
Special Article - Vitamin D Deficiency
Austin J Nutr Metab. 2017; 4(2): 1047.
Effects of Vitamin D Supplementation on Health Outcomes in Young Healthy Population: A Systematic Review and Metaregression of Randomized Controlled Trials
Farrokhyar F1,2*, Sivakumar G3, Savage K1, Easterbrook B1, Chaudhry S1, Koziarz A2, Hong BY4, Fathalla Z2 and Reid S1
¹Department of Surgery, McMaster University, Canada
²Department of Health, Evidence and Impact, McMaster University, Canada
³Schulich School of Medicine and Dentistry, University of Western Ontario, Canada
4Faculty of Medicine, University of Ottawa, Canada
*Corresponding author: Forough Farrokhyar, Department of Surgery, McMaster University, 39 Charlton Avenue East Hamilton, ON L8N 1Y3, Canada
Received: April 26, 2017; Accepted: May 30, 2017; Published: June 15, 2017
Abstract
Background/Objective: Level 1 evidence on impact of vitamin D supplementation and fortified food on serum 25-hydroxyvitamin D (25(OH)D), and its consequent effects on musculoskeletal health and specific diseases in needed. The aim of this meta-analysis is to evaluate the effects of vitamin D supplementation on 25(OH)D and health outcomes in adolescent and young individuals.
Methods: The participants include healthy population aged 10 to 40 years. The intervention includes vitamin D supplementation or fortified food (any dosage, duration, schedule or formulation) with or without calcium. The comparison includes lower vitamin D dosages or placebo. The outcomes include 25(OH)D, musculoskeletal or vascular health, physical performance, injuries and infection. Multiple electronic literature searches are completed. Review process will be completed independently and in duplicate to avoid bias. Due to between study heterogeneity, studies will be stratified by moderator variables and pooled using a random effects model. Absolute mean differences and relative risk with 95% confidence intervals will be reported. A random effects meta-regression will be performed to examine the contribution of moderator variables on the mean serum 25(OH)D concentrations. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guidelines and the Cochrane Risk of Bias assessment tool will be used to ensure rigorous methodology.
Implication: The findings will guide clinical practice worldwide by providing the most comprehensive analysis of the benefits/risks associated with vitamin D supplementation. The knowledge dissemination will inform health policy decision makers worldwide by calling attention to Vitamin D deficiency and appropriate supplementation as a global health issue.
Review Registration Number: CRD42016048788.
Keywords: Vitamin D; 25-hydroxyvitamin D; Adolescents; Healthy adults
Introduction
Physiology and sources of vitamin D
Vitamin D has an intrinsic role in maintaining the structural integrity and function of the musculoskeletal system [1]. It is classically known to orchestrate processes associated with calcium and phosphorous homeostasis, as well as bone and mineral metabolism [2]. There are two main forms of vitamin D, vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol), which are obtained from different sources such as ultraviolet B (UVB) radiation exposure, diet, and supplements. Both forms of vitamin D are transformed in the liver to 25-hydroxyvitamin [25(OH)D] which can be measured in blood serum samples [3]. Vitamin D is predominantly produced in the skin as vitamin D3 after exposure to ultraviolet B (UVB) radiation from sunlight [4,5] and approximately 10% of the required vitamin D is derived from dietary supplements and foods including oil-rich fish, mushrooms, and fortified foods such as cereals, breads, juices, and dairy products [6-10]. Vitamin D generated from the dermis and diet is biologically inactive. Precursors of vitamin D are metabolized by the liver into an inactive form known as 25(OH)D, the main form of vitamin D metabolite in circulation. In the kidney, the 25(OH) D is further transformed into the biologically active compound, calcitriol (1,25(OH)2-D) [2,7]. Most cells and tissues possess vitamin D receptors (VDRs) to convert vitamin D to its active form [7,11]. The calcitriol binds to VDR in the cell nucleus before exerting a broad range of actions within the body [9-11].
Recommended daily intake of vitamin D
There is debate among experts surrounding healthy serum 25(OH)D concentrations and the minimum required daily vitamin D intake. The Endocrine Society Committee (ESC) has defined concentrations of ≥30 ng/mL (≥75 nmol/L) as sufficient, 21-29 ng/ mL (52.5-72.5 nmol/L) as insufficient and ≤20 ng/mL (≤50 nmol/L) as deficient [8,12]. The 2016 global recommendations suggested >20 ng/mL (>50 nmol/L) concentrations as sufficient, 12-20 ng/mL (30- 50 nmol/L) as insufficient and <12 ng/mL (<30 nmol/L) as deficient [13]. Vitamin D toxicity and hypercalcemia occur when the serum 25(OH)D concentration reaches beyond >100 ng/mL (>250 nmol/L). Holick [14] suggested that it would take years of very high dose of vitamin D to cause vitamin D toxicity and hypercalcemia. Although exceedingly rare, the adverse effects of toxicity are considerable and may include gastrointestinal bleeding, infectious diseases and aseptic necrosis of the hip [14]. For healthy adolescents up to 18 years and adults aged 19 to 70 years, the ESC recommends daily vitamin D intake of 600-1000 IU with an upper limit of 4000 IU and 1,500-2,000 IU with an upper limit of 10,000 IU, respectively, while, the Institute of Medicine (IOM) suggests a vitamin D intake of 600 IU with an upper limit of 4000 IU for ages 9 to 70 years [15].
Epidemiology of vitamin D deficiency
Vitamin D insufficiency has been characterized as a global health problem. More than 1 billion people are estimated to be vitamin D deficient or insufficient worldwide [7,14,16]. Children, adolescents, women and middle-aged adults are equally susceptible to developing inadequacies in vitamin D concentrations [8,17]. Hypovitaminosis D in children and youth has been reported in North America [18- 21], Australia and Oceana [16], Europe [22,23], Midland China [24], Korea [25], the Middle East [26-28], India [29] and South America [8]. Our recent publications have suggested higher rates of vitamin D insufficiency in athletes, particularly in higher altitudes, in late fall and wintertime, and with indoor activities. In addition, lower extremity stress fractures seem to be related to vitamin D insufficiency [30-32].
Contributing factors to vitamin D deficiency
As mentioned previously, sunlight-driven cutaneous production of vitamin D3 is the primary source of vitamin D as only a minority of foods naturally contain vitamin D or are fortified with vitamin D [7,8]. As such, inadequate exposure or penetration of sunlight is often the major cause of vitamin D deficiency or insufficiency [4,33]. In addition, season and latitude substantially impact vitamin D3 synthesis [13,17]. During the winter season, the sun’s rays enter earth at an oblique angle; resulting in a low number of UVB photons striking the earth, reducing vitamin D3 synthesis [5]. While latitudes above 37° face a steep decline in the number of UVB photons contacting the earth’s surface during the winter months, locations below 37° latitude maintain steady, adequate concentrations of vitamin D3 production despite seasonal variations [17]. People with dark skin and body mass index of greater than 30 kg/m² require longer natural sun exposure and thus, these factors are associated with vitamin D deficiency [8]. Individuals such as elite athletes who partake in intense physical activities, particularly indoor activities and at higher latitudes, are prone to vitamin D deficiency [17,34]. Black, et al. [35] conducted a meta-analysis of vitamin D food fortification and found that a mean intake of 400 IU (~11 ug) per day increased 25(OH)D concentrations by 19.6 nmol/L.
Health consequences of vitamin D deficiency
Vitamin D deficiency ensues in aberrancies associated with calcium and phosphorus absorption, resulting in increased serum parathyroid hormone (PTH) concentrations [7,8,36]. The body counteracts hyperparathyroidism by balancing serum concentrations of calcium through metabolizing calcium from bone leading to a disruption in bone metabolism, which can ultimately result in osteopenia and osteoporosis [8,36]. Severe abnormalities in serum vitamin D concentrations can cause muscular atrophy and skeletal disorders such as rickets in children and osteomalacia in adults [37,38]. Recent evidence also indicates that vitamin D is capable of acting on the immunological landscape to stimulate innate and adaptive immune responses [8,10]. The pleiotropic functionalities of vitamin D have been further confirmed by studies which have associated vitamin D deficiency with cardiovascular diseases, various types of cancers, autoimmune disorders, multiple sclerosis, and inflammatory bowel disease [3,7].
Global Impact of Vitamin D deficiency
The prevalence of vitamin D deficiency and insufficiency among all age groups was announced a global problem based on the findings from a systematic review in 2014 [39]. This is of concern, considering many diseases have been linked to vitamin D deficiency. Rickets, for example, which was an epidemic of the 19th century, is making a come-back in regions of the world wherefrom it was thought the disease had been all but completely eradicated [3,40] due to the fortification of milk with vitamin D. While taking into consideration that cardiovascular disease, cancer, respiratory infections, respiratory diseases, tuberculosis and diabetes mellitus were “vitamin D-sensitive diseases” that accounted for more than half of global mortality rates, a study undertaken by Grant [41] concluded that increasing serum 25-hydroxyvitamin D concentrations was the most cost-effective way to reduce global mortality rates. Despite the fact that vitamin D deficiency is widely considered to be a major global health problem [33,39,42], very few policies and interventions aimed at combating low serum 25(OH)D concentrations are in place. Those that are in place are in need of updating based on changing population dynamics. Given that vitamin D deficiency contributes to the global burden of morbidity and mortality which, naturally, results in dire global economic consequences, it is imperative that an appropriate assessment of the impact of vitamin D on serum 25(OH)D be conducted so as to inform present and future global health policies.
Rationale and importance of conducting this systematic review
Current systematic reviews and meta-analyses present interesting findings on targeted and specific populations or outcomes, but are neither inclusive nor comprehensive. Cashman, et al. [43] included 44 randomized controlled trials (RCT) of oral administration of vitamin D3 <2,000 IU/d (50 ug/d), with or without calcium on healthy populations and excluded RCTs of vitamin D2 and those including higher doses of vitamin D3. They compared latitudes 40-49.5°N to >49.5°N and found that an intake of 930 IU/d would maintain 25(OH)D >50 nmol/L concentrations. Three additional metaanalyses assessed the relationship of all forms and doses of vitamin D supplementation on muscle strength (17 RCTs) [44], bone density (23 RCTs) [45], and muscle strength and injuries (10 and 4 RCTs, respectively) [46,47] on healthy adults of 18 years or older and found no strong association. A meta-analysis by Muir, et al. [47] included elderly adults of >60 years of age and found 800-1,000 IU of vitamin D to be beneficial for muscle strength and balance. The most recent meta-regression [48] included 88 RCTs of neonates, infants and adolescents with vitamin D deficiency to assess the effect of a high dose vitamin D regimen (>1000 IU) on 25(OH)D concentrations.
The researchers, who considered disease status, baseline 25(OH) D and age, found the rapid normalization of vitamin concentrations (>75 nmol/L) is best achieved by using a loading dose of >50,000 IU; however, they found that loading doses of >300 000 IU should be avoided until the risks and benefits are evaluated. Our group has previously found a high prevalence of vitamin D deficiency in athletes [17] and that providing three months of 3,000 IU vitamin D supplementation to athletes with vitamin D insufficiency at baseline achieved sufficiency [49]. However, the supporting evidence assessing the effects of daily doses of vitamin D supplementation or fortified food intake on serum 25(OH)D and its consequent and concurrent effects on other biomarkers, musculoskeletal health and specific diseases and conditions on healthy adolescent and young healthy adults is lacking. Hence, we aim to evaluate the effects of all forms and dosages of vitamin D on the concentrations of 25(OH) D, biomarkers, vascular health, musculoskeletal health, physical performance and infection in healthy human beings aged 10 to 40 years. This systematic review will be the most comprehensive and address different outcomes in different subgroups.
Research Question, Objectives and Hypotheses
In young population of 10-40 years old, what is the impact of vitamin D supplementation or fortified food intake on serum 25(OH) D and other health outcomes? The primary objective is to compare the mean serum 25(OH)D between vitamin D supplementation/ vitamin D fortified food and placebo/control. The secondary objectives are to compare the following outcomes between the study groups:
• parathyroid hormone concentrations
• physical performance
• musculoskeletal health
• vascular health
• infectious diseases
• fractures and stress fractures
• muscle and soft tissue injuries
We hypothesize that there is a dose-response effect of vitamin D on 25(OH)D concentrations and the effect might differ by season, latitude and baseline status of vitamin D levels in an adolescent and young healthy population.
Methods
Design
This systematic review of RCTs will be conducted according to predefined criteria with trained reviewers as registered on PROSPERO (CRD42016048788). The systematic review will utilize the PRISMA (the Preferred Reporting Items for Systematic Reviews and Meta-Analysis) Statement [50], the Cochrane risk of bias assessment tool [51] (www.cochrane.org) and Covidence platform (https://community.cochrane.org/tools/review-production-tools/ covidence) to ensure a rigorous methodology and reporting. The PRISMA Statement consists of a flow diagram and a checklist. The 27 PRISMA checklist items pertain to title, abstract, methods, results, discussion and funding of the meta-analysis. Covidence is an online Cochrane primary screening and data extraction tool that has built-in key steps including the PRISMA flow diagram and the Cochrane risk of bias assessment tool.
Eligibility criteria
The PICOT approach is used to frame the research question. The population (P) of interest includes healthy males and females aged 10 to 40 years. The age range was decided based on our previous publication on the prevalence of vitamin insufficiency in athletes [17]. Of 23 included studies, the age of healthy athletes varied from 11 to 39 years of age. Populations with chronic illness or comorbidities that could influence serum 25(OH)D concentrations or alter responses to vitamin D are excluded. Studies on specific populations, such as pregnant women, elite athletes, or military personnel are also excluded because of their unique situation, circumstances, lifestyle practices and differential needs of vitamin D intake.
The intervention (I) includes vitamin D2 or D3 supplementation, or fortified food. Any dosage, duration, schedule, and formulation (tablets, chewable, oral sprays, or emulsified oils), with or without calcium, is acceptable. Trials assessing solar radiation or multivitamin supplementation are excluded to avoid heterogeneity due to different methods of intervention assessment.
The comparison (C) intervention includes placebo, different dosage(s) of vitamin D, ultraviolet light exposure or no supplementation.
The outcome measures (O) primarily include serum 25(OH)D, and secondarily musculoskeletal health, physical activities, injuries, infection and any other outcomes assessed in the trial. The follow-up time (T) is not limited and will be approximated in weeks or months, whichever appropriate.
Only RCTs are included to ensure the best methodological quality and the highest level of evidence available. Quasi-randomized and observational designs are excluded. Abstracts, conference proceedings and non-published data are excluded due to limited information on quality and outcome assessment and incomplete data. Review articles, basic science research articles and non-human studies are also excluded. Identified studies not in the English language will be excluded during the screening process.
Before the start of the search process, a workshop was conducted to train reviewers and data abstractors to ensure consistency and accuracy of the review process.
Search methods for identification of relevant articles
A search algorithm is developed and an electronic literature search of Medline, CINAHL, EMBASE, SPORT Discus and The Cochrane Library databases are completed, with the guidance of a professional medical librarian, from inception to April 30, 2016. The search terms include: cholecalciferol, ergocalciferol, Vitamin D*, vitamin D derivative, 25 hydroxyvitamin D, 25-OH Vitamin D, Serum 25 hydroxyvitamin D, 25-hydroxycholecalciferol, and serum 25-OH-D, and 25 (OH)D. The developed search strategies are tailored to the relevant subject headings of each database search engine to be most inclusive. No limitations on language are applied. Examples of the search process are shown in Appendix 1. The search results from all databases are merged and all duplicate articles are removed using EndNote software. The references of the published systematic reviews are hand-searched to retrieve unidentified relevant trials. The search will be updated before final analysis to retrieve the most recent publications.
Screening process, eligibility assessment and study selection
The Covidence platform is used for screening and eligibility assessment of the retrieved citations. The citations from the initial search, after excluding duplicates using EndNote, are uploaded into Covidence. The review articles are cross-referenced to identify additional articles to include in Covidence. Titles and abstracts are reviewed for eligibility, in duplicate and independently by two reviewers. The full texts of the eligible articles are uploaded onto Covidence. Two reviewers independently review the full-texts of the selected articles for eligibility to ascertain that studies are designed as RCTs and meet the predefined inclusion criteria. In the title and abstract screening phase, the articles are rated as include (when all eligibility criteria is met), exclude (when one or more of the eligibility criteria was not met) or maybe (when the reviewer is not certain). In full-text review phase, the articles are listed as include (all eligibility criteria are met) or exclude (one or more of the eligibility criteria was not met). The reasons for exclusion are entered in Covidence using the predefined criteria. Disagreements are resolved through consensus with a third reviewer.
Risk of bias assessment of included studies
The Covidence platform, with its inbuilt but modified Cochrane risk of bias quality assessment will be used for methodological quality assessment of the included trials. Box 1 demonstrates the modified risk of bias assessment domains relevant to selection, performance, detection, attrition, and other sources of biases. Selection bias is best avoided if an unbiased method of random sequence generation and an optimal method of concealing the treatment allocation from all involved are utilized. Performance and detection biases are prevented when the treatment allocation is fully masked from participants, research personnel, caregivers and outcome assessors. In other words, all individuals involved are blinded to treatment allocation. Attrition bias is minimized when complete data for all outcomes is collected and all participants were followed up until the end of the study. This requires rigorous follow-up data collection and documentation of the reasons for losses to follow-up and withdrawals. For the purpose of this systematic review, the other sources of bias will include an adequate report of power calculation, report of intention-to-treat analysis, an adequate flow diagram of the search process, and clear reporting of sources of funding. The methodological quality of the included studies will be assessed independently and in duplicate and scored as low-risk if the process was adequately described, highrisk if the process was not described and unclear risk if the process was inadequately described. Comments on the rationale for the decisions made in each domain will be included by each reviewer. Disagreements will be resolved by discussion between the two reviewers regarding the comments and further discordance will be settled by a third reviewer.
Data extraction and data management
The data extraction sheets for baseline characteristics, as well as primary and secondary outcome measures, are created using Microsoft Excel software based on our previous systematic reviews on the topic [17,30,49]. The data extraction sheets are piloted on 10 articles to ensure complete data collection. One reviewer will collect the data from all articles. The second reviewers will verify the data for accuracy and highlight discrepancies. Disagreements will be resolved by the third reviewer. Data collection forms include information on study and participant demographics, methods, interventions and measured outcomes.
Information on the studies’ geographical location, latitudes, design, placebo controlled or open-label, the start and end date of the trial and year of publication will be collected. Latitudes will be retrieved (www.worldatlas.com) if not reported in the article. Data pertaining to participants’ demographics (mean age, standard deviation (SD), gender proportions), vitamin D (unit, type, dosage, product and duration), and outcome measures (mean, SD, unit, method of laboratory analysis) at baseline and at follow-ups for each group will be extracted (Table 1,2). The SDs will be extracted from range, standard errors, confidence interval or p-value if not reported. The authors will be contacted and mean values will be requested if median values were reported. If no response is received from authors after two reminders, data will be considered missing.
Variable name
Description
Study variables
Authors
Title
Abstract
Year of publication
Year
Geographical location
Country
Latitude
Degree
Design
Randomized
Season
Fall/winter or spring/summer
No. of study arms
1,2,3,…
Patients randomized
Total sample size
Study duration
Start and end date
Vitamin D and placebo
Company and method used for analysis. placebo standardized for absence of vitamin
Serum 25(OH)D and other measures
Company and method used for analysis
Participants variables per arm
Randomized
Number randomized per arm
Age
Mean, SD, range
Age categories
Adolescent (10-18 years), adults (>18 Years)
Gender
Number of men
Occupation/activity
Student, college student, employed, etc.
BMI
Mean, SD, range
Weight
Unit, mean, SD, range
Height
Unit, mean, SD, range
Vitamin D status
Number insufficient, deficient
Bone Mass Density
Unit, mean, SD
Body fat
Unit, mean, SD
Intervention variables per arm
Vitamin D arm
Type
Supplementation, fortified food
Dose in IU
Dose in IU, convert to IU if reported in ug
From
Tablets, spray, capsules, powder liquid, food.
Daily dose
Convert in daily dose if otherwise
Frequency
Daily, weekly, one loading dose, or other
Duration
The length of total treatment
Calcium
Unit, dose, etc.
Control arm
Vitamin D
Dose, unit, frequency
Ultraviolet
Hours, time, etc.
Other
Outcome measures per arm
At baseline and at each follow- up
Number of lost to follow-up
Total number lost to follow-up per arm
25(OH)D per arm
Number analysed, unit, mean, SD, range
Parathyroid hormone
Number analysed, unit, mean, SD, range
Calcium absorption, osteocalcin
Number analysed, unit, mean, SD, range
Cytokine concentrations
Number analysed, unit, mean, SD, range
Any other biomarkers
Number analysed, unit, mean, SD, range
Any physical activity/ performance
Like hand grip, pinch grip strength, jump height (number analysed, unit, mean, SD, range)
Infection
Respiratory, gastrointestinal, urinary or any other infection event
Musculoskeletal outcomes
Any muscle, bone, soft tissue managements (Number analysed, unit, mean, SD, range )
Number analysed, unit, mean, SD, range
Vascular health
Cardiovascular fitness, cardiac wall thickness, blood pressure, cholesterol levels, any other measures (number analysed, unit, mean, SD, range)
Any other outcome assessed
Number analysed, unit, mean, SD, range
Table 1: Data extraction strategy and variables collected on study, participants, interventions outcome measures per study arm whenever required.
1. Was the random sequence generation adequately explained?
2. Was the treatment allocation adequately concealed from all involved in the study?
3. Were participants and research personnel adequately blinded to treatment allocation?
4. Were outcome assessors adequately blinded to treatment allocation?
5- Were outcomes data complete? If not, was the loss to follow and withdrawal reported?
6- Was a power calculation adequately reported in a way that it is reproducible?
7- Was intention-to-analysis reported?
8- Was a CONSORT flow diagram of the search process adequately reported?
Table 2: The cochrane modified risk of bias assessment tool.
Vitamin D intake is measured in international units (IU) or micrograms (μg) where 100 IU is equal to 2.5 μg [52]. The units will be converted (www.nafwa.org/vitamind.php) and reported in IU for consistency. The dosing regimen, timing and frequency will likely vary between trials. They will be converted into daily dosages when reported weekly or in other time intervals for quantitative analysis. The ESC recommended cut-off of 600-1,000 IU for 9-13 years and 1,500-2,000 IU for adults aged 19 or older [15] will be used as our criteria.
Serum 25(OH)D is measured in nanogram per milliliter (ng/mL) or nanomoles per liter (nmol/L), where 1 ng/mL is equal to 2.496 nmol/L [34].
The units will be converted (https://www.endmemo.com/medical/ unitconvert/Vitamin__D.php) and reported in both units, but ng/ mL unit will be used for data analysis for consistency. The cut-off of >30 ng/mL (>75 nmol/L) for vitamin D sufficiency recommended by ESC for young and healthy population [8,12] will be used for the analysis. The unit of biomarkers and different physical performance measures will be standardized. For the definition and classification of the dichotomous outcome measures, we will ensure that the data is pooled using similar definitions and classifications.
For further assurance, each review stage is piloted on approximately 15 articles. At this time, the process of screening and selection of the articles is completed and a total of 148 articles are included (Figure 1). The bias assessment criteria and baseline and outcome data collection sheets are piloted and revised accordingly.
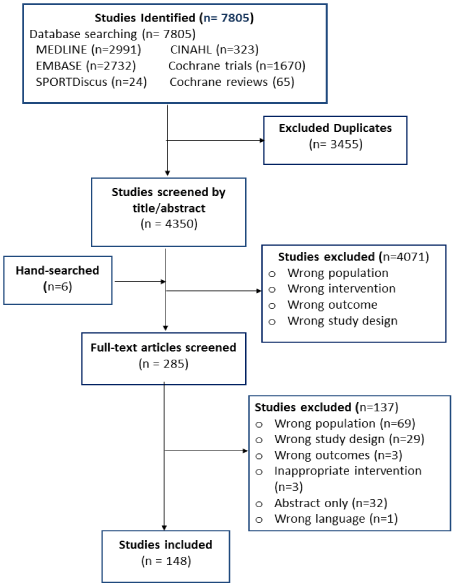
Figure 1: PRISMA flow diagram of search strategy and study identification
process.
Data stratification
It was noted in previous systematic reviews that latitudes have a significant impact on vitamin D concentrations (particularly in fall and winter when sun exposure is minimal), and that some studies target those with insufficient vitamin D status while others include any vitamin D status [17,30,35,43,49]. Also noted was that the recommended vitamin D dosage is likely to be age-dependent. For homogeneity and consistency, we will estimate the treatment effect magnitude using the following strata: latitude (>40° versus >40°), season (fall and winter versus spring and summer), baseline vitamin D status (insufficient versus sufficient), and mean age (<18 years and >18 years). The follow-up period for treatment effect estimation will be approximated at 1 week, 6 weeks, 12 weeks, 24 weeks and >24 weeks. We will also examine the overall effect of vitamin D supplementation adjusting for these moderator variables as well as vitamin D dosage, formulation, type and duration through meta-regression analysis.
Data synthesis
The baseline characteristics will be summarized. Clinical heterogeneity (population, dosing regimen, and outcome measurement features) and methodological heterogeneity (risk of bias) will be described in tables and figures and considered in the data synthesis. Data on the outcome measures will be pooled and absolute means differences and relative risks with 95% confidence intervals (CI) will be reported for each stratum taking the following approaches.
For within-group effects, the change in mean 25(OH)D concentration from pre-supplementation to post-supplementation for the vitamin D and control groups will be calculated for each study and the mean change in 25(OH)D concentrations with 95% CI for the vitamin D and control group will be pooled at each follow-up. For between-group effects, the absolute mean differences (AMD) in mean 25(OH)D concentrations between the vitamin D and control groups at each follow-up will be estimated using a random effects model with an inverse variance approach assuming large between-study heterogeneity. Heterogeneity will be tested using the Cochran’s Q test with p-value set at 0.1 for significance and quantified using the I2 statistic (I2 <40% as low, 40-60% as moderate and >60% as substantial heterogeneity). Pooled AMDs with 95% CIs will be reported. The synthesis of the secondary outcome measures will be conducted taking a similar approach and methodology. The mean differences for continuous outcomes and relative risk with 95% CIs for dichotomous outcomes will be reported. SPSS (www.ibm.com) and review manager version 3 (www.cochrane.org) will be used for data synthesis.
To examine the contribution of moderator variables on the mean serum 25(OH)D concentrations, a random effects metaregression model will be applied using ‘metareg’ macro available for STATASE 12.0 software (www.stata.com). The dependent variable will be the absolute mean difference in 25(OH)D concentrations between the vitamin D and control arms at each follow-up. The moderator variables will include study location, latitude, season, baseline vitamin D status, mean age and its corresponding SD, male proportion, vitamin D type, formulation, dosage, and duration as well as the Cochrane methodological quality components and sample size. First, a univariable random-effects meta-regression will be performed and then variables with a significance level of 0.1 will be tested in a multivariable meta-regression analysis. Between moderators, interaction will be considered for adjustment and included in the multivariable meta-regression analysis. The findings from the metaregression will be robust with an anticipated 148 included RCTs.
Sensitivity analysis will be performed by excluding trials with >15% of the missing outcome data and trials with high risk of bias for treatment allocation. A p-value of 0.05 will be used for statistical significance. A funnel plot will be used for visual assessment of potential publication bias. The PRISMA checklist will be completed to adhere to the Cochrane reviews guidelines.
The GRADE recommendation for treatment effects of vitamin D
The GRADE (grading of recommendations, assessment, development and evaluations) recommendation criteria [53] will be utilized to determine the strength of recommendation of vitamin D supplementation in adolescents and healthy young adults based on the current evidence. The GRADE recommendation determines the extent of confidence that the benefits of vitamin D supplementation outweigh no supplementation. According to GRADE, data from randomised controlled trials will produce high quality evidence, but the quality can be diminished due to the robustness of the included studies. The following key factors will be considered in determining the strength of the recommendation: risk of bias, magnitudes, inconsistency, indirectness, imprecision of the treatment effects and publication bias. Another key factor will be dose-response vitamin D gradient. The GRADE Profiler 3.36.1 (www.cochrane.org) will be used to develop the recommendation.
Discussion
Vitamin D deficiency and its impact on health outcomes is a global concern. Some systematic reviews have been conducted for specific populations, but the supporting evidence assessing the effects of daily doses of vitamin D supplementation or fortified food on serum 25(OH)D concentrations, and its consequent and concurrent effects on other health outcomes in healthy adolescents and young adults, is lacking. The aim of this review is to examine the effects of all forms and dosages of vitamin D on the concentrations of 25(OH) D and the consequent effects on vascular and musculoskeletal health, physical performance, injuries, and infection in healthy adolescent and adult individuals. This systematic review will be the most comprehensive to date, and will address different outcomes in different subgroups. This protocol uses the GRADE framework to summarise confidence in estimates of vitamin D dosing effects, uses a rigorous methodology and will be reported according to the PRISMA guidelines. By pooling available data from a number of RCTs, this systematic review will have enough power to evaluate patient-relevant outcomes and address the safety and efficacy of vitamin D supplementation on the healthy population worldwide. The findings from this systematic review will guide clinical practice worldwide by providing the most comprehensive analysis of the benefits and the risks associated with vitamin D supplementation. The knowledge dissemination will call attention to vitamin D deficiency as a global health issue and inform health policy and intervention planning worldwide.
Acknowledgment
We express our special gratitude to Ms. Laura Banfield, a professional librarian at the School of Health Sciences at McMaster University, for guiding KS in literature search process.
Authorship Contribution
F Farrokhyar was involved in formulating the research question, designing the study, supervising the review guidelines development, screening, modifying CONSORT bias assessment criteria and designing the data collection forms, and writing and editing the manuscript.
G Sivakumar was involved in editing the review guidelines, screening titles and abstracts, and full texts for study selection, designing and piloting the data collection forms, and writing and editing the manuscript.
K Savage was involved in editing the review guidelines, assisting in literature search process, uploading the articles in Endnote and Covidence, screening titles and abstracts, and full texts for study selection, piloting the data collection forms, and editing the manuscript.
S Chaudhry was involved in revising the review guidelines, and revising the data collection forms, piloting the data collection forms, and writing and editing the manuscript.
B Easterbrook was involved in screening titles and abstracts, and full texts for study selection, piloting the data collection forms and editing the manuscript.
A. Koziarz was involved in screening titles and abstracts, and full texts for study selection, bias assessment process and editing the manuscript.
B.Y. Hong was involved in screening titles and abstracts, and full texts for study selection, bias assessment process and editing the manuscript
Z. Fathalla was involved in screening titles and abstracts, and full texts for study selection, piloting the data collection forms and editing the manuscript.
S Reid was involved in formulating the research question, designing the study, and editing the manuscript.
References
- Pfeifer M, Begerow B, Minne HW. Vitamin D and muscle function. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2002; 13: 187-194.
- DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004; 80: 1689S-96S.
- Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011; 86: 50-60.
- Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D-lightful story. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2007; 22: V28-33.
- Moan J, Porojnicu AC, Dahlback A, Setlow RB. Addressing the health benefits and risks, involving vitamin D or skin cancer, of increased sun exposure. Proceedings of the National Academy of Sciences of the United States of America. 2008; 105: 668-673.
- Rosen CJ, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, et al. IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab. 2012; 97: 1146-1152.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357: 266-281.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metabolism. 2011; 96: 1911-1930.
- Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M; Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008; 122: 398-417.
- Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013; 5: 2502-2521.
- Adams JS1, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010; 95: 471-478.
- Ross AC. The 2011 report on dietary reference intakes for calcium and vitamin D. Public health nutrition. 2011; 14: 938-939.
- Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J Clin Endocrinol Metab. 2016; 101: 394-415.
- Holick MF. Vitamin D Is Not as Toxic as Was Once Thought: A Historical and an Up-to-Date Perspective. Mayo Clin Proc. 2015; 90: 561-564.
- Golden NH, Carey DE. Vitamin D in Health and Disease in Adolescents: When to Screen, Whom to Treat, and How to Treat. Adolescent medicine: state of the art reviews. 2016; 27: 125-139.
- Thacher TD, Fischer PR, Strand MA, Pettifor JM. Nutritional rickets around the world: causes and future directions. Annals of tropical paediatrics. 2006; 26: 1-16.
- Farrokhyar F, Tabasinejad R, Dao D, Peterson D, Ayeni OR, Hadioonzadeh R, et al. Prevalence of vitamin D inadequacy in athletes: a systematic-review and meta-analysis. Sports Med. 2015; 45: 365-378.
- Calvo MS, Whiting SJ, Barton CN. Vitamin D fortification in the United States and Canada: current status and data needs. Am J Clin Nutr. 2004; 80: 1710S- 6S.
- Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001-2004. Pediatrics. 2009; 124: e362-370.
- Turer CB, Lin H, Flores G. Prevalence of vitamin D deficiency among overweight and obese US children. Pediatrics. 2013; 131: e152-161.
- Whiting SJ, Langlois, KA, tanparast H, Greene-Finestone LS. The vitamin D status of Canadians relative to the 2011 Dietary Reference Intakes: an examination in children and adults with and without supplement use. The American journal of clinical nutrition. 2011; 94: 128-135.
- Ceroni D, Anderson de la Llana, R., Martin X, Lamah L, De Coulon G, Turcot K, Dubois-Ferriere V. Prevalence of vitamin D insufficiency in Swiss teenagers with appendicular fractures: a prospective study of 100 cases. Journal of children’s orthopaedics. 2012; 6: 497-503.
- Lehtonen-Veromaa M, Mottonen, T, Irjala K, Karkkainen M, Lamberg-Allardt C, Hakola P, Viikari J. Vitamin D intake is low and hypovitaminosis D common in healthy 9- to 15-year-old Finnish girls. Eur J Clin Nutr. 1999; 53: 746-751.
- Zhang W, Stoecklin E, Eggersdorfer M. A glimpse of vitamin D status in Mainland China. Nutrition. 2013; 29: 953-957.
- Lee YA, Kim HY, Hong H, Kim JY, Kwon HJ, Shin CH, Yang SW. Risk factors for low vitamin D status in Korean adolescents: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008-2009. Public health nutrition. 2014; 17: 764-771.
- Badawi A, Arora P, Sadoun E, Al-Thani AA, Thani MH. Prevalence of vitamin d insufficiency in qatar: a systematic review. J Public Health Res. 2012; 1: 229-235.
- Korchia G, Amitai Y, Moshe G, Korchia L, Tenenbaum A, Rosenblum J, Schechter A. Vitamin D deficiency in children in Jerusalem: the need for updating the recommendation for supplementation. Isr Med Assoc J. 2013; 15: 333-338.
- Racinais S, Hamilton B, Li CK, Grantham J. Vitamin D and physical fitness in Qatari girls. Arch Dis Child. 2010; 95: 854-855.
- Marwaha RK, Tandon N, Reddy DR, Aggarwal R, Singh R, Sawhney RC, et al. Vitamin D and bone mineral density status of healthy schoolchildren in northern India. Am J Clin Nutr. 2005; 82: 477-482.
- Dao D, Sodhi S, Tabasinejad R, Peterson D, Ayeni OR, Bhandari M, et al. Serum 25-Hydroxyvitamin D Levels and Stress Fractures in Military Personnel: A Systematic Review and Meta-analysis. Am J Sports Med. 2014.
- Davey T, Lanham-New SA, Shaw AM, Hale B, Cobley R, Berry JL, et al. Low serum 25-hydroxyvitamin D is associated with increased risk of stress fracture during Royal Marine recruit training. Osteoporos Int. 2016; 27: 171- 179.
- Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin d supplementation decreases incidence of stress fractures in female navy recruits. J Bone Mine Res. 2008; 23: 741-749.
- Holick MF. Vitamin D: a D-Lightful health perspective. Nutr Rev. 2008; 66: S182-194.
- Ogan D, Pritchett K. Vitamin D and the athlete: risks, recommendations, and benefits. Nutrients. 2013; 5: 1856-1868.
- Black LJ, Seamans KM, Cashman KD, Kiely M. An updated systematic review and meta-analysis of the efficacy of vitamin D food fortification. J Nutr. 2012; 142: 1102-1108.
- Heaney RP. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am J Clin Nutr. 2004; 80: 1706S-9S.
- Bouillon R. [Vitamin D and human health]. Presse medicale (Paris, France: 1983). 2009; 38: 3-6.
- Bouillon R1, Bischoff-Ferrari H, Willett W. Vitamin D and health: perspectives from mice and man. J Bone Miner Res. 2008; 23: 974-979.
- Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014; 144: 138-145.
- Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006; 116: 2062-2072.
- Grant WB. An estimate of the global reduction in mortality rates through doubling vitamin D levels. Eur J Clin Nutr. 2011; 65: 1016-1026.
- Naeem Z. Vitamin d deficiency- an ignored epidemic. Int J Health Sci (Qassim). 2010; 4: V-VI.
- Cashman KD, Fitzgerald AP, Kiely M, Seamans KM. A systematic review and meta-regression analysis of the vitamin D intake-serum 25-hydroxyvitamin D relationship to inform European recommendations. Br J Nutr. 2011; 106: 1638-1648.
- Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL. Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2011; 22: 859-871.
- Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta-anaysis. Lancet. 2014; 383: 146-155.
- Redzic M, Lewis RM, Thomas DT. Relationship between 25-hydoxyvitamin D, muscle strength, and incidence of injury in healthy adults: a systematic review. Nutr Res. 2013; 33: 251-258.
- Muir SW, Montero-Odasso M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: a systematic review and metaanalysis. J Am Geriatr Soc. 2011; 59: 2291-2300.
- McNally JD, Iliriani K, Pojsupap S, Sampson M, O’Hearn K, McIntyre L, et al. Rapid normalization of vitamin D levels: a meta-analysis. Pediatrics. 2015; 135: e152-166.
- Farrokhyar F, Sivakumar G, Savage K, Koziarz A, et al. Effects of Vitamin D Supplementation on Serum 25-Hydroxyvitamin D Concentrations and Physical Performance in Athletes: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Sports Med. 2017.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Sur. 2010; 8: 336-341.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0
- Angeline ME, Gee AO, Shindle M, Warren RF, Rodeo SA. The effects of vitamin D deficiency in athletes. Am J Sports Med. 2013; 41: 461-464.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008; 336: 924-926.