
Review Article
Austin J Nutr Metab. 2016; 3(2): 1039.
The Path to Serum Total 25-hydroxyvitamin D Assay Standardization
Sempos CT1,2*, Carter GD³, Merkel JM¹ and Binkley N4
¹Office of Dietary Supplements, National Institutes of Health, USA
²Department of Population Health Sciences, University of Wisconsin-Madison, USA
³Charing Cross Hospital, UK
4Institute on Aging, University of Wisconsin-Madison, USA
*Corresponding author: Sempos CT, Office of Dietary Supplements, National Institutes of Health, 6100 Executive Blvd, Rm 2b01, MSC 7517, Bethesda, MD, 20892-7517, USA
Received: August 23, 2016; Accepted: October 03, 2016; Published: October 05, 2016
Abstract
Widespread variation in assay measurement of total 25-hydroxyvitamin D [25(OH)D] confounds international efforts to develop evidence-based clinical and public health guidelines. The Vitamin D Standardization Program (VDSP) was founded in 2010 to develop methods to help alleviate this problem. Its goal is to promote the standardized laboratory measurement of serum total 25(OH)D in order to improve clinical and public health practice around the world. VDSP’s main objective for achieving that goal is to promote the development and maintenance of standardized laboratory measurement in both commercially and individual laboratory developed assay systems. Two distinct VDSP protocols exist for standardizing 25(OH)D measurements: one for current and future laboratory assays and another for retrospectively standardizing serum total 25(OH)D concentrations from studies conducted in the past. In this paper, we will focus on the protocol that laboratories can use now to standardize their 25(OH)D assays.
Keywords: 25-hydroxyvitamin D; CAP; CDC; Ghent University; DEQAS; NIST; ODS; PT/EQA; VDSP; Vitamin D
Abbreviations
25(OH)D: 25-hydroxyvitamin D; CAPABVD: College of American Pathologists Accuracy-Based Vitamin D; CDC: Centers for Disease Control and Prevention; DEQAS: Vitamin D External Quality Assessment Scheme; LC-MS/MS: liquid chromatographytandem mass spectrometry; NIST: National Institute for Standards and Technology; ODS: Office of Dietary Supplements; PT/EQA: performance testing or external quality assessment; RMP: Reference Measurement Procedure; SRM: standard reference material; VDSP: Vitamin D Standardization Program
Introduction
Serum total 25-hydroxyvitamin D [25(OH)D] is considered to be the best biological marker of an individual’s vitamin D status [1-3]. It is the sum of the serum concentrations of 25(OH)D2 and 25(OH)D3. A number of medical societies and government agencies nutrition groups have issued vitamin D guidelines, however, two very different sets of guidelines for defining clinical states of vitamin D deficiency, insufficiency, sufficiency and excess have caused a great deal of controversy [1,2].
A fundamental factor that confounds efforts to develop consensus clinical/public health guidelines for interpreting the serum 25(OH) D concentration is the substantial variability in the many assays that have been used over the years to measure 25(OH)D in vitamin D research studies [4-7]. The lack of assay standardization is the underlying source of bias thwarting attempts to pool research results in order to develop consensus cut-points [8].
The Vitamin D Standardization Program (VDSP) was developed to alleviate this problem [9].This paper will provide an overview of the VDSP, and its standardization procedures that can be used now and in the future to help guarantee that 25(OH)D assays are producing accurate and precise values compared to the gold standard Reference Measurement Procedures (RMPs).
Standardization
Before describing the VDSP, it is important to clarify the meaning of assay standardization. In terms of 25(OH)D a standardized laboratory measurement is one that is “comparable across measurement system, location and time” [10]. In other words, every laboratory regardless of type of assay utilized, location or time (now or in the future) would obtain the same 25(OH)D result from the same sample -within proscribed statistical limits -as would be obtained using one of the internationally recognized gold standard RMPs developed by the National Institute for Standards and Technology (NIST), Ghent University and the Centers for Disease Control and Prevention (CDC) [11-13].That is each standardized laboratory reports the true concentration of serum total 25(OH)D.
Standardization allows for the development of and consistent application of evidenced-based guidelines [14]. It is the first and essential step in comparing and contrasting results from different studies whether clinical trials or observational epidemiological studies. With standardization it is possible to achieve long-term stability of measurement results both in research and patient care.
Overview of the VDSP
The VDSP has developed a reference measurement system that is the backbone for standardizing 25(OH)D measurement in current and future assay systems [15,16].The components of this reference measurement system include the gold standard RMPs, NIST Standard Reference Materials (SRMs) [17], the VDSP’s Standardization Certification Program developed and conducted by CDC[18, 19]and the accuracy-based performance testing or external quality assessment schemes (PT/EQA) conducted by the College of American Pathologists or CAP Accuracy-Based Vitamin D (ABVD) Performance Testing Scheme and the Vitamin D External Quality Assessment Scheme or DEQAS [20,21]. Finally, but importantly, the VDSP includes a set of laboratory performance guidelines for both gold standard RMPs and for routine laboratories [22].
Accuracy-based surveys are a very special type of PT/EQA [23]. While there are several Vitamin D PT/EQA programs around the world, to our knowledge there are only two accuracy-based programs, i.e., CAP ABVD and DEQAS. By accuracy-based we mean that each laboratory’s determinations for the different PT/EQA samples are compared to the true value as determined by one of the three recognized RMPs. CDC assigns target values to the CAP ABVD materials using their RMP and NIST does the same for DEQAS. The VDSP suggests that all laboratories participate in an accuracy-based PT/EQA simply because a laboratory can determine the bias in their assay versus the true concentration there by help promote assay standardization.
Protocol for standardizing current and future measurements
The steps to standardizing an existing assay system are to: 1) Calibrate currently existing commercial assay systems so that the assay is standardized to yield the same value as obtained using the gold standard RMP. 2) Calibrate individual clinical and research assays to the RMP; 3) Verify that the performance of each laboratory is meeting the VDSP guidelines over time [16].
Reference materials and single donor serum samples with concentrations assigned to them using the RMP can then be used to develop an unbroken chain of traceability from the routine clinical/ research laboratory, to the assay manufacturer and finally back to the gold standard RMPs (Figure 1) [14]. Traceability ensures that the clinical sample result determined with a routine assay is equivalent to the result that would be obtained using the RMP [10,14]. Routine assays are thereby calibrated or standardized to the RMP. Standard Reference Material (SRM) 2972a 25-Hydroxyvitamin D2 and D3 Calibration Solutions” in ethanol is especially useful in calibrating chromatographic-based assays, e.g. LC-MS/MS assays (Table 1). Periodic use of NIST SRMs and participation in an accuracy-based PT/EQA program can help to monitor the calibration of the assay over time, i.e., Trueness Controls (Table 2 and 3). For the in-house developed 25(OH)D assay all of the responsibility for assuring that the assay is traceable rests with the assay developer to incorporate all of the steps indicated in Figure 1.
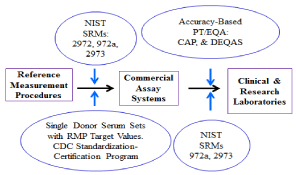
Figure 1: VDSP calibration traceability scheme.
SRM 972a consists of four separate ethanolic solutions
Vitamin D Metabolite
Concentration (ng/g)
25-(OH)D3 in Ethanol Level 1
410.0 ± 14.9
25(OH)D3 in Ethanol Level 2
812.0 ± 29.2
25(OH)D2 in Ethanol
293.6 ± 9.1
3-epi-25(OH)D3 in Ethanol
293.4 ± 13.5
1SRM 2972a is a replacement for SRM 2972 which has been redesigned.
2Ethanolic solutions can be diluted to prepare calibration curves.
Table 1: SRM 2972a¹ 25-hydroxyvitamin D calibrating² solutions.
- SRM 972a Vitamin D Metabolites in Frozen Human Serum
- SRM 1950 Metabolites in Human Plasma
- SRM 968e Fat Soluble Vitamins, Carotenoids, and Cholesterol in Human Serum
- SRM 2973 Vitamin D Metabolites in Frozen Human Serum
Table 2: NIST SRMs for vitamin D metabolites in human serum/plasma.
SRM
Total 25(OH)D
25-(OH)D2
25-(OH)D3
3-epi-25-(OH)D3
24,25(OH)2D3
972a
Level 1
29.3 ± 1.1
--
28.8 ± 1.1
1.81 ± 0.10
2.66 ± 0.10
Level 2
18.9 ± 0.4
0.81 ± 0.06
18.1 ± 0.4
1.28 ± 0.09
1.41 ± 0.05
Level 3
33.2 ± 0.5
13.3 ± 0.3
19.8 ± 0.4
--
1.62 ± 0.06
Level 4
30.0 ± 0.9
--
29.4 ± 0.9
26.0 ± 2.2
2.64 ± 0.09
2973
40.1 ± 0.8
0.65 ± 0.02
39.4 ± 0.8
2.10 ± 0.08
3.13 ± 0.11
Table 3: Assigned values of SRMs 972a and 2973 (ng/mL).
Finally, but of extreme importance, the third step is verification of “end-user” performance and assuring consistency across the different types of assays which can be performed by certification through CDC’s Vitamin D Standardization-Certification Program for commercial assay manufacturers and large commercial/clinical laboratories and by accuracy-based performance testing for routine laboratories [16,23]. Assessing “end-user” performance requires the setting of quantitative performance criteria or limits. As part of the RMP development, the VDSP has accepted performance limits based on biological variation for both reference and routine laboratories estimated by Stckl, et al. [22] (Table 4). Specifically, for routine laboratories, these current performance limits are CV≤10% and Bias≤5%. Currently, the VDSP is reviewing these limits and in the future may suggest revisions of them.
Measurements
CV (%)
Bias (%)
Reference Laboratories
≤5%
≤1.7%
Routine Laboratories
≤10%
≤5%
1Stöckl et al. [22]
Table 4: VDSP Assay performance limits based on biological variation1
As most serum total 25(OH)D laboratory measurements are made using commercially developed measurement systems, one of the initial goals of the VDSP was to encourage commercial assay manufacturers to work with the VDSP to standardize their assay to the NIST-Ghent-CDC RMPs. Currently, commercial assays from around the world have been certified by CDC as being standardized [19]. In addition, several large commercial clinical laboratories and a number of research laboratories have serum total 25(OH)D assays which are certified as being standardized.
Steps to standardize an assay
If a commercially developed assay system is utilized, there is very little that an individual laboratory can do to correctly calibrate the system. However, individual labs can participate in the CAP ABVD and/or DEQAS PT/EQA and monitor local results over time. If the mean bias (%) is >|5%| or if while the mean bias (%) is<|5%| most or a large proportion of values are outside the bounds of +5% and -5% then it is necessary to contact the assay manufacturer’s representative to come and re-calibrate the instrument or correct mistakes being made when using the system. In addition, one can periodically insert blindly samples of SRM 972a and 2973 or to save money use old samples from CAP or DEQAS to test the accuracy of the system. Again, if it does not seem to be working properly then it is necessary to contact the manufacturer’s representative.
For research laboratories which use their assay systems only periodically, it can be much more difficult. If using a commercial assay system, the procedure noted in the previous paragraph should be followed. In addition, sets of single donor serum samples from CDC or alternative sources should be used to determine if the assay system meets VDSP performance criteria of Total CV≤10% and Mean Bias≤5%. For researchers who use an in-house assay system, the VDSP is preparing a detailed set of guidelines for setting up and using it in measuring the concentration of 25(OH)D in study samples.
Summary and Conclusion
The VDSP has developed a set of tools which routine clinical and research laboratories can use to check assay accuracy and precision when setting them up for the first time and for verifying an assay’s calibration over time. The tools include using NIST SRMs and/or samples from the CAP ABVD and/or DEQAS as blinded “trueness” controls, and participation in one or both accuracy-based PT/EQA surveys –CAP ABVD and DEQAS. In addition to the above, assay manufacturers, large commercial laboratories and large clinical/ epidemiological laboratories should participate and maintain certification in the VDSPs’ Standardization Certification Program developed and conducted by CDC.
References
- Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press. 2011.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011; 96:1911-1930.
- Scientific Advisory Committee on Nutrition (SACN). Vitamin D and Health. Public Health England; 2016.
- Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KE, et al. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab. 2004; 89: 3152-3157.
- Carter GD. Accuracy of 25-hydroxyvitamin D assays: confronting the issues. Curr Drug Targets. 2011; 12: 19-28.
- Wallace AM, Gibson S, de la Hunty A, Lamberg-Allardt C, Ashwell M. Measurement of 25-hydroxyvitamin D in the clinical laboratory: current procedures, performance characteristics and limitations. Steroids. 2010; 75: 477-488.
- Le Goff C, Cavalier E, Souberbielle JC, González-Antuña A, Delvin E. Measurementofcirculating²5-hydroxyvitaminD:Ahistorical review. Pract LabMed. 2015; 2: 1-14.
- Sempos CT, Durazo-Arvizu RA, Binkley N, Jones J, Merkel JM, Carter GD. Developing vitamin D dietary guidelines and the lack of 25-hydroxyvitamin D assay standardization: The ever-present past. J Steroid Biochem Mol Biol. 2015.
- Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM. Vitamin D status as an international issue: national surveys and the problem of standardization. Scand J Clin Lab Invest Suppl. 2012; 243: 32-40.
- Vesper HW, Thienpont LM. Traceability in laboratory medicine. Clin Chem. 2009; 55:1067-75.
- Tai SS, Bedner M, Phinney KW. Development of a candidate reference measurement procedure for the determination of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem. 2010; 82: 1942- 1948.
- Stepman HC, Vanderroost A, Van Uytfanghe K, Thienpont LM. Candidate reference measurement procedures for serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 by using isotope-dilution liquid chromatographytandem mass spectrometry. Clin Chem. 2011; 57: 441-448.
- Mineva EM, Schleicher RL, Chaudhary-Webb M, Maw KL, Botelho JC, Vesper HW, et al. A candidate reference measurement procedure for quantifying serum concentrations of 25-hydroxyvitamin D(3) and 25-hydroxyvitamin D(2) using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2015; 407: 5615-5624.
- Myers GL. Introduction to standardization of laboratory results. Steroids. 2008; 73: 1293-1296.
- Bunk DM. Reference materials and reference measurement procedures: an overview from a national metrology institute. Clin Biochem Rev. 2007; 28:131-137.
- Binkley N, Sempos CT, Vitamin DSP. Standardizing vitamin D assays: the way forward. J Bone Miner Res. 2014; 29:1709-1714.
- Phinney KW, Bedner M, Tai SS, Vamathevan VV, Sander LC, Sharpless KE, et al. Development and certification of a standard reference material for vitamin D metabolites in human serum. Anal Chem. 2012; 84: 956-962.
- Thienpont LM, Stepman HC, Vesper HW. Standardization of measurements of 25-hydroxyvitamin D3 and D2. Scand J Clin Lab Invest Suppl. 2012; 243: 41-49.
- Centers for Disease Control and Prevention (CDC) Laboratory Quality Assurance and Standardization Programs. Hormone Standardization Program. 2016.
- College of American Pathologists. Accuracy-Based Vitamin D (ABVD) Proficiency Testing Program 2016.
- Carter GD. DEQAS (Vitamin D External Quality Assessment Scheme) 2016.
- Stockl D, Sluss PM, Thienpont LM. Specifications for trueness and precision of a reference measurement system for serum/plasma 25-hydroxyvitamin D analysis. Clin Chim Acta. 2009; 408: 8-13.
- Miller WG, Jones GR, Horowitz GL, Weykamp C. Proficiency testing/external quality assessment: current challenges and future directions. Clin Chem. 2011; 57: 1670-1680.