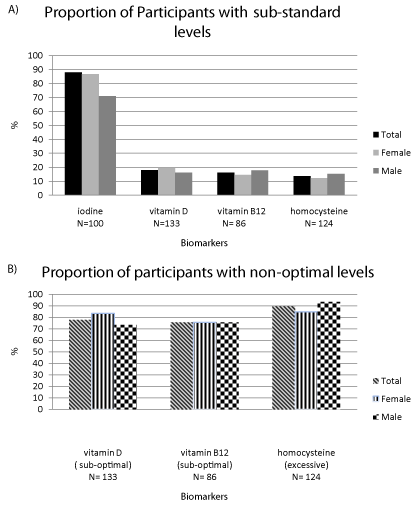
Figure 1: Proportion of participants within sub-standard (A) and non-optimal
(B) levels of vital biomarkers.
Vitamin D: optimal =75nmol/L, standard reference range =50μmol/L iodine:
optimal/standard reference range= 100-199 µg/L; vitamin B12: optimal = 500
pg/ml-1300 pg/ml, standard reference range = 200-700 pg/ml; homocysteine
optimal =7µmol/L, standard reference range = 5.0-12µmol/L.
Abbreviations: nmol/L= nanomoles/L; µg/L= microgram per litre; pg/ml=
picogram per millilitre; µmol/L= micromoles per litre
The mean iodine level was 73.8µg/L, below the optimal range of 100-199µg/L, with a significant difference between males and females (p=0.042). On average, iodine levels were highest in the 40-59 year age group, but lowest in the over 60 year age group. BMI levels only slightly influenced iodine levels. Average iodine levels increased with BMI, approaching optimal levels in the overweight and obese subgroups (Table 2). Further analyses revealed a strong significant positive correlation between female iodine levels and triiodothyronine (T3) (r= 0.50, p= 0.009).
A negative correlation was found between homocysteine levels and folate levels (r = -0.29, p=0.08) or vitamin B12 levels (r = -0.32, p=0.03). The mean vitamin B12 level was 449.13 pg/ml below the optimal range of 500 pg/ml-1300 pg/ml. Vitamin B12 levels remained consistent across all age subgroups for each gender. The reported median was 434.00 pg/ml and was positively skewed (skewness of 1.72). BMI did not appear to have a clear effect although the proportion of participants in the optimal range dropped to as low as 11% as BMI levels increased (Table 2).
The mean homocysteine level was 9.39µmol/L, 34.33% above the optimum level (7µmol/L Males had a significantly higher homocysteine level than females in the <39years age category (p=0.05). Homocysteine gradually increased by age in females (by 2.32µmol/L, <39 years compared to subgroup to>60 age subgroup, p= 0.08).
Discussion
The study summarised test results of the NIIM Health Check revealing suboptimal levels in vital biomarkers, including vitamin D, iodine, vitamin B12, and homocysteine.
Suboptimal Vitamin D levels were present in every age group except for young males (<39 years). Body Mass index was inversely correlated to vitamin D levels with a significant difference between healthy weight males and obese males. Moreover, vitamin D levels were correlated to seasonal changes particularly in significant difference females.
The definition of what constitutes optimal vitamin D levels has varied, and cut-off points have not been developed by a scientific consensus process. It has been proposed that 25(OH) vitamin D levels at >75nmol/L (used in the study) and even as high as 200nmol/L are associated with optimal health [7-9]. This higher value is based on the level below which parathyroid hormone concentrations begin to rise and the risk of fractures and chronic diseases increases [16]. Rather, higher vitamin D levels (>75nmo/L) have been associated with decreased risk of osteoporosis, cancers, autoimmune disease, osteoarthritis and diabetes [7-9].
The main cause of suboptimal vitamin D levels in Australians is insufficient exposure to sunlight [17-19], the time of day exposed (early morning sun is weaker than during mid-afternoon), lower than recommended vitamin D intake, genetic factors including kidneys inadequately converting the oral 25(OH) D to its active form, or low absorption of vitamin D in the digestive tract. Vitamin D deficient diets are those with milk allergy, lactose intolerance, ovovegetarianism, and veganism [20].
Vitamin D deficiency is more common in winter, and in females compared to males in line with the variability found amongst females in our study [21]. Low vitamin D levels often lead to seasonal affective disorder and low mood [22]. Vitamin D deficiency has also been linked to bone and muscle weakness, increased risk of cardiovascular disease and cognitive impairments [7].
Our findings are consistent with Australian population studies, whereby an estimated vitamin D deficiency is present in 15-52% of older Australians [23,24], due to younger individuals <50years being more capable of storing vitamin D for 6 months during winter [25]. This age difference is also partly explained by age-related thinning of the skin and a reduction in synthesizing Vitamin D efficiently [26,27].
Higher BMI was associated with lower vitamin D levels which is likely linked with a sedentary lifestyle and reduced outdoor activities [28,13]. Obese individuals may need larger than usual intakes of vitamin D to achieve optimal 25(OH) D levels compared to those with ideal BMI [14]. Obesity does not affect the skin’s capacity to synthesize vitamin D, but greater amounts of subcutaneous fat sequester more of the vitamin and alter its release into the circulation [14].
Secondly, females demonstrated significantly lower iodine levels than our male sample. Age and BMI were inversely correlated with iodine levels. Optimal iodine level recommendations range from 100-199µg/L [29,30]. Iodine plays a central role in healthy function of the thyroid gland. Iodine deficiency has been linked to preventable mental retardation worldwide [29], and thyroid enlargement (goiter).
Iodine deficiency in Australia is associated with the poor iodine levels in the soil, leading to low levels in foods and hence low dietary intakes [31]. Iodine is found in a range of foods, dairy products, seafood, seaweed (kelp), eggs, bread, some vegetables and iodised salt [32]. Our results may be due to low consumption of iodine-rich foods, especially within females. Fluorinated water consumption, lack of iodide supplementation in the food and agricultural industry, are further explanations for iodine depletion [31].
Our sample of women had significantly lower iodine levels than men, and deficiency decreased with age, consistent with population studies [33,34]. The National Health Measures Survey (2011-2012), also indicated that iodine levels tended to increase with increasing BMI, also consistent with our results. Furthermore, the negative correlation between triiodothyronine (T3) levels and iodine levels found in female sample is indicative of a deficient iodine level, consistent with early signs of hypothyroidism.
Thirdly, vitamin B12 levels were below optimal levels in our sample, and inversely correlated to BMI, consistent with population studies [35,36]. Recommended levels for vitamin B12 deficiency vary in different countries. When the serum level drops below 500 or 550 pg/ml the cerebrospinal fluid level can become deficient [37]. A lack of vitamin B12 is associated with dementia, brain atrophy [38], various neurological disorders, neuralgia, neuritis and bursitis [15,39]. Some experts suggested the current recommended range of vitamin B12 is too low and that the normal range should be at least 500 - 1,300 pg/ ml [10]. Brain atrophy, associated with dementia is reversible with adequate vitamin B12 levels [38,40].
Vegan diets and diets low in vitamin B12 found in red meats, fish, dairy and eggs result in diminished stores of vitamin B12 [33,34,40]. Low levels of acetyl-carnitine and folic acid as well as antacids & antibiotics chronic overuse may be responsible [41]. Absorption of vitamin B12 can be compromised by microwaving food.
There was an inverse correlation between low vitamin B12 levels and homocysteine levels. Homocysteine levels below 7µmol/L are ideal, a sharp increase in stroke incidence occurs when homocysteine levels exceed 11µmol/L [11]. Elevated homocysteine levels may lead to early heart attack and stroke, narrowing the carotid artery and Alzheimer’s disease and other types of dementia [42]. Risk factors for high homocysteine levels include male gender, smoking, coffee consumption, increasing age, high blood pressure, an unfavourable lipid profile, and high creatinine. Variables such as physical activity, moderate alcohol consumption, and an adequate folate or vitamin B12 status are associated with lower homocysteine concentrations [43].
Our study results are in line with similar studies whereby homocysteine levels in males aged less than 39 years were lower than in females and increasing with age [44-46]. Changes in renal function [45] and impaired renal metabolism of homocysteine play a role [47], as well as differences in BMI, estrogen status, vitamin status, creatine production, folate, vitamin B12, and vitamin B6 status [48].
There were some limitations in the present study. Although 139 participants were recruited across four year duration, these participants may not be representative of the general population. Data was collected in one clinic located in an inner city suburb of Melbourne. A larger sample size, with participants recruited from various regions of the state would result in a sample more representative of the typical Melbourne/Australian population.
To better guide practitioners and patients in determining individual’s optimal levels, some biomarkers, such as Vitamin B12 should be presented in reference ranges by sex and age groups, as needs vary across the lifespan.
Conclusion
Our results are in line with the literature, which indicated that Australian adults are prone to suboptimal vitamin D and vitamin B12 levels, sub-standard iodine levels and high homocysteine levels. Regular monitoring, taking into account age, gender, BMI and seasonal differences would help prevent associated problems and illnesses. Health check programs such as the NIIM Health Check have the potential in determining significant deficiencies and health concerns by a standard suite of simple blood tests.
Acknowledgement
We would like to thank all of the doctors and nurses who have been involved in the NIIM Health Check.
References
- Chronic Diseases are the leading causes of death and disability in Australia: The Department of Health. 2012.
- National Health Survey: summary of Results, 2007-08: Australian Bureau of Statistics. 2008.
- AIHW. Australia's Health 2012. 13th edn. Canberra: AIHW. 2012.
- Morabia A, Zhang FF. History of medical screening: from concepts to action. Postgrad Med J. 2004; 80: 463-469.
- Organization WH. Constitution: World Health Organization. 1960.
- Britt H, Henderson J, Bayram C, Harrison C, Valenti L, Wong C, et al. General practice activity in Australia 2013–14. Sydney. 2014.
- Joshi D, Center JR, Eisman JA. Vitamin D deficiency in adults. Aust Press. 2010; 33: 103-106.
- Pludowski P, Kryskiewicz E, Karczmarewicz E. Vitamin D provision and supplementation standards. Standardy Medyczne/Pediatria. 2012.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357: 266-281.
- Iso H, Moriyama Y, Sato S, Kitamura A, Tanigawa T, Yamagishi K, et al. Serum total homocysteine concentrations and risk of stroke and its subtypes in Japanese. Circulation. 2004; 109: 2766-2772.
- Working Group of the Australian and New Zealand Bone and Mineral Society; Endocrine Society of Australia; Osteoporosis Australia. Vitamin D and adult bone health in Australia and New Zealand: a position statement. Med J Aust. 2005; 182: 281-285.
- https://niim.com.au/healthcheck/healthchecks.
- Ervin RB, Wang CY, Wright JD, Kennedy-Stephenson J. Dietary intake of selected minerals for the United States population: 1999-2000. Adv Data. 2004; 1-5.
- World Health Organization. Assessment of iodine deficiency disorders and monitoring their elimination. A guide for programme managers. 2007.
- Hattersley JG. High-dose vitamin B12 in the treatment of dementia. 2007.https://www.ncbi.nlm.nih.gov/pubmed/16958558
- Webb AR, Engelsen O. Calculated ultraviolet exposure levels for a healthy vitamin D status. Photochem Photobiol. 2006; 82: 1697-1703.
- Rhodes LE, Webb AR, Fraser HI, Kift R, Durkin MT, Allan D, et al. Recommended summer sunlight exposure levels can produce sufficient (> or =20 ng ml(-1)) but not the proposed optimal (> or =32 ng ml(-1)) 25(OH)D levels at UK latitudes. J Invest Dermatol. 2010; 130: 1411-1418.
- Fuller KE, Casparian JM. Vitamin D: balancing cutaneous and systemic considerations. South Med J. 2001; 94: 58-64.
- Del Valle HB, Yaktine AL, Taylor CL, Ross AC. Dietary reference intakes for calcium and vitamin D. Nat Acad Press. 2011.
- Holick MF. Environmental factors that influence the cutaneous production of vitamin D. Am J Clin Nutr. 1995; 61: 638S-645S.
- Garnefski N, Teerds J, Kraaij V, Legerstee J, van den Kommer T. Cognitive emotion regulation strategies and depressive symptoms: Differences between males and females. Pers Individ Dif. 2004; 36: 267-276.
- Bruce DG, St John A, Nicklason F, Goldswain PR. Secondary hyperparathyroidism in patients from Western Australia with hip fracture: relationship to type of hip fracture, renal function, and vitamin D deficiency. J Am Geriatr Soc. 1999; 47: 354-359.
- Flicker L, Mead K, MacInnis RJ, Nowson C, Scherer S, Stein MS, et al. Serum vitamin D and falls in older women in residential care in Australia. J Am Geriatr Soc. 2003; 51: 1533-1538.
- Holick MF. Vitamin D and bone health. J Nutr. 1996; 126: 1159S-64S.
- Holick MF. Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health. Curr Op Endo, Diab Obes. 2002; 9: 87-98.
- Sanders KM, Nowson CA, Kotowicz MA, Briffa K, Devine A, Reid IR; Working group: Australian and New Zealand Bone and Mineral Society and Osteoporosis Australia. Calcium and bone health: position statement for the Australian and New Zealand Bone and Mineral Society, Osteoporosis Australia and the Endocrine Society of Australia. Med J Aust. 2009; 190: 316-320.
- Benson J, Skull S. Hiding from the sun - vitamin D deficiency in refugees. Aust Fam Physician. 2007; 36: 355-357.
- Brock K, Wilkinson M, Cook R, Lee S, Bermingham M. Associations with Vitamin D deficiency in "at risk" Australians. J Steroid Biochem Mol Biol. 2004; 89-90: 581-8.
- Delange F, de Benoist B, Burgi H; ICCIDD Working Group International Council for Control of Iodine Deficiency Disorders. Determining median urinary iodine concentration that indicates adequate iodine intake at population level. Bull World Health Organ. 2002; 80: 633-636.
- Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001; 101: 294-301.
- Pennington JA, Schoen SA. Total diet study: estimated dietary intakes of nutritional elements, 1982-1991. Int J Vitam Nutr Res. 1996; 66: 350-362.
- Als C, Keller A, Minder C, Haldimann M, Gerber H. Age- and gender-dependent urinary iodine concentrations in an area-covering population sample from the Bernese region in Switzerland. Eur J Endocrinol. 2000; 143: 629-637.
- Iodine: Australian Bureau of Statistics. 2013.
- Karatela RA, Sainani GS. Plasma homocysteine in obese, overweight and normal weight hypertensives and normotensives. Indian Heart J. 2009; 61: 156-159.
- Baltaci D, Kutlucan A, ozturk S. Evaluation of vitamin B-12 level in middle-aged obese women with metabolic and non-metabolic syndrome: case-control study. Tur J Med Sci. 2012; 42: 802-809.
- Van Tiggelen CJ, Peperkamp JP, Tertoolen HJ. Assessment of vitamin B12 status in CSF. Am J Psychiatry. 1984; 141: 136-137.
- Smith AD, Smith SM, de Jager CA, Whitbread P, Johnston C, Agacinski G, et al. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS One. 2010; 5: e12244.
- Dommisse J. Subtle vitamin-B12 deficiency and psychiatry: a largely unnoticed but devastating relationship? Med Hypotheses. 1991; 34: 131-140.
- Mitsuyama Y, Kogoh H. Serum and cerebrospinal fluid vitamin B12 levels in demented patients with CH3-B12 treatment--preliminary study. Jpn J Psychiatry Neurol. 1988; 42: 65-71.
- Douaud G, Refsum H, de Jager CA, Jacoby R, Nichols TE, Smith SM, et al. Preventing Alzheimer's disease-related gray matter atrophy by B-vitamin treatment. Proc Natl Acad Sci U S A. 2013; 110: 9523-9528.
- West B. Brain function and antacids. Health Alert. 1995; 12: 3-4.
- Varga EA, Sturm AC, Misita CP, Moll S. Cardiology patient pages. Homocysteine and MTHFR mutations: relation to thrombosis and coronary artery disease. Circulation. 2005; 111: e289-293.
- Refsum H, Nurk E, Smith AD, Ueland PM, Gjesdal CG, Bjelland I, et al. The Hordaland Homocysteine Study: a community-based study of homocysteine, its determinants, and associations with disease. J Nutr. 2006; 136: 1731S-1740S.
- Bathum L, Petersen I, Christiansen L, Konieczna A, Sørensen TI, Kyvik KO. Genetic and environmental influences on plasma homocysteine: results from a Danish twin study. Clin Chem. 2007; 53: 971-979.
- Jacques PF, Rosenberg IH, Rogers G, Selhub J, Bowman BA, Gunter EW, et al. Serum total homocysteine concentrations in adolescent and adult Americans: results from the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 1999; 69: 482-489.
- Nygård O, Vollset SE, Refsum H, Stensvold I, Tverdal A, Nordrehaug JE, et al. Total plasma homocysteine and cardiovascular risk profile. The Hordaland Homocysteine Study. JAMA. 1995; 274: 1526-1533.
- Guttormsen AB, Ueland PM, Svarstad E, Refsum H. Kinetic basis of hyperhomocysteinemia in patients with chronic renal failure. Kidney Int. 1997; 52: 495-502.
- Brattström L, Lindgren A, Israelsson B, Andersson A, Hultberg B. Homocysteine and cysteine: determinants of plasma levels in middle-aged and elderly subjects. J Intern Med. 1994; 236: 633-641.