
Review Article
Austin J Nutr Metab. 2015; 2(3): 1024.
An Insight into the Role of Vitamins other than Vitamin D on Bone
Salazar RE1 and Banu J1,2*
¹Coordinated Program in Dietetics, University of Texas, USA
²Department of Biology, University of Texas, USA
*Corresponding author: Jameela Banu, Department of Biology, College of Health Sciences and Human Services, University of Texas - Pan American, 1201, W University Drive, Edinburg, TX 78539-2999, USA
Received: July 08, 2015; Accepted: August 09, 2015; Published: August 11, 2015
Abstract
Vitamins are essential micronutrients for normal development. Great emphasis has been placed on vitamin D for bone development and maintenance. However, other vitamins also influence bone health. While some of them are more beneficial to bone and increase bone mass by increasing bone formation, calcium deposition and stimulate osteoblastogenesis, higher concentrations of others have deleterious effects causing fragile bones and increasing the risk of fractures. Knowledge about the effects of these vitamins will help in better maintenance of bone. This review focuses on the information available on vitamins A,B,C,E and K on bone health. Existing information supports vitamin C and K to play a role in bone formation and calcification. Vitamin E in low amounts and some of the B vitamins may also be beneficial to bone. There is very limited data supporting the favorable effects of vitamin A.
Keywords: Vitamin A; Vitamin B; Vitamin C; Vitamin E; Vitamin K; Bone
Abbreviations
ALP: Alkaline Phosphatase; αTF: α tocopherol; BMC: Bone Mineral Content; BMD: Bone Mineral Density; BMP: Bone Morphogenetic Protein; COX2: Cyclooxygenase 2; FN: Femoral Neck; G-CSF: Granulocyte Colony Stimulating Factor; IGF-I: Insulin Like Growth Factor I; IL: Interleukin; MTHFR: Methylenetetrahydrofolate reductase; NFAT: Nuclear Factor Activated T Cells; OVX: Ovariectomized; PTH: Parathyroid Hormone; PPAR: Peroxisome Proliferator-Activated Receptor; PGE: Prostaglandin E; PUFA: Polyunsaturated Fatty Acid; RAR: Retinoic Acid Receptor; RXR: Retinoid X Receptor; RA: Retinoic Acid; RANKL: Receptor Activated Nuclear Factor Kappa Ligand; SVCT: Sodium Dependent Vitamin C Transporter; IEC-6: Small Intestine Epithelial Cells; SD: Sprague Dawley; TGF: Tumor Growth Factor; TRAP: Tartrateresistant Acid Phosphatase
Introduction
An individual’s well-being is dependent on several factors such as: diet, physical activity, availability of resources etc. Among diet, the balance in intake of macronutrients such as carbohydrates, proteins, lipids and micronutrients like vitamins and minerals is important. Vitamins form some of the most essential micronutrients. They are implicated in many diseases that can be reversed by supplementing or limiting the intake of vitamins.
Vitamins are divided into water soluble (B vitamins, C) or fat soluble (vitamins A, D, E, K) depending on their solubility. Their categorization also refers to the mode by which they are absorbed in the body after digestion. Water soluble vitamins are absorbed with the help of sodium dependent transporters, while fat soluble vitamins follow the fat absorption pathway and are packed in chylomicrons for delivery to the organs. The availability and storage of these vitamins is dependent on ones intake. They serve as important catalyst for many enzymatic activities; are converted to essential compounds required for normal metabolism or they can activate and deactivate important signaling pathways. Therefore, adequate vitamin intake is necessary for normal development of all the different organ systems, in an individual, including the skeletal system.
Bone is an active organ undergoing modeling and remodeling throughout life. During childhood and adolescence, bone modeling takes place helping the bones to grow. Throughout this period, bone formation is dominant and needs balanced nutrients to increase bone mass. After attaining peak bone mass, the skeletal system is maintained by bone remodeling and bone resorption increases, steadily decreasing bone mass with age. Several vitamins play a role in building bones and maintaining them through the years. An imbalance or decrease intake of these vitamins can negatively impact the bone remodeling process by increasing bone resorption. When bones do not get the nourishment required for normal growth and development, they are weak and are easily susceptible to fractures putting individuals at risk of developing osteoporosis. Osteoporosis is a medical condition that is seen in one out of two women and one out of four men [1]. People diagnosed with osteoporosis have low bone mass and fragile bones that breaks with minor trauma. In women, bone loss is accelerated during menopause and post menopause. However, close to half the trabecular bone is lost in women and men before the age of 50 [2].
Bone formation and resorption are sequestered events involving many different proteins. Bone formation is accomplished by osteoblasts. Osteoblasts differentiate from bone marrow stem cells (mesenchymal stem cells) under the influence of growth factors(insulin-like growth factor I (IGF-I)), cytokines (Interleukin (IL) -18 (IL-18)), several transcriptional regulators (homeodomain proteins, surfactant proteins, Runt homology domain transcriptional factors), hormones (estrogen, parathyroid hormone (PTH), vitamin D), prostaglandin E1 (PGE1), on costatin M, adrenomedullin and leptin [3]. Mature osteoblasts first form the collagen matrix and then with the help of osteocalcin start the mineralization process [3]. On the other hand, cells involved in bone resorption, are multinucleated cells called osteoclasts and differentiate from hematopoietic cells. Their differentiation takes place under the influence of several cytokines (IL-1, IL-6, IL-7, and tumor necrosis factor-α (TNFα), hormones (PTH, estrogen and vitamin D) and prostaglandin E2 (PGE2). These cells, dissolve the matrix proteins [4] and release the minerals from the bone leading to cavities and increasing the fragility of the bone [3].
This review focuses on the influence of vitamins on bone health. Articles were collected from Pubmed and Medline databases. The keywords used were: vitamin A and bone, retinoic acid (RA) and bone, vitamin B and bone, thiamine and bone, riboflavin and bone, vitamin B3 and bone, vitamin B5 and bone, vitamin B6 and bone, vitamin B7 and bone, vitamin B9 and bone, vitamin B12 and bone, vitamin C and bone, vitamin E and bone, and vitamin K and bone. As the role of vitamin D on bone health is very well established, this review focuses on the influence of other vitamins on bone strength, bone mineral density (BMD), micro architecture and pathways of bone.
Water Soluble Vitamins
There are two water soluble vitamins – vitamin B complex and vitamin C. Both vitamin B complex and C are not stored in the body and have to be supplemented in the diet regularly. They are absorbed with the help of sodium dependent transporters [5].
Vitamin B complex
B Vitamins are composed of 8 compounds: vitamin B1 (thiamine), vitamin B2 (riboflavin), vitamin B3 (niacin), vitamin B5 (pantothenic acids), vitamin B6 (pyridoxine, pyridoxal, pyridoxamine), vitamin B7 (biotin), vitamin B9 (folic acid), vitamin B12 (cobalamine) [5]. Off these, thiamine prevented malformations of the palate related to teratogony [6]. Studies on the direct effects of thiamine on bone are lacking. Sources of the different B vitamin are listed in Table 1 [7-9].
Vitamin
Source
Vitamin B1 (Thiamine)
Found in cereals (rice, wheat, maida, rava, poha, etc.) breads, fortified cereals and pasta, pulses or lentils (dals such as moong dal, masoor dal, chana dal etc), legumes (whole pulses such as whole moong, channa, chowli, rajmah), dark green leafy vegetables such as spinach, fenugreek, lettuce, cabbage, asparagus etc. soy foods, whole grains like wheat germ, fish, egg, milk, meat, pork ham etc, nuts such as almonds and pecans. Daily value: 1mg for men; 0.8mg for women.
Vitamin B2 (Riboflavin)
Some of the best sources of riboflavin are chicken, fish, eggs, legumes (like peas and lentils), milk and milk products such as yogurt and cheese, nuts, green leafy vegetables like spinach, broccoli, asparagus, and fortified cereals also supply significant amounts of riboflavin to the diet. Daily value: 1.3mg for men; 1.1mg for women.
Vitamin B3 (Niacin)
It is found in chicken, salmon and in fishes like canned tuna – they are an excellent source of niacin. Vegetarians can get their source of niacin from legumes, pasta and whole wheat. Daily value: 17mg for men; 13mg for women.
Vitamin B6 (Pyridoxine)
Foods like potatoes, beans, red meat, poultry, eggs and fortified cereals contain are very high in vitamin B6. Daily value: 1.4mg for men; 1.2mg for women.
Vitamin B5 (Pantothenic Acid)
Yogurt and avocado are both excellent sources of pantothenic acid, but it is also available in a wide variety of foods such as legumes including lentils and split peas, sweet potatoes, mushrooms and broccoli. Daily Value: 4-7 mg for adults.
Vitamin B7 (Biotin)
Liver and egg yolks are the richest dietary sources of biotin, but fortunately this B vitamin is well distributed throughout the food supply, so it is doubtful that anyone eating a balanced, varied diet will experience a deficiency. Salmon, pork and avocado are good sources; most fruits and vegetables contain a little biotin, as do cheeses and grain foods. Daily value: 30-100?g for adolescents and adults.
Vitamin B9 (Folate, folic acid, or folacin)
To remember which foods are high in folate, remember that the word folate has the same root as the word foliage. Leafy greens such as spinach, fenugreek, turnip greens, asparagus, etc and other fresh fruits and vegetables are all excellent sources of folate. Liver, dried beans and other legumes, and orange juice are good sources of this vitamin. So are fortified bread, rice, and cereals. Daily value: 0.2mg for adults.
Vitamin B12 (Cobalamin)
Animal foods are the only natural source of vitamin B12. It is found naturally in fish, red meat, poultry, milk, milk products, cheese, and eggs. But, many products, including soy products and cereals, are fortified with B12 so it is widely available in the food supply. Other good natural sources include shellfish, such as clams, mussels and crab, fin fish and beef. Daily value: 0.0015mg for adults.
Table 1: Sources of the various vitamin B compounds [7-9].
In vitro studies using Vitamin B2 (riboflavin) in MC3T3 cells, which can be differentiated to become osteoblasts, have shown to enhance the differentiation process [10]. Osteoclastogenesis was also decreased by riboflavin through the expression of osteoprotegrin which is secreted by the osteoblastic stromal cells [10]. In patients with elevated homocysteine, due to MTHFR C677T polymorphism, riboflavin was not associated with fracture risk [11] especially in the femoral neck [12].
In vitro studies using vitamin B3 (niacin and niacin amide) have shown no effect on the bone mineral content (BMC) in male leghorn chicken, and it also decreased bone strength [13,14]. This may be because niacin can stimulate oxidative stress in bone marrow cells [15] – a factor that stimulates bone resorption. However, in diabetic mice, niacin supplementation improved blood flow after ischemia [16] which may be beneficial to bone health.
Limited studies have reported the effects of vitamin B5 (pantothenic acid) on bone. Deficiency of this vitamin changed the osteogenesis in the tibia of rats, in a time dependent manner. Reports concluded that, longer deficiency caused more damage to the tibia [17,18]. Additionally, longer periods of pantothenic acid deficiency, led to decreased osteoblast proliferation, osteogenesis and increased trabecular bone resorption which led to loss of trabeculae [17].
The effects of vitamin B6 (pyridoxine, pyridoxal and pyridoxamine), on bone health, has been reported in a few in vivo studies. Studies on male Wister rats showed that vitamin B6 deficiency decreased cross-link intermediates and impaired crosslink formation [19]. Similar effects were also reported in chick embryo. When treated with vitamin B6 antagonist there was decreased crosslinking of collagen supporting vitamin B6 supplementation during pregnancy [20]. In addition, vitamin B6 deficiency decreased alkaline phosphatase (ALP), bone healing and tibial strength [21].
Vitamin B7 (biotin) deficiency affects bone formation. In broiler chicken, vitamin B7 deficiency decreased periosteal bone apposition, bone formation rate, osteoid perimeter and IGF-I [22,23]. Biotin may require riboflavin to maintain tibial BMD, strength and stiffness as demonstrated in young turkey chicks [24].
Vitamin B9 (folic acid and folate) is well known for their effects on the nervous system. In the rat embryo skull bone, folic acid decreased TGFβ1 and TGF-1 expression [25]. Otherwise, there is no evidence that folic acid directly affects bone. However, when adequate amount of folate is not consumed, there is decreased BMD and trabecular thickness in the vertebra of postmenopausal women which can be reversed with folate supplementation [26,27]. Additional studies, showed that folic acid could reduce the accumulation of aluminum in organs such as bone, kidney and brain, thereby, reducing the toxicity [28].
Vitamin B12 (cobalamine) can act on both osteoblasts and osteoclasts [29-31]. Its deficiency decreased bone mass by stimulating osteoclastogenesis mainly by increasing methyl malonic acid and homocysteine [29]. It also influenced ALP secretion in patients’ deficient with vitamin B12 and decreased BMD [30-34]. In vegetarians decreased intake of vitamin B12 increased bone turnover [35] which may lead to net loss of bone.
Decreased consumption of vitamin B12 and folic acid decreased BMD in older men which led to greater bone loss [26,36,37]. One of the functions of vitamin B12 and B6 is to regulate homocysteine. Hyperhomocysteinemia is caused by deficiency of folate and B12 [38]. For every mmol/l of homocysteine there is 4% increase in fracture risk [32,39]. Universally, homocysteine is associated inversely with BMD, as it reduced BMD in older women [33,40-44]. Increased homocysteine and low vitamin B12 were significantly associated with high levels of bone turnover markers and increased fracture risk [45]. High concentrations of homocysteine may also interfere with the cross linking of collagen, thereby, decreasing the stability and strength of collagen network [46]. Additional evidence showed that homocysteine may increase osteoclast formation and activity [46- 48], bone turnover [44] and bone marker levels [44]. Due to plasma homocysteine levels association with osteoporosis [49], it has been suggested that levels of circulating homocysteine can be used as a marker of fragility in females>75 years of age [44]. In male rats, however, there was no change in the BMD in the tibia, although there was decreased blood flow to the bone and reduced biochemical properties [50]. Homocysteine is reported to bind with collagen resulting in decreased bone strength [32,51]. Supplementation with folic acid, vitamins B12 and vitamin B6 effectively reduced homocysteine levels [46].
Except for vitamin B3, other vitamin B compounds are beneficial to bone health. It was interesting to note that there were scanty reports on vitamin B1 and bone. Vitamin B12 and B6 plays a major role in lowering homocysteine levels, thereby reducing osteoclast activity and decreasing bone turnover.
Vitamins C (ascorbic acid)
Vitamin C is absorbed in the small intestine into the brush border cells using the sodium dependent vitamin C transporter (SVCT) proteins. When this transporter is overexpressed, it is able to increase mineralization and calcium deposition. It is also reported to help with the bone marrow stromal cells to differentiate into osteoblasts as knockdown of this transporter inhibitedosteogenesisin these cells [52]. In bone marrow aspirates from athymic BALB/C ru mice with subcutaneous implant, ALP and calcium deposition on scaffolds increased [53] with vitamin C supplementation. A list of the sources, serving sizes and recommended daily value of vitamin C is given in Table 2 [7,9].
Source
Serving Size
Vitamin Content (mg)
Guava, raw
½ cup
188
Red sweet pepper, raw
½ cup
142
Red Sweet pepper, cooked
½ cup
116
Kiwi fruit
1 medium
70
Orange, raw
1 medium
70
Orange juice, ¾ cup
¾ cup
61-93
Green pepper, sweet, raw
½ cup
60
Green pepper, sweet, cooked
½ cup
51
Grapefruit juice
¾ cup
50-70
Vegetable juice cocktail
¾ cup
50
Strawberries, raw
½ cup
49
Brussel sprouts, cooked
½ cup
48
Cantaloupe
¼ medium
47
Papaya, raw
¼ medium
47
Kohlrabi, cooked
½ cup
45
Daily value: 90 mg for men; 75 mg for women
Table 2: List of sources, serving size, and vitamin C content in foods [7-9].
In male Sprague Dawley (SD) rats, there was increased bioavailability of calcium as corbate compared to calcium acetate [54]. In female guinea pigs, lower intake of vitamin C was associated with decreased collagen synthesis resulting in lower levels of collagen, ALP and osteocalcin [55,56]. Decreased intake of vitamin C also increased bone turnover and reduced transition of salts to the crystalline form [57], whereas, long term deficiency of vitamin C, decreased skeletal maturation and showed bone abnormalities in female guinea pigs [58]. Total deprivation of vitamin C in guinea pigs led to destruction of the proximal tibia with micro fractures. Additionally, damage to the diaphysis led to Genu Varums (bow legs) [59]. Treatment with vitamin C restored the tibia and formation of trabecular bone at the growth cartilage by sub periosteal thickening [59] and development of trabecular callus. However in dogs, vitamin C supplementation did not modify BMC [60] and the tibial breaking strength or egg shell thickness in hens also was not altered due to vitamin C supplementation [61]. Inversely, in chicks, it is reported that ascorbic acid may be involved in mobilizing calcium and phosphorus soon after injection but had no effect on bone resorption or the bone formation process [62].
Vitamin C deficiency accelerated bone loss and increased fractures, in the femur of senescence marker protein 30 knockout mice [63]. It is known to help in the differentiation of osteoblasts and deficiency promotes osteoblasts to transition into adipocytes [63]. It may accomplish this by upregulating the expression of proximal proliferator-activated receptor gamma (PPARγ) [63] which is an important cofactor required for the hydroxylation of collagen and is necessary for osteoblast proliferation [64]. ALP expression is also dependent on as corbate levels. It influenced collagen organization to form the protein matrix for the deposition of minerals [64]. In addition, it is established that vitamin C deficiency inhibits calcification process [64].
In postmenopausal women, BMD decreased in response to lower vitamin C intake in a dose dependent manner, however, there was no such effect in males [65]. Another study reported a 3% increase in BMD at the ultra-distalmidshaft, radii, hip and lumbar spine in postmenopausal women [66]. It also increased ALP and enhanced collagen synthesis as well as stimulated procollagen [66]. Short term (7 weeks) vitamin C supplementation in children (boys) did not show any changes in biochemical markers like pyridoxiline and deoxypyridoxiline [67].
Fluoride toxicity in monkeys and copper toxicity in rabbits were attenuated by supplementation with vitamin C [68,69]. Vitamin C and Zinc positively influenced bone geometry, size and strength in children [70].
Overall, vitamin C is required for osteoblast differentiation and is important for bone formation. It is also an anti-oxidant, therefore, may act by reducing oxidative stress and positively influencing bone.
Fat Soluble Vitamins
All fat soluble vitamins are absorbed along with fatty acid and delivered to the liver as fatty acid. It is dependent on bile salts and can be transported in chylomicrons. These vitamins are stored in the body just like fatty acids.
Vitamin A (retinoids)
Vitamin A consists of a group of compounds – retinal, retinyl ester, retinol and RA [5]. Vitamin A can affect the translational processes in cells and nuclear receptors like retinoic acid receptor (RAR) and retinoid X receptor (RXR) help in this process. The sources, serving sizes and recommended intake of vitamin A and pro vitamin A are listed in Table 3 [7,9].
Sources
Serving Size
Vitamin Content (IU)
Liver, beef, cooked*
3 oz.
27,185
Liver, chicken, cooked*
3 oz.
12,325
Carrot juice, canned*
½ cup
22,567
Carrots, boiled*
½ cup slices
13,418
Spinach, frozen, boiled*
½ cup
11,458
Kale, frozen, boiled
½ cup
9558
Carrots, raw
7 ½ in
8666
Vegetable soup, canned, chunky, ready-to-serve
1 cup
5820
Cantaloupe
1 cup cubes
5411
Spinach, raw
1 cup
2813
Apricots with skin, juice pack
½ cup
2063
Daily value: 5000IU for adults and children age 4 and older
*high in retinol activity equivalents.
Table 3: List of sources, serving size, and vitamin A content in foods [7-9].
RA treatment of growth plate chondrocytes, induced annexins II, V and VI to form Ca2+ channels and influx calcium into the cells. It also stimulated the release of ALP to initiate mineralization [71]. In addition, RA stimulated chondrocyte differentiation by upregulating the expression of bone morphogenetic protein (BMP) [72]. In human and murine cells, RA inhibited Receptor Activator of Nuclear Factor kappa-B Ligand (RAN?L) stimulated osteoclast differentiation [73] and in the fish, Atlantic cod, RA decreased bone resorption [74]. On the other hand, there are also reports that RA decreased BMP2 expression thereby stimulating osteoclastogenesis [75] and induced bone loss. Interestingly, it has been used as a method to induce bone loss in female rats [76]. There are also reports showing reduction of mineralization with RA supplementation [77] and RA has a negative effect on the oral bone mass in rats [78].
One of the receptors, retinoic acid receptor γ (RARγ) is a potent inhibitor of osteoclastogenesis as it decreased nuclear factor activated T cells (NFAT) activation, granulocyte colony stimulating factor (G-CSF) and RANKL, and also increased bone formation [79]. Carotenoids from fruits and vegetables act through the anti-oxidant pathway to reduce bone resorption [80].
Vitamin A supplementation in female rats, increased BMD but decreased trabecular area with no change in cortical thickness or stiffness and levels of osteocalcin [81]. In IEC-6 cells, vitamin A supplementation up regulated ALP [82]. Vitamin A can also promote bone formation through the BMP2/7 heterodimer [83]. It is also reported that vitamin A supplementation in postmenopausal women, had no age related increase in risk for fractures or any changes in BMD [84] and the mechanical properties of the bone [85]. However, the beneficial effect of vitamin A is more in the trabecular bone than the cortical bone in aged rats [85]. When vitamin A is consumed with polyunsaturated fatty acids (PUFAs) they act on different pathways and while vitamin A can cause malformations, PUFAs increased bone formation independent of each other [86].
However, some reports concluded that supplementation with vitamin A did not show any beneficial or detrimental effects in humans and animal models. In women, decreased intake of vitamin A did not have a detrimental effect on bone [84] and in men, short term supplementation with vitamin A did not influence bone turnover [87]. Similarly, studies on dogs supplemented with vitamin A also did not show any detrimental effects on bone [88].
Another vitamin A compound, retinol has a negative effect on bone as it decreased BMD in mice and rats [89-94]. Increased intake of retinol increased bone fragility and fracture risk at different sites including the femur neck, Ward’s triangle, trochanter region of the proximal femur, lumbar spine [93,95]. In cases where there is decreased vitamin D, increased intake of retinol further increased fracture risk [96]. However, retinol with retinol binding protein 4 showed decreased osteoclast activity in humans (males) [97]. Early studies have shown that decreased vitamin A increased osteoblast activity [98].
In addition, there are studies that have reported several negative effects on bone, especially when vitamin A was given to children. Problems reported include building of fontanelle, increase in intracranial pressure, anoxia, drowsiness, instability followed by brain damage [99,100]. Dhem et al. have reported several other detrimental effects of vitamin A supplementation including lesions in the endocortical surface of the tibia, cavities and demineralization on the periosteal surface and cancellous bone, enlargement of Haversian canals [77,91,101] and hypervitaminosis A which is associated with long bone fractures [77,91]. High intake of cod liver oil also increased the risk of fractures [102].
Although vitamin A has shown some bone protective effects by up-regulating BMP, and RAR inhibited osteoclastogenesis, there are many reports indicating that consuming high levels of this vitamin may be deleterious to both long and parietal bones.
Vitamin E (tocopherols)
Vitamin E consists of the tocopherols and tocotrienols. Each of them include4 vitamers but α tocopherol (αTF) is the biologically active form and has been studied very extensively. Table 4 lists the sources, caloric content with the recommended daily value intake of vitamin E [7,9].
Source
Serving Size
Vitamin Content (mg)
Sunflower seeds, dry roasted
1 oz.
7.4
Sunflower oil, high linoleic
1 Tbsp.
5.6
Cottonseed oil
1 Tbsp.
4.8
Safflower oil, high oleic
1 Tbsp.
4.6
Hazelnuts (filberts)
1 oz.
4.3
Mixed nuts, dry roasted
1 oz.
3.1
Turnip greens, frozen, cooked
½ cup
2.9
Tomato paste
¼ cup
2.8
Pine nuts
1 oz.
2.6
Peanut butter
2 Tbsp.
2.5
Tomato Puree
½ cup
2.5
Tomato sauce
½ cup
2.5
Canola oil
1 Tbsp.
2.4
Wheat germ, toasted, plain
2 Tbsp.
2.3
Daily value: 30IU for adults and children above age 4.
Table 4: List of sources, serving sizes, and Vitamin E content in foods [7-9].
Vitamin E levels have been directly related to the bone status in humans as osteoporotic subjects have less circulating vitamin E levels [103,104]. Many in vivo studies have reported the benefits of αTF. Supplementation with αTF increased mineralizing surface, bone formation rates and strength of bones in OVX rats [105,106]. In addition, it also decreased osteoclast surface at medium dose as well as in low doses and reduced oxidative stress [107-109]. In postmenopausal rat model, trabecular bone volume, trabecular number increased and trabecular separation decreased with αTF supplementation [106,107]. Biochemical markers like ALP, osteocalcin and IGF-I expression increased with αTF [108,110] and decreased TRAP. In male rats αTF prevented bone loss due to disuse of hind limb by decreasing cyclooxygenase – 2 (COX-2) [111]. Older mice showed increased yield stress, ultimate load and stiffness of bone [110], however this effect was not seen in younger mice with αTF supplementation. However, the effect of vitamin E is site specific as it positively correlates with BMD in the spine and not in the long bones [112]. Young (6 months old) and old (24 months old) C57Bl/6 mice when fed vitamin E showed increased bone strength and matrix protein with no change in BMD. This effect was more in older mice than younger mice. There was also decreased PGE2 and increased IGF-I formation [110]. In female rats, vitamin E also increased calcium content [113] and also increased calcium availability and decreased certain free radical production; however, αTF did not improve calcium in the bone [114]. The beneficial effects of vitamin E are attributed to the tocotrienols in the vitamin E mixture used in the study. The ratio of vitamin E to lipids is significantly associated with decreased BMD of the lumbar spine of in postmenopausal women [115,116]. Moreover, αTF may modulate γTF by reducing its levels and affect bone formation [115].
In lower concentrations, αTF can protect bone and at high concentrations is detrimental to bone as they can interfere with the function of vitamin K and other isoforms of vitamin E [117-119]. Moreover, the source of vitamin E may also make a difference [109].
Vitamin K
Vitamin K includes two compounds – vitamin K1 (Phylloquinone) and K2 (Menaquinone). The major function of vitamin K is to promote coagulation of blood but recently there is evidence that it is also involved in maintaining bone health [120,121] by increasing bone formation and reducing bone resorption [122]. Table 5 has the list of sources and serving size with the daily value for vitamin K [7,9].
Source
Serving Size
Vitamin Content (?cg)
Brussel sprouts
½ cup
460
Turnips greens, raw, chopped
1 cup
364
Broccoli
½ cup
248
Lentils, dry
½ cup
214
Cauliflower
½ cup
150
Kale, cooked
½ cup
150
Spinach, raw, chopped
½ cup
149
Garbanzo beans, dry
½ cup
132
Swiss chard
½ cup
124
Daily value: 75?g for men and women
Table 5: List of sources, serving sizes, and Vitamin K content in foods [7-9].
Vitamin K is implicated in carboxylating a couple of proteins that are involved in the bone formation process - matrix Gla proteins and osteocalcin [123-125]. Matrix Gla protein has high affinity to calcium and functions as a modulator of calcium availability [126]. Osteocalcin is a protein secreted by osteoblasts and is an important bone biochemical marker associated with increase in bone formation [127-129]. Age related decrease in γ carboxylation of osteocalcin takes place [130].
Several studies have shown that osteocalcin is under carboxylated and does not mobilize the calcium for mineralization, thereby, decreasing bone formation and vitamin K supplementation attenuated this effect by carboxylating osteocalcin [131,132], therefore, is beneficial to bone [133].
Many in vivo studies on animal models have shown the benefits of vitamin K supplementation. Male rats, supplemented with menaquinone, showed decreased under carboxylated osteocalcinalong with increased mineral crystallinity and hardness in the tibiae [134]. OVX rats, fed vitamin K, showed higher bone strength and decreased risk of fractures in the tibia and femur by increasing trabecular bone volume [135,136] trabecular number and decreasing trabecular separation, osteoclast number/bone surface and osteoclast surface/bone surface [137]. Vitamin K also promoted bone healing in a rodent model of osteotomy [138]. In gastroectomized SD rats, vitamin K2 supplementation attenuated the decrease in ultimate force and increased stiffness of the femoral diaphysis [139]. Male orchidectomized rats, showed increased bone MAR, BFR, BV/ TV and BV [140-142] and improved trabecular micro architecture with vitamin K supplementation [132]. Similar benefits of vitamin K supplementation on the trabecular structure and bone volume in tail suspended rats [143] was also reported.
Children with better vitamin K status had increased bone mass [144]. In early menopausal women, under caroboxylated osteocalcin was negatively associated with BMD in the total hip, femoral neck, and lumbar spine [145,146]. Elderly institutionalized men and women are recommended to consume higher amounts of vitamin K than the recommended adequate intake to maintain skeletal health [147]. In healthy postmenopausal women, supplementation of vitamin K2, significantly, decreased loss in vertebral height and increased bone strength, BMC and BMD in the lumbar spine, femoral neck and hip bone geometry [148-151]. Biochemical markers of bone metabolism like serum under carboxylated osteocalcin decreased and γ carboxylated osteocalcin increased, with vitamin K supplementation [152,153]. Healthy women in the ages of 30-88 years showed decreased under carboxylated osteocalcin especially in older women, among the cohort studied [154]. However, another study reported decreased bone turnover in both the young and elderly, when supplemented with vitamin K [155]. A direct relationship between BMD and lower concentrations of phylloquinone in postmenopausal women was also reported [156]. Plasma phylloquinone levels were inversely associated with N- terminal telopeptide and under carboxylated osteocalcin in girls (3-16 years of age) [157]. Interestingly, decreased vitamin K intake decreased BMD in female but not in males [158]. Vitamin K also induced IL-1α, PGE2 [159] in patients with femur neck fractures or lower BMD had decreased levels of circulating vitamin K [160,161]. Vitamin K supplementation also protected bone by modulating bone turnover during space flight [162] and osteoporotic patients with vitamin K2 supplementation showed increased lumbar BMD [163].
Vitamin K when supplemented with alendronate increased total and trabecular BMD at the distal metaphysis of the femur, in mice. It also increased the strength of the bone [164]. Combined treatment with risendronate and vitamin K prevented glucocorticoid induced bone loss and decreased bone formation and bone erosion in rats [165-167]. In addition, when OVX rats were given vitamin K in combination with bisphosphonates, pretreatment with vitamin K improved the strength, BMD, BMC and trabecular structure in the femur [168,169]. OVX rats with, vitamin K2 and Raloxifene showed greater strength in the femoral neck [170]. Vitamin K supplementation may also reduce drug induced osteoporosis [155].
Although there are many studies showing the benefits of vitamin K on bone, there are some studies that did not show any benefits. Early menopausal women did not show any benefits with vitamin K supplementation for 1 year [171]. Six months of vitamin K supplementation did not show any significant increase in BMD in pre and perimenopausal women [172]. In OVX rats, vitamin K did not change the BMD in the distal femur [173]. This may be due to the short term treatment or the concentration of vitamin K given to the subjects or there might have been drug/nutrient interactions.
There is ample evidence to show that vitamin K is essential for the carboxylation of osteocalcin and improves calcium deposition in the matrix. Therefore, adequate vitamin K is necessary to maintain the mineral content and strength in bones.
Combination of Vitamins on Bone
Combination of vitamins C and E partially prevented bone destruction due to heparin [174]. αTF interacts with vitamin K and other vitamin E isomers to increase pro-oxidative effects. Vitamin D and K may have synergistic effects [175-177]. Decreased vitamin E and C increased fracture risk in smokers [95,103]. No influence of vitamin E supplementation on BMD of the lumbar and femoral neck (FN) has also been reported in postmenopausal women [178].
Conclusions
In summary, among the water soluble vitamins, research supports that vitamin C was most beneficial to bone. This may be because it is a potent anti-oxidant and can help in osteoblast differentiation. Of the different B vitamins, riboflavin, B6, biotin, folic acid and B12 had a positive influence on bone metabolism. This may be by different mechanism such as: inhibition of osteoclastogenesis by decreasing in IGF-I levels, and interfering with the cross linking of proteins. Combination of B vitamins like folic acid, B12 and B6 is probably more effective in inhibiting the action of hyperhomocysteinemia. However, vitamins B3 had a negative effect on bone because of their pro oxidative effects. Of the fat soluble vitamins, vitamin K and E influenced bone formation positively, while vitamin A has been reported to have more deleterious effects on bone especially when given in high concentrations during infancy and childhood. So far, based on the available literature, after vitamin D, vitamin K supplementation is the most promising. Vitamin K in combination with several bisphosphonates increased BMD and strength of long bones. Further studies should be conducted to see if vitamin K can maintain bone mass with decreased concentrations of bisphosphonates. This will reduce the side effects of bisphosphonates considerably. Moreover, as pretreatment with vitamin K before bisphosphonates improves the strength, BMC and BMD, it may also benefit individuals after withdrawal of bisphosphonate treatment. Vitamin K may also help diabetic patients, where the matrix is compromised. However, more detailed studies on the effects of various vitamins have to be conducted to elucidate the actual benefits of the different vitamins in maintaining bone health. The concise effects of vitamins are summarized in Figure 1.
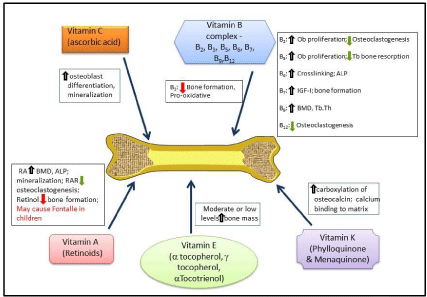
Figure 1: Text Here.
An important fact to note is that most of the studies reporting the effects of vitamins in humans are self reporting surveys. A better understanding of the benefits of vitamins may be obtained by interventional controlled studies. It is also important to remember that each vitamin has a recommended intake, which should be taken into consideration to decrease any undesirable side effects and may help maintaining healthy bones. But most importantly, interactions of drugs and other nutrients with vitamins should be carefully studied before any vitamin supplementation is taken.
Acknowledgement
The authors thank the assistance of Ms. Vaijayanthi Rajendran for collecting the literature. We also thank the Undergraduate Research Institute, UTPA for supporting Mr. Robert E. Salazar.
References
- National Osteoporosis Foundation. What is Osteoporosis? 2015 [cited 2015 5/30/2015].
- Seeman E. Structural basis of growth-related gain and age-related loss of bone strength. Rheumatology (Oxford). 2008; 47 Suppl 4: iv2-8.
- Banu J, Varela E, Fernandes G. Alternative therapies for the prevention and treatment of osteoporosis. Nutr Rev. 2012; 70: 22-40.
- Delaisse JM, Andersen TL, Engsig MT, Henriksen K, Troen T, Blavier L. Matrix metalloproteinases (MMP) and cathepsin K contribute differently to osteoclastic activities. Microscopy research and technique. 2003; 61: 504-513.
- Gropper SS, Smith JL. Advanced Nutrition and Human Metabolism. 6th ed. California: Wadsworth; 2013.
- Bienengraber V, Fanghanel J, Malek FA, Kundt G. Application of thiamine in preventing malformations, specifically cleft alveolus and palate, during the intrauterine development of rats. The Cleft palate-craniofacial journal: official publication of the American Cleft Palate-Craniofacial Association. 1997; 34: 318-324.
- Office of Dietary Supplements. 2015 [07/01/2015].
- National Health Service. 2015 [07/01/2015].
- Mahan LK, Escott-Stump S, Raymond JL. Krause's Food & The Nutrition Care Process. 13 ed. Alexopoulos Y, editor. USA: Elsevier 2012.
- Chaves Neto AH, Yano CL, Paredes-Gamero EJ, Machado D, Justo GZ, Peppelenbosch MP, et al. Riboflavin and photoproducts in MC3T3-E1 differentiation. Toxicol In Vitro. 2010; 24: 1911-1919.
- Yazdanpanah N, Uitterlinden AG, Zillikens MC, Jhamai M, Rivadeneira F, Hofman A, et al. Low dietary riboflavin but not folate predicts increased fracture risk in postmenopausal women homozygous for the MTHFR 677 T allele. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2008; 23: 86-94.
- Abrahamsen B, Madsen JS, Tofteng CL, Stilgren L, Bladbjerg EM, Kristensen SR, et al. Are effects of MTHFR (C677T) genotype on BMD confined to women with low folate and riboflavin intake? Analysis of food records from the Danish osteoporosis prevention study. Bone. 2005; 36: 577-583.
- Johnson NE, Harland BF, Ross E, Gautz L, Dunn MA. Effects of dietary aluminum and niacin on chick tibiae. Poult Sci. 1992; 71: 1188-1195.
- Johnson NE, Qiu XL, Gautz LD, Ross E. Changes in dimensions and mechanical properties of bone in chicks fed high levels of niacin. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 1995; 33: 265-271.
- Tang K, Sham H, Hui E, Kirkland JB. Niacin deficiency causes oxidative stress in rat bone marrow cells but not through decreased NADPH or glutathione status. The Journal of nutritional biochemistry. 2008; 19: 746-753.
- Huang PH, Lin CP, Wang CH, Chiang CH, Tsai HY, Chen JS, et al. Niacin improves ischemia-induced neovascularization in diabetic mice by enhancement of endothelial progenitor cell functions independent of changes in plasma lipids. Angiogenesis. 2012; 15: 377-389.
- NELSON MM, SULON E, et al. Changes in endochondral ossification of the tibia accompanying acute pantothenic acid deficiency in young rats. Proc Soc Exp Biol Med. 1950; 73: 31-36, illust.
- WAINWRIGHT WW, NELSON MM. Effect of acute pantothenic acid deficiency on the tibia of young rats. J Dent Res. 1946; 25: 185.
- Fujii K, Kajiwara T, Kurosu H. Effect of vitamin B6 deficiency on the crosslink formation of collagen. FEBS Lett. 1979; 97: 193-195.
- Bird TA, Levene CI. The effect of a vitamin B-6 antagonist, 4-deoxypyridoxine, on the cross-linking of collagen in the developing chick embryo. Biochem J. 1983; 210: 633-638.
- Reynolds TM. Vitamin B6 deficiency may also be important. Clin Chem. 1998; 44: 2555-2556.
- Bain SD, Newbrey JW, Watkins BA. Biotin deficiency may alter tibiotarsal bone growth and modeling in broiler chicks. Poult Sci. 1988; 67: 590-595.
- Báez-Saldaña A, Gutiérrez-Ospina G, Chimal-Monroy J, Fernandez-Mejia C, Saavedra R. Biotin deficiency in mice is associated with decreased serum availability of insulin-like growth factor-I. Eur J Nutr. 2009; 48: 137-144.
- Hocking PM, Stevenson E, Beard PM. Supplementary biotin decreases tibial bone weight, density and strength in riboflavin-deficient starter diets for turkey poults. Br Poult Sci. 2013; 54: 801-809.
- Santoso MI, Rohman MS. Decreased TGF-beta1 and IGF-1 protein expression in rat embryo skull bone in folic acid-restricted diet. J Nutr Biochem. 2006; 17: 51-56.
- Cagnacci A, Bagni B, Zini A, Cannoletta M, Generali M, Volpe A. Relation of folates, vitamin B12 and homocysteine to vertebral bone mineral density change in postmenopausal women. A five-year longitudinal evaluation. Bone. 2008; 42: 314-320.
- Rejnmark L, Vestergaard P, Hermann AP, Brot C, Eiken P, Mosekilde L. Dietary intake of folate, but not vitamin B2 or B12, is associated with increased bone mineral density 5 years after the menopause: results from a 10-year follow-up study in early postmenopausal women. Calcif Tissue Int. 2008; 82: 1-11.
- Baydar T, Nagymajtényi L, Isimer A, Sahin G. Effect of folic acid supplementation on aluminum accumulation in rats. Nutrition. 2005; 21: 406-410.
- Vaes BL, Lute C, Blom HJ, Bravenboer N, de Vries TJ, Everts V, et al. Vitamin B(12) deficiency stimulates osteoclastogenesis via increased homocysteine and methylmalonic acid. Calcif Tissue Int. 2009; 84: 413-422.
- Carmel R, Lau KH, Baylink DJ, Saxena S, Singer FR. Cobalamin and osteoblast-specific proteins. N Engl J Med. 1988; 319: 70-75.
- Kim GS, Kim CH, Park JY, Lee KU, Park CS. Effects of vitamin B12 on cell proliferation and cellular alkaline phosphatase activity in human bone marrow stromal osteoprogenitor cells and UMR106 osteoblastic cells. Metabolism: clinical and experimental. 1996; 45: 1443-1446.
- Clarke M, Ward M, Strain JJ, Hoey L, Dickey W, McNulty H. B-vitamins and bone in health and disease: the current evidence. Proc Nutr Soc. 2014; 73: 330-339.
- Morris MS, Jacques PF, Selhub J. Relation between homocysteine and B-vitamin status indicators and bone mineral density in older Americans. Bone. 2005; 37: 234-242.
- Tucker KL, Hannan MT, Qiao N, Jacques PF, Selhub J, Cupples LA, et al. Low plasma vitamin B12 is associated with lower BMD: the Framingham Osteoporosis Study. J Bone Miner Res. 2005; 20: 152-158.
- Herrmann W, Obeid R, Schorr H, Hübner U, Geisel J, Sand-Hill M, et al. Enhanced bone metabolism in vegetarians--the role of vitamin B12 deficiency. Clin Chem Lab Med. 2009; 47: 1381-1387.
- McLean E, de Benoist B, Allen LH. Review of the magnitude of folate and vitamin B12 deficiencies worldwide. Food Nutr Bull. 2008; 29: S38-51.
- Naharci I, Bozoglu E, Karadurmus N, Emer O, Kocak N, Kilic S, et al. Vitamin B(12) and folic acid levels as therapeutic target in preserving bone mineral density (BMD) of older men. Arch Gerontol Geriatr. 2012; 54: 469-472.
- Herrmann M, Wildemann B, Wagner A, Wolny M, Schorr H, Taban-Shomal O, et al. Experimental folate and vitamin B12 deficiency does not alter bone quality in rats. J Bone Miner Res. 2009; 24: 589-596.
- van Wijngaarden JP, Doets EL, SzczeciÅĄska A, Souverein OW, Duffy ME, et al. Vitamin B12, folate, homocysteine, and bone health in adults and elderly people: a systematic review with meta-analyses. J Nutr Metab. 2013; 2013: 486186.
- Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996; 312: 1254-1259.
- Gjesdal CG, Vollset SE, Ueland PM, Refsum H, Drevon CA, Gjessing HK, et al. Plasma total homocysteine level and bone mineral density: the Hordaland Homocysteine Study. Arch Intern Med. 2006; 166: 88-94.
- Kim BJ, Koh JM, Ahn SH, Lee SH, Kim EH, Bae SJ, et al. High serum total homocysteine levels accelerate hip bone loss in healthy premenopausal women and men. Bone. 2013; 52: 56-62.
- Zhu K, Beilby J, Dick IM, Devine A, Soos M, Prince RL. The effects of homocysteine and MTHFR genotype on hip bone loss and fracture risk in elderly women. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2009; 20: 1183-1191.
- Gerdhem P, Ivaska KK, Isaksson A, Pettersson K, Väänänen HK, Obrant KJ, et al. Associations between homocysteine, bone turnover, BMD, mortality, and fracture risk in elderly women. J Bone Miner Res. 2007; 22: 127-134.
- Dhonukshe-Rutten RA, Pluijm SM, de Groot LC, Lips P, Smit JH, van Staveren WA. Homocysteine and vitamin B12 status relate to bone turnover markers, broadband ultrasound attenuation, and fractures in healthy elderly people. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2005; 20: 921-929.
- Green TJ, McMahon JA, Skeaff CM, Williams SM, Whiting SJ. Lowering homocysteine with B vitamins has no effect on biomarkers of bone turnover in older persons: a 2-y randomized controlled trial. Am J Clin Nutr. 2007; 85: 460-464.
- Koh JM, Lee YS, Kim YS, Kim DJ, Kim HH, Park JY, et al. Homocysteine enhances bone resorption by stimulation of osteoclast formation and activity through increased intracellular ROS generation. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2006; 21: 1003-1011.
- Kang AH, Trelstad RL. A collagen defect in homocystinuria. J Clin Invest. 1973; 52: 2571-2578.
- Bozkurt N, Erdem M, Yilmaz E, Erdem A, Biri A, Kubatova A, et al. The relationship of homocyteine, B12 and folic acid with the bone mineral density of the femur and lumbar spine in Turkish postmenopausal women. Arch Gynecol Obstet. 2009; 280: 381-387.
- Tyagi N, Vacek TP, Fleming JT, Vacek JC, Tyagi SC. Hyperhomocysteinemia decreases bone blood flow. Vasc Health Risk Manag. 2011; 7: 31-35.
- Herrmann M, Tami A, Wildemann B, Wolny M, Wagner A, Schorr H, et al. Hyperhomocysteinemia induces a tissue specific accumulation of homocysteine in bone by collagen binding and adversely affects bone. Bone. 2009; 44: 467-475.
- Fulzele S, Chothe P, Sangani R, Chutkan N, Hamrick M, Bhattacharyya M, et al. Sodium-dependent vitamin C transporter SVCT2: expression and function in bone marrow stromal cells and in osteogenesis. Stem Cell Res. 2013; 10: 36-47.
- Kim H, Suh H, Jo SA, Kim HW, Lee JM, Kim EH, et al. In vivo bone formation by human marrow stromal cells in biodegradable scaffolds that release dexamethasone and ascorbate-2-phosphate. Biochem Biophys Res Commun. 2005; 332: 1053-1060.
- Cai J, Zhang Q, Wastney ME, Weaver CM. Calcium bioavailability and kinetics of calcium ascorbate and calcium acetate in rats. Exp Biol Med (Maywood). 2004; 229: 40-45.
- Mahmoodian F, Gosiewska A, Peterkofsky B. Regulation and properties of bone alkaline phosphatase during vitamin C deficiency in guinea pigs. Arch Biochem Biophys. 1996; 336: 86-96.
- Kipp DE, McElvain M, Kimmel DB, Akhter MP, Robinson RG, Lukert BP. Scurvy results in decreased collagen synthesis and bone density in the guinea pig animal model. Bone. 1996; 18: 281-288.
- Thornton PA. Effect of ascorbic acid deprivation in guinea pigs on skeletal metabolism. J Nutr. 1968; 95: 388-392.
- Kipp DE, Grey CE, McElvain ME, Kimmel DB, Robinson RG, Lukert BP. Long-term low ascorbic acid intake reduces bone mass in guinea pigs. J Nutr. 1996; 126: 2044-2049.
- SHEPHERD RH, SHOLL DA, VIZOSO A. The size relationships subsisting between body length, limbs and jaws in man. J Anat. 1949; 83: 296-302, pl.
- Pointillart A, Denis I, Colin C, Lacroix H. Vitamin C supplementation does not modify bone mineral content or mineral absorption in growing pigs. J Nutr. 1997; 127: 1514-1518.
- Rowland LO Jr, Roland DA Sr, Harms RH. Ascorbic acid as related to tibia strength in spent hens. Poult Sci. 1973; 52: 347-350.
- Thornton PA. Influence of exogenous ascorbic acid on calcium and phosphorus metabolism in the chick. J Nutr. 1970; 100: 1479-1485.
- Park JK, Lee EM, Kim AY, Lee EJ, Min CW, Kang KK, et al. Vitamin C deficiency accelerates bone loss inducing an increase in PPAR-γ expression in SMP30 knockout mice. Int J Exp Pathol. 2012; 93: 332-340.
- Ganta DR, McCarthy MB, Gronowicz GA. Ascorbic acid alters collagen integrins in bone culture. Endocrinology. 1997; 138: 3606-3612.
- Kaptoge S, Welch A, Mc Taggart A, Mulligan A, Dalzell N, Day NE, et al. Effects of dietary nutrients and food groups on bone loss from the proximal femur in men and women in the 7th and 8th decades of age. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2003; 14: 418-428.
- Morton DJ, Barrett-Connor EL, Schneider DL. Vitamin C supplement use and bone mineral density in postmenopausal women. J Bone Miner Res. 2001; 16: 135-140.
- Munday K, Fulford A, Bates CJ. Vitamin C status and collagen cross-link ratios in Gambian children. Br J Nutr. 2005; 93: 501-507.
- Reddy GS, Srikantia SG. Effect of dietary calcium, vitamin C and protein in development of experimental skeletal fluorosis. I. Growth, serum chemistry, and changes in composition, and radiological appearance of bones. Metabolism: clinical and experimental. 1971; 20: 642-656.
- Hunt CE, Carlton WW, Newberne PM. Interrelationships between copper deficiency and dietary ascorbic acid in the rabbit. Br J Nutr. 1970; 24: 61-69.
- Laudermilk MJ, Manore MM, Thomson CA, Houtkooper LB, Farr JN, Going SB. Vitamin C and zinc intakes are related to bone macroarchitectural structure and strength in prepubescent girls. Calcif Tissue Int. 2012; 91: 430-439.
- Wang W, Kirsch T. Retinoic acid stimulates annexin-mediated growth plate chondrocyte mineralization. J Cell Biol. 2002; 157: 1061-1069.
- Li X, Schwarz EM, Zuscik MJ, Rosier RN, Ionescu AM, Puzas JE, et al. Retinoic acid stimulates chondrocyte differentiation and enhances bone morphogenetic protein effects through induction of Smad1 and Smad5. Endocrinology. 2003; 144: 2514-2523.
- Hu L, Lind T, Sundqvist A, Jacobson A, Melhus H. Retinoic acid increases proliferation of human osteoclast progenitors and inhibits RANKL-stimulated osteoclast differentiation by suppressing RANK. PloS one. 2010; 5: e13305.
- Lie KK, Moren M. Retinoic acid induces two osteocalcin isoforms and inhibits markers of osteoclast activity in Atlantic cod (Gadus morhua) ex vivo cultured craniofacial tissues. Comparative biochemistry and physiology Part A, Molecular & integrative physiology. 2012; 161: 174-184.
- Tanaka K, Tanaka S, Sakai A, Ninomiya T, Arai Y, Nakamura T. Deficiency of vitamin A delays bone healing process in association with reduced BMP2 expression after drill-hole injury in mice. Bone. 2010; 47: 1006-1012.
- OrÅ¡olić‡ N, Goluža E, Dikić D, LisiÄćić D, SaÅ¡ilo K, RoÄ‘ak E, et al. Role of flavonoids on oxidative stress and mineral contents in the retinoic acid-induced bone loss model of rat. Eur J Nutr. 2014; 53: 1217-1227.
- Lind T, Sundqvist A, Hu L, Pejler G, Andersson G, Jacobson A, et al. Vitamin a is a negative regulator of osteoblast mineralization. PLoS One. 2013; 8: e82388.
- Hotchkiss CE, Latendresse J, Ferguson SA. Oral treatment with retinoic acid decreases bone mass in rats. Comp Med. 2006; 56: 502-511.
- Green AC, Poulton IJ, Vrahnas C, Hääusler KD, Walkley CR, Wu JY, et al. RARγ is a negative regulator of osteoclastogenesis. J Steroid Biochem Mol Biol. 2015; 150: 46-53.
- Wattanapenpaiboon N, Lukito W, Wahlqvist ML, Strauss BJ. Dietary carotenoid intake as a predictor of bone mineral density. Asia Pac J Clin Nutr. 2003; 12: 467-473.
- Lind PM, Larsson S, Johansson S, Melhus H, Wikström M, Lindhe O, et al. Bone tissue composition, dimensions and strength in female rats given an increased dietary level of vitamin A or exposed to 3,3',4, 4',5-pentachlorobiphenyl (PCB126) alone or in combination with vitamin C. Toxicology. 2000; 151: 11-23.
- Nikawa T, Rokutan K, Nanba K, Tokuoka K, Teshima S, Engle MJ, et al. Vitamin A up-regulates expression of bone-type alkaline phosphatase in rat small intestinal crypt cell line and fetal rat small intestine. J Nutr. 1998; 128: 1869-1877.
- Bi W, Gu Z, Zheng Y, Zhang X, Guo J, Wu G. Heterodimeric BMP-2/7 antagonizes the inhibition of all-trans retinoic acid and promotes the osteoblastogenesis. PLoS One. 2013; 8: e78198.
- Rejnmark L, Vestergaard P, Charles P, Hermann AP, Brot C, Eiken P, et al. No effect of vitamin A intake on bone mineral density and fracture risk in perimenopausal women. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2004; 15: 872-880.
- Wray AE, Okita N, Ross AC. Cortical and trabecular bone, bone mineral density, and resistance to ex vivo fracture are not altered in response to life-long vitamin A supplementation in aging rats. J Nutr. 2011; 141: 660-666.
- Villeneuve LA, Gisbert E, Moriceau J, Cahu CL, Zambonino Infante JL. Intake of high levels of vitamin A and polyunsaturated fatty acids during different developmental periods modifies the expression of morphogenesis genes in European sea bass (Dicentrarchus labrax). Br J Nutr. 2006; 95: 677-687.
- Kawahara TN, Krueger DC, Engelke JA, Harke JM, Binkley NC. Short-term vitamin A supplementation does not affect bone turnover in men. J Nutr. 2002; 132: 1169-1172.
- Cline JL, Czarnecki-Maulden GL, Losonsky JM, Sipe CR, Easter RA. Effect of increasing dietary vitamin A on bone density in adult dogs. J Anim Sci. 1997; 75: 2980-2985.
- Nallamshetty S, Wang H, Rhee EJ, Kiefer FW, Brown JD, Lotinun S, et al. Deficiency of retinaldehyde dehydrogenase 1 induces BMP2 and increases bone mass in vivo. PLoS One. 2013; 8: e71307.
- Frankel TL, Seshadri MS, McDowall DB, Cornish CJ. Hypervitaminosis A and calcium-regulating hormones in the rat. J Nutr. 1986; 116: 578-587.
- Dhem A, Goret-Nicaise M. Effects of retinoic acid on rat bone. Food Chem Toxicol. 1984; 22: 199-206.
- Hough S, Avioli LV, Muir H, Gelderblom D, Jenkins G, Kurasi H, et al. Effects of hypervitaminosis A on the bone and mineral metabolism of the rat. Endocrinology. 1988; 122: 2933-2939.
- Conaway HH, Henning P, Lerner UH. Vitamin a metabolism, action, and role in skeletal homeostasis. Endocr Rev. 2013; 34: 766-797.
- Johansson S, Lind PM, Hakansson H, Oxlund H, Orberg J, Melhus H. Subclinical hypervitaminosis A causes fragile bones in rats. Bone. 2002; 31: 685-689.
- Melhus H, Michaëlsson K, Kindmark A, Bergström R, Holmberg L, Mallmin H, et al. Excessive dietary intake of vitamin A is associated with reduced bone mineral density and increased risk for hip fracture. Ann Intern Med. 1998; 129: 770-778.
- Mata-Granados JM, Cuenca-Acevedo R, Luque de Castro MD, Sosa M, Quesada-Gomez JM. Vitamin D deficiency and high serum levels of vitamin A increase the risk of osteoporosis evaluated by Quantitative Ultrasound Measurements (QUS) in postmenopausal Spanish women. Clinical biochemistry. 2010; 43: 1064-1068.
- Högström M, Nordström A, Nordström P. Retinol, retinol-binding protein 4, abdominal fat mass, peak bone mineral density, and markers of bone metabolism in men: the Northern Osteoporosis and Obesity (NO2) Study. Eur J Endocrinol. 2008; 158: 765-770.
- Gallina AM, Helmboldt CF, Frier HI, Nielsen SW, Eaton HD. Bone growth in the hypovitaminotic A calf. J Nutr. 1970; 100: 129-141.
- de Francisco A, Chakraborty J, Chowdhury HR, Yunus M, Baqui AH, Siddique AK, et al. Acute toxicity of vitamin A given with vaccines in infancy. Lancet. 1993; 342: 526-527.
- Miller DR. Nutritional toxicology. JN H, editor. New York: Academic Press; 1982.
- Clark L. The effect of excess vitamin A on longbone growth in kittens. J Comp Pathol. 1970; 80: 625-634.
- Forsmo S, Fjeldbo SK, Langhammer A. Childhood cod liver oil consumption and bone mineral density in a population-based cohort of peri- and postmenopausal women: the Nord-Trondelag Health Study. Am J Epidemiol. 2008; 167: 406-411.
- Chin KY, Ima-Nirwana S. The effects of α-tocopherol on bone: a double-edged sword? Nutrients. 2014; 6: 1424-1441.
- Maggio D, Barabani M, Pierandrei M, Polidori MC, Catani M, Mecocci P, et al. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab. 2003; 88: 1523-1527.
- Feresin RG, Johnson SA, Elam ML, Kim JS, Khalil DA, Lucas EA, et al. Effects of vitamin e on bone biomechanical and histomorphometric parameters in ovariectomized rats. J Osteoporos. 2013; 2013: 825985.
- Hermizi H, Faizah O, Ima-Nirwana S, Ahmad Nazrun S, Norazlina M. Beneficial effects of tocotrienol and tocopherol on bone histomorphometric parameters in sprague-dawley male rats after nicotine cessation. Calcif Tissue Int. 2009; 84: 65-74.
- Muhammad N, Luke DA, Shuid AN, Mohamed N, Soelaiman IN. Two different isomers of vitamin e prevent bone loss in postmenopausal osteoporosis rat model. Evid Based Complement Alternat Med. 2012; 2012: 161527.
- Norazlina M, Ima-Nirwana S, Gapor MT, Khalid BA. Palm vitamin E is comparable to alpha-tocopherol in maintaining bone mineral density in ovariectomised female rats. Experimental and clinical endocrinology & diabetes: official journal, German Society of Endocrinology [and] German Diabetes Association. 2000; 108: 305-310.
- Maniam S, Mohamed N, Shuid AN, Soelaiman IN. Palm tocotrienol exerted better antioxidant activities in bone than alpha-tocopherol. Basic Clin Pharmacol Toxicol. 2008; 103: 55-60.
- Arjmandi B, Juma S, Beharka A, Bapna M, Akhter M, Meydani S. Vitamin E improves bone quality in the aged but not in young adult male mice. J Nutr Biochem. 2002; 13: 543.
- Smith BJ, Lucas EA, Turner RT, Evans GL, Lerner MR, Brackett DJ, et al. Vitamin E provides protection for bone in mature hindlimb unloaded male rats. Calcif Tissue Int. 2005; 76: 272-279.
- Chan R, Woo J, Lau W, Leung J, Xu L, Zhao X, et al. Effects of lifestyle and diet on bone health in young adult Chinese women living in Hong Kong and Beijing. Food Nutr Bull. 2009; 30: 370-378.
- Norazlina M, Chua CW, Ima-Nirwana S. Vitamin E deficiency reduced lumbar bone calcium content in female rats. Med J Malaysia. 2004; 59: 623-630.
- Norazlina M, Ima-Nirwana S, Abul Gapor MT, Abdul Kadir Khalid B. Tocotrienols are needed for normal bone calcification in growing female rats. Asia Pac J Clin Nutr. 2002; 11: 194-199.
- Hamidi MS, Corey PN, Cheung AM. Effects of vitamin E on bone turnover markers among US postmenopausal women. J Bone Miner Res. 2012; 27: 1368-1380.
- Mata-Granados JM, Cuenca-Acebedo R, Luque de Castro MD, Quesada Gómez JM. Lower vitamin E serum levels are associated with osteoporosis in early postmenopausal women: a cross-sectional study. J Bone Miner Metab. 2013; 31: 455-460.
- Takahashi O, Ichikawa H, Sasaki M. Hemorrhagic toxicity of d-alpha-tocopherol in the rat. Toxicology. 1990; 63: 157-165.
- Wheldon GH, Bhatt A, Keller P, Hummler H. d,1-alpha-Tocopheryl acetate (vitamin E): a long term toxicity and carcinogenicity study in rats. International journal for vitamin and nutrition research Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung Journal international de vitaminologie et de nutrition. 1983; 53: 287-296.
- Frank J, Weiser H, Biesalski HK. Interaction of vitamins E and K: effect of high dietary vitamin E on phylloquinone activity in chicks. International journal for vitamin and nutrition research Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung Journal international de vitaminologie et de nutrition. 1997; 67: 242-247.
- Pearson DA. Bone health and osteoporosis: the role of vitamin K and potential antagonism by anticoagulants. Nutrition in clinical practice: official publication of the American Society for Parenteral and Enteral Nutrition. 2007; 22: 517-544.
- Shearer MJ, Bach A, Kohlmeier M. Chemistry, nutritional sources, tissue distribution and metabolism of vitamin K with special reference to bone health. J Nutr. 1996; 126: 1181S-6S.
- Iwamoto J, Takeda T, Sato Y. Effects of vitamin K2 on the development of osteopenia in rats as the models of osteoporosis. Yonsei Med J. 2006; 47: 157-166.
- Vermeer C, Jie KS, Knapen MH. Role of vitamin K in bone metabolism. Annu Rev Nutr. 1995; 15: 1-22.
- Vermeer C, Gijsbers BL, CrÄĀciun AM, Groenen-van Dooren MM, Knapen MH. Effects of vitamin K on bone mass and bone metabolism. J Nutr. 1996; 126: 1187S-91S.
- Douglas AS, Robins SP, Hutchison JD, Porter RW, Stewart A, Reid DM. Carboxylation of osteocalcin in post-menopausal osteoporotic women following vitamin K and D supplementation. Bone. 1995; 17: 15-20.
- Cancela ML, Conceição N, Laizé V. Gla-rich protein, a new player in tissue calcification? Adv Nutr. 2012; 3: 174-181.
- Bharadwaj S, Naidu AG, Betageri GV, Prasadarao NV, Naidu AS. Milk ribonuclease-enriched lactoferrin induces positive effects on bone turnover markers in postmenopausal women. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2009; 20: 1603-1611.
- Jagtap VR, Ganu JV, Nagane NS. BMD and Serum Intact Osteocalcin in Postmenopausal Osteoporosis Women. Indian J Clin Biochem. 2011; 26: 70-73.
- Bandeira F, Costa AG, Soares Filho MA, Pimentel L, Lima L, Bilezikian JP. Bone markers and osteoporosis therapy. Arq Bras Endocrinol Metabol. 2014; 58: 504-513.
- Shearer MJ. Role of vitamin K and Gla proteins in the pathophysiology of osteoporosis and vascular calcification. Curr Opin Clin Nutr Metab Care. 2000; 3: 433-438.
- Zhang C, Li D, Wang F, Dong T. Effects of dietary vitamin K levels on bone quality in broilers. Arch Tierernahr. 2003; 57: 197-206.
- Iwamoto J, Takeda T, Sato Y. Menatetrenone (vitamin K2) and bone quality in the treatment of postmenopausal osteoporosis. Nutr Rev. 2006; 64: 509-517.
- Hamidi MS, Gajic-Veljanoski O, Cheung AM. Vitamin K and bone health. J Clin Densitom. 2013; 16: 409-413.
- Matsumoto T, Miyakawa T, Yamamoto D. Effects of vitamin K on the morphometric and material properties of bone in the tibiae of growing rats. Metabolism. 2012; 61: 407-414.
- Shiraishi A, Higashi S, Masaki T, Saito M, Ito M, Ikeda S, et al. A comparison of alfacalcidol and menatetrenone for the treatment of bone loss in an ovariectomized rat model of osteoporosis. Calcif Tissue Int. 2002; 71: 69-79.
- Mawatari T, Miura H, Higaki H, Moro-Oka T, Kurata K, Murakami T, et al. Effect of vitamin K2 on three-dimensional trabecular microarchitecture in ovariectomized rats. J Bone Miner Res. 2000; 15: 1810-1817.
- Akiyama Y, Hara K, Kobayashi M, Tomiuga T, Nakamura T. Inhibitory effect of vitamin K2 (menatetrenone) on bone resorption in ovariectomized rats: a histomorphometric and dual energy X-ray absorptiometric study. Jpn J Pharmacol. 1999; 80: 67-74.
- Iwamoto J, Seki A, Sato Y, Matsumoto H, Tadeda T, Yeh JK. Vitamin K2 promotes bone healing in a rat femoral osteotomy model with or without glucocorticoid treatment. Calcif Tissue Int. 2010; 86: 234-241.
- Iwamoto J, Sato Y, Matsumoto H. Vitamin K2 improves femoral bone strength without altering bone mineral density in gastrectomized rats. J Nutr Sci Vitaminol (Tokyo). 2014; 60: 71-77.
- Iwasaki Y, Yamato H, Murayama H, Sato M, Takahashi T, Ezawa I, et al. Combination use of vitamin K(2) further increases bone volume and ameliorates extremely low turnover bone induced by bisphosphonate therapy in tail-suspension rats. J Bone Miner Metab. 2003; 21: 154-160.
- Iwamoto J, Yeh JK, Takeda T. Effect of vitamin K2 on cortical and cancellous bones in orchidectomized and/or sciatic neurectomized rats. J Bone Miner Res. 2003; 18: 776-783.
- Iwamoto J, Takeda T, Yeh JK, Ichimura S, Toyama Y. Effect of vitamin K2 on cortical and cancellous bones in orchidectomized young rats. Maturitas. 2003; 44: 19-27.
- Iwasaki Y, Yamato H, Murayama H, Sato M, Takahashi T, Ezawa I, et al. Maintenance of trabecular structure and bone volume by vitamin K(2) in mature rats with long-term tail suspension. J Bone Miner Metab. 2002; 20: 216-222.
- van Summeren MJ, van Coeverden SC, Schurgers LJ, Braam LA, Noirt F, Uiterwaal CS, et al. Vitamin K status is associated with childhood bone mineral content. Br J Nutr. 2008; 100: 852-858.
- Rassweiler J, Schmidt A, Gumpinger R, Mayer R, Eisenberger F. ESWL for ureteral calculi. Using the Dornier HM 3, HM 3+ and Wolf Piezolith 2,200. J Urol (Paris). 1990; 96: 149-153.
- Yamauchi M, Yamaguchi T, Nawata K, Takaoka S, Sugimoto T. Relationships between undercarboxylated osteocalcin and vitamin K intakes, bone turnover, and bone mineral density in healthy women. Clin Nutr. 2010; 29: 761-765.
- Kuwabara A, Fujii M, Kawai N, Tozawa K, Kido S, Tanaka K. Bone is more susceptible to vitamin K deficiency than liver in the institutionalized elderly. Asia Pac J Clin Nutr. 2011; 20: 50-55.
- Knapen MH, Drummen NE, Smit E, Vermeer C, Theuwissen E. Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2013; 24: 2499-2507.
- Iwamoto J, Sato Y, Takeda T, Matsumoto H. High-dose vitamin K supplementation reduces fracture incidence in postmenopausal women: a review of the literature. Nutr Res. 2009; 29: 221-228.
- Knapen MH, Schurgers LJ, Vermeer C. Vitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal women. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2007; 18: 963-972.
- Iwamoto I, Kosha S, Noguchi S, Murakami M, Fujino T, Douchi T, et al. A longitudinal study of the effect of vitamin K2 on bone mineral density in postmenopausal women a comparative study with vitamin D3 and estrogen-progestin therapy. Maturitas. 1999; 31: 161-164.
- Koitaya N, Ezaki J, Nishimuta M, Yamauchi J, Hashizume E, Morishita K, et al. Effect of low dose vitamin K2 (MK-4) supplementation on bio-indices in postmenopausal Japanese women. J Nutr Sci Vitaminol (Tokyo). 2009; 55: 15-21.
- Binkley NC, Krueger DC, Engelke JA, Foley AL, Suttie JW. Vitamin K supplementation reduces serum concentrations of under-gamma-carboxylated osteocalcin in healthy young and elderly adults. The American journal of clinical nutrition. 2000; 72: 1523-1528.
- Tsugawa N, Shiraki M, Suhara Y, Kamao M, Tanaka K, Okano T. Vitamin K status of healthy Japanese women: age-related vitamin K requirement for gamma-carboxylation of osteocalcin. Am J Clin Nutr. 2006; 83: 380-386.
- Onodera K, Takahashi A, Sakurada S, Okano Y. Effects of phenytoin and/or vitamin K2 (menatetrenone) on bone mineral density in the tibiae of growing rats. Life Sci. 2002; 70: 1533-1542.
- Booth SL, Broe KE, Peterson JW, Cheng DM, Dawson-Hughes B, Gundberg CM, et al. Associations between vitamin K biochemical measures and bone mineral density in men and women. J Clin Endocrinol Metab. 2004; 89: 4904-4909.
- Kalkwarf HJ, Khoury JC, Bean J, Elliot JG. Vitamin K, bone turnover, and bone mass in girls. Am J Clin Nutr. 2004; 80: 1075-1080.
- Booth SL, Broe KE, Gagnon DR, Tucker KL, Hannan MT, McLean RR, et al. Vitamin K intake and bone mineral density in women and men. Am J Clin Nutr. 2003; 77: 512-516.
- Vogl TP, Cheskin H, Blumenfeld TA, Speck WT, Koenigsberger MR. Effect of intermittent phototherapy on bilirubin dynamics in Gunn rats. Pediatr Res. 1977; 11: 1063-1068.
- Liu G, Peacock M. Age-related changes in serum undercarboxylated osteocalcin and its relationships with bone density, bone quality, and hip fracture. Calcif Tissue Int. 1998; 62: 286-289.
- Kanai T, Takagi T, Masuhiro K, Nakamura M, Iwata M, Saji F. Serum vitamin K level and bone mineral density in post-menopausal women. Int J Gynaecol Obstet. 1997; 56: 25-30.
- Heer M. Nutritional interventions related to bone turnover in European space missions and simulation models. Nutrition. 2002; 18: 853-856.
- Shiraki M, Shiraki Y, Aoki C, Miura M. Vitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2000; 15: 515-521.
- Sasaki H, Miyakoshi N, Kasukawa Y, Maekawa S, Noguchi H, Kamo K, et al. Effects of combination treatment with alendronate and vitamin K(2) on bone mineral density and strength in ovariectomized mice. J Bone Miner Metab. 2010; 28: 403-409.
- Iwamoto J, Matsumoto H, Tadeda T, Sato Y, Yeh JK. Comparison of the effect of vitamin K(2) and risedronate on trabecular bone in glucocorticoid-treated rats: a bone histomorphometry study. Yonsei Med J. 2009; 50: 189-194.
- Iwamoto J, Takeda T, Sato Y, Yeh JK. Additive effect of vitamin K2 and risedronate on long bone mass in hypophysectomized young rats. Exp Anim. 2007; 56: 103-110.
- Iwamoto J, Takeda T, Sato Y, Shen CL, Yeh JK. Beneficial effect of pretreatment and treatment continuation with risedronate and vitamin K2 on cancellous bone loss after ovariectomy in rats: a bone histomorphometry study. J Nutr Sci Vitaminol (Tokyo). 2006; 52: 307-315.
- Matsumoto Y, Mikuni-Takagaki Y, Kozai Y, Miyagawa K, Naruse K, Wakao H, et al. Prior treatment with vitamin K(2) significantly improves the efficacy of risedronate. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2009; 20: 1863-1872.
- Kobayashi M, Hara K, Akiyama Y. Effects of vitamin K2 (menatetrenone) on calcium balance in ovariectomized rats. Jpn J Pharmacol. 2002; 88: 55-61.
- Iwamoto J, Yeh JK, Schmidt A, Rowley E, Stanfield L, Takeda T, et al. Raloxifene and vitamin K2 combine to improve the femoral neck strength of ovariectomized rats. Calcif Tissue Int. 2005; 77: 119-126.
- Emaus N, Gjesdal CG, Almas B, Christensen M, Grimsgaard AS, Berntsen GK, et al. Vitamin K2 supplementation does not influence bone loss in early menopausal women: a randomised double-blind placebo-controlled trial. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2010; 21: 1731-1740.
- Volpe SL, Leung MM, Giordano H. Vitamin K supplementation does not significantly impact bone mineral density and biochemical markers of bone in pre- and perimenopausal women. Nutr Res. 2008; 28: 577-582.
- Binkley N, Krueger D, Engelke J, Crenshaw T, Suttie J. Vitamin K supplementation does not affect ovariectomy-induced bone loss in rats. Bone. 2002; 30: 897-900.
- Turan B, Can B, Delilbasi E. Selenium combined with vitamin E and vitamin C restores structural alterations of bones in heparin-induced osteoporosis. Clin Rheumatol. 2003; 22: 432-436.
- Matsunaga S, Ito H, Sakou T. The effect of vitamin K and D supplementation on ovariectomy-induced bone loss. Calcif Tissue Int. 1999; 65: 285-289.
- Takee A, Hirano J, Uchikura C, Ohmori S, Kodera M, Kotani A, et al. Effects of drug treatment on bone strength and structural changes with aging: an experimental study in rats. J Orthop Sci. 2002; 7: 544-548.
- Hirano J, Ishii Y. Effects of vitamin K2, vitamin D, and calcium on the bone metabolism of rats in the growth phase. J Orthop Sci. 2002; 7: 364-369.
- Macdonald HM, New SA, Golden MH, Campbell MK, Reid DM. Nutritional associations with bone loss during the menopausal transition: evidence of a beneficial effect of calcium, alcohol, and fruit and vegetable nutrients and of a detrimental effect of fatty acids. The American journal of clinical nutrition. 2004; 79: 155-165.