
Research Article
Austin J Nutr Metab. 2019; 6(1): 1067.
Shelf Life Characteristics of Composite Gluten-Free Cookies Fortified with Fermented and Unfermented Agaricus bisporus Polysaccharide Flours
Sulieman AA*
Department of Food Science, Jiangnan University, China
*Corresponding author: Abdellatief A. Sulieman, School of Food Science & Technology, Jiangnan University, China
Received: September 28, 2019; Accepted: October 28, 2019; Published: November 04, 2019
Abstract
In this work, shelf life characteristics were evaluated for Composite Gluten- Free (CGF) cookies enriched with Fermented and unfermented Agaricus bisporus Polysaccharide (FABP and UABP) flours and stored at 25°C for 4 and 6 months. Incorporation of both FABP flour and UABP flour in CGF flour reduced aw, moisture content and whiteness, and increased redness and yellowness, while addition of FABP flour decreased the syneresis. Furthermore, most of stored CGF cookie formulations had higher aw, and moisture content, as well as lower fracture strength and hardness compared to the control. UABP flour Formulation (F3) showed additional fat proton with highest relaxation time (403.70-932.60 ms, T24), whereas FABP flour F3 exhibited strong linked proton (0.01-1.32 ms), with an increase in T22 (P2) of the other stored CGF cookies. FTIR spectroscopy showed the presence of C=O group and glycosidic linkages (C-O-C and C-O-H) in FABP flour and CGF cookies, with different intensities.
Keywords: Fermented Agaricus bisporus Polysaccharide Flour; Composite Gluten-Free Cookies; texture; 1H NMR Spectroscopy; FTIR Spectroscopy
Introduction
Gluten-free products are very important and useful for individuals suffering from Celiac Disease (CD). CD is caused by ingestion of gluten proteins, which are encountered in wheat, barley, rye and their crossbreeds [1]. Recent epidemiological studies have shown that the prevalence of CD has been significantly underestimated in many countries, including China [2]. Gluten-free products are commonly consumed as staple foods in a number of countries, where CD persist. Gallagher reported that over 90% of people with CD in the United Kingdom obtained their gluten-free food on prescription. Furthermore, gluten-free products commercialization has grown at an annual rate of 28% in the last years [3]. Gluten-free flours, such as rice, corn, potato and soy with high fat powders produced cookie dough that was sheetable, and cookies of comparable quality to wheat cookies. The development of appealing gluten-free products from composite flour of sweet potatoes will therefore play a major role in raising awareness on the potential of the crop.
Cookies have been suggested as a good way to utilize composite flours as they are ready-to-eat convenient foods, provide a good source of energy, and are consumed widely throughout the world [4]. The term cookies or biscuits, which are called in many countries in the world, refer to a baked product generally consist of the three major ingredients flour, sugar and fat. These ingredients are mixed together with other minor ones to form dough [5]. In the USA, the cookie and cracker manufacturing industry includes about 300 companies with combined annual revenue of about $11 billion [6].
As a health food, edible mushrooms are preferred by people worldwide because they are low in calories and high in nutrients. Recently, several studies have shown interest in isolating and characterizing new functional compounds from mushrooms, such as polysaccharides, polysaccharide-peptide complexes and proteins [6,7]. Mushroom polysaccharides are well-known to possess diverse health benefits for human body. Due to their strong antioxidant activities and free radical scavenging abilities, many polysaccharides have been detected for development into safe and effective medicines. Jeong et al. stated that polysaccharide isolated from Agaricus bisporus possesses excellent inhibiting action against human breast cancer [8]. Furthermore, Chinese quince seed meal polysaccharides obtained by different processes had good DPPH and superoxide anion-scavenging activities [9].
Fermentation is a biotechnological process brought out by microorganisms, such as bacteria, fungi, yeast, or a combination of them in anaerobic conditions used by human for long period of time. The main goal of fermentation was primarily on food preservation and to extend the shelf life, while safety, nutritional value and organoleptic quality of the foods are concurrently succeeded [10]. Lactic Acid Bacteria (LAB) are generally the prominent microorganisms utilized in food industry. LAB promote the nutritional value, flavor, tastiness and texture of various types of fermented foods [11]. Therefore, in this study, Agaricus bisporus Polysaccharide (ABP) flour was fermented by Lactobacillus plantarum and incorporated in Composite Gluten- Free (CGF) cookies to improve their shelf life characteristics.
Several studies have been performed on improvement in quality of bakery products, but researches on extension of shelf life of these products are lacking. Shelf life of bakery product is mostly characterized by both sensorial changes and microbial spoilage, in addition to other factors that affecting the shelf life, such as rancidity, crystallization, grittiness, syneresis of jellies, chocolate bloom, structural weakness, fade color and water mobility. Ruan and Chen reported that food materials containing higher proteins and/or carbohydrates could decrease water mobility, while lower contents could increase the mobility [12]. To extend the shelf life of the bakery products, the above factors must be controlled by suitable preservation methods. Preservation in bakery means the retardation of spoilage including the texture staling. A hygroscopic substance has the effect of keeping the food material moist. In addition, moisture content and water mobility of product are key measuring factors in deciding when a particular product reaches the end of its sensory life. The relationship between the water activity and the microbial shelf lives of bakery products is well established with mold growth being the main limiting factor. The present work aims to study CGF cookies enriched with Fermented Agaricus bisporus Polysaccharide (FABP) flour and Unfermented Agaricus bisporus (UABP) flour and to evaluate their shelf life characteristics, such as water activity, moisture content, texture and 1H NMR spectroscopy.
Materials and Methods
Materials
Sweet potato roots (white variety) and glutinous rice flour (13.37 g/100 g moisture, 8.03 g/100 g protein, 1.06 g/100 g ash, and 1.03 g/100 g fat) were purchased from local supermarket. Freeze-dried button mushroom powder (8.47 g/100 g moisture, 27.91 g/100 g protein, 7.29 g/100 g ash and 2.22 g/100 g fat) was obtained from Longhai Union Food Co., Ltd. (Zhangzhou, China), and xanthan gum manufactured by Danisco Co., Denmark. In addition, De Man Rogosa Sharpe (MRS) broth medium was obtained from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). All baking ingredients (baking powder, sodium bicarbonate, sugar, margarine and skim milk powder) were purchased from local supermarkets.
Preparation of sweet potato flour
Sweet potato flour was prepared according to the method described by Sulieman et al. [13]. Briefly, the roots after peeling, washing and slicing, the slices were blanched, and then dried at 60°C for 10 h in convection oven (Dasol Scientific Co. Ltd., Seoul, Korea). The dried slices were milled into flour using a laboratory-scale mill (Tianjin Taisite Instrument Co., Ltd., Tianjin, China), sieved through an 80-mesh sieve, packed, and sealed in high-density polyethylene bags for further use.
Extraction of Agaricus Bisporus Polysaccharide (ABP) flour
Using the procedure described by Fan et al., with some modifications, freeze-dried button mushroom powder (200 g) was boiled with 4 L distilled water in a vessel for 4 h with stirred regularly [14]. The solid residue was filtered through a 200-mesh nylon cloth, and the residue was re-extracted twice with 2 L distilled water to obtain the polysaccharide fraction. All extracted liquid fractions were combined and concentrated at 50°C using a rotary evaporator under reduced pressure (Wuxi Shenke Instrument, Wuxi, China). The concentrated fraction was precipitated overnight with four portions of 70% ethanol at room temperature and centrifuged at 2080×g for 10 min. The resulting pellet was freeze-dried (Free Zone, Labconco Co., Ltd., Kansas, USA) at -55°C and 0.123 mbar (pressure) for 96 h to obtain ABP flakes, which were then ground in a coffee grinder (Baijie, Wuxi, China). Finally, the ABP flour was stored in sealed bags at 4°C until further use.
Fermentation of ABP flour
MRS broth medium was prepared and dispensed (100 mL) in conical flask (250 mL), sterilized in an autoclave at 121°C (200 kPa) for 15 min, and then cooled to room temperature (24±1°C). Lactobacillus plantarum was grown in MRS at 37°C for 24 h. The cells were harvested and re-suspended in sterilized tryptone (2%) and adjusted to 106 CFU/mL. The cells were then used as an inoculum for ABP flour fermentation.
The extracted ABP flour (200 g, wet weight basis) was put tightly into screw-capped plastic vessel and inoculated with 2 mL L. plantarum suspension (106 CFU/mL), without mixing. The fermentation process was carried out at 37°C for 72 h. The final pH of the fermented product was 3.6. The product was freeze-dried and ground as described above for ABP flour, to obtain Fermented Agaricus bisporus Polysaccharide (FABP) flour and stored in sealed bags at 4°C for further analyzes.
Preparation of Composite Gluten-Free (CGF) flours and cookies
Three blends were prepared as follows: Sweet Potato Flour (SPF) was blended with a constant level of Glutinous Rice Flour (GRF), and then it was fortified with different percentages of FABP flour, and UABP flour (86.5/10/3, 83.5/10/6, and 80.5/10/9). These blends were referred to the formulations (F1, F2, and F3, for both FABP flour and UABP flour). Control sample was composed of SPF (89.5%) and GRF (10%), as well as xanthan gum was added to all flour blends at 0.5% by the total weight. The CGF flours were kept in sealed bags at 4°C for analyzes.
CGF cookies were produced using the method described by Sai Manohar and Haridas Rao, with modifications [15]. The dough was prepared in a laboratory dough mixer. The margarine (120 g) and ground sugar (130 g) were creamed in a Hobart mixer (model SM-5D, Sinmag Machine Co. Ltd., Wuxi, China) with a flat beater for 2 min at 61 rpm and to the cream, water (60 mL) containing sodium bicarbonate (3 g), baking powder (3 g) and sodium chloride (3 g) was added and mixed more for 5 min at 125 rpm to obtain a homogeneous cream. The CGF flour (300 g) and skim milk powder (15 g) were added to the cream and mixed continuously to form the final dough and then sheeted to a thickness 5.0 mm with a rolling pin and using aluminium platform and frame. The cookies were shaped with a cutter (diameter 55 mm), and baked on aluminium trays at 200°C for 12 min, cooled for 30 min and stored in air-tight containers for further analyzes.
Water activity, moisture content and color of CGF flour
Water activity (aw) of CGF flour was determined using a Novasina Thermo-constanter model Lab swift-aw (Lucerne, Switzerland) according to the manufacturer’s instructions. The moisture content of the CGF flour was measured according to the method of AOAC [16]. The color of the CGF flour was determined using a chromameter (CR-400, Konica Minolta, Japan), which calibrated with a white standard plate. The values were L*, a*, and b*; The L* values (white 100/ black 0), the a* values (red positive/ green negative), and the b* values (yellow positive/blue negative)
Syneresis of CGF flour
Retrogradation of flour starch measured by syneresis was carried out according to the method described by Kuar et al., with minor modifications [17]. Flour suspension (6% flour in water, w/w) was heated at 85°C for 30 min in a temperature controlled water bath, and then cooled to room temperature in an ice water bath for 5 min. The cooled samples were stored at 4°C for 1-6 days. The ratio of the weight of liquid separated from the sample to the total weight of the sample before centrifugation at 2149×g for 15 min, and multiplied by 100 was calculated as the percentage of syneresis of stored sample.
Texture characteristics of stored CGF cookies
Fracture strength and hardness of stored CGF cookies were measured using a texture analyzer TA-XT2i (Stable Micro Systems, London, England). The distance between the two beams was 50 mm. Another identical beam was brought down from above at a pretest speed of 10 mm/s, test speed of 1 mm/s, post-test speed of 10 mm/s and distance of 5 mm to contact the cookie. The downward movement was continued till the cookie breaks. The peak force (g) was reported as fracture strength [18].
1H NMR spectroscopy of stored CGF cookies
Spin-Spin Relaxation Time (T2) was analyzed using NMR (MesoMR-23-060V-1, Shanghai Niumag Company Ltd., Shanghai, China) operated at 25°C and a frequency of 21 MHz for 1H, with a dead time of 18 μs to observe the water mobility in polysaccharide flours and fresh and stored CGF cookies at 25°C for 4 months. Transverse relaxation (T2) was measured using the Carr-Purcell- Meiboom-Gill (CPMG) pulse sequence [19,20]. The sample (1.5 g) was weighted in a small sealed tube and placed inside a 25 mm NMR tube. The parameters of the CPMG test are as follows: the pulse widths of the 90° and the 180° pulse were 7.52 and 15 μs, respectively, the number of points selected to measure the sample was 2000, the number of echoes was 1000, the number of scans was 4 and the relaxation decay time was 3s. The CPMG data were fitted with analysis software from Niumag Instruments. (Shanghai, China). All analyzes were performed in triplicate and variation coefficients less than 10%.
FT-IR spectroscopy of CGF cookies
FT-IR spectroscopy was performed using alkali metal halide pellets according to the method described by Jouraiphy et al. [21]. Powdered CGF cookie (2 mg) was mixed with anhydrous potassium bromide (200 mg) in a small crucible to generate a homogenous mixture. The mixtures were compressed using a hydraulic press to form a transparent pellet, which was then measured by FT-IR (Spectrum 400, Perkin Elmer, USA) from wavenumbers of 4000 to 500 cm-1 at 0.2 cm/s.
Volatile compounds of CGF cookies
Extraction of volatile compounds was achieved using the method proposed in Aponte et al., with some modifications [22]. In brief, powdered CGF cookie (2 g) was transferred to a 20 mL headspace vial, and 200 μL of an aqueous toluene solution (250 mL/L) was added. The vial was placed in a thermostatic block at 40°C with continuous agitation and the fiber was inserted and maintained in the sample head space for 30 min; the fiber was then removed and immediately inserted into a Gas Chromatography-Mass Spectroscopy (GC-MS) injector for compound desorption. Volatile compounds from the samples were isolated using Solid-Phase Micro-Extraction (SPME) and separated on a CP-Sil-8CB (Varian, Walnut Creek, CA, USA) fused silica capillary column. Aroma compounds were identified by comparison of mass spectra and retention time of MS database.
Results and Discussion
Water activity, moisture content and color of CGF flour
Water activity (aw), moisture content and color parameters of UABP flour, FABP flour and CGF flour are presented in Table 1. Significant differences were observed in aw, moisture content and color values among all the samples. Addition of both UABP flour and FABP flour decreased aw, moisture content and whiteness (L* values), and increased redness (a* values), yellowness (b* values) and Δ E values of CGF flour. Moreover, FABP flour formulations had higher aw, moisture content and whiteness, and lower redness, yellowness and Δ E values than UABP flour formulations. The lowest aw, moisture content and lightness, and highest redness and yellowness of FABP flour and UABP flour added, as well as higher their nutritional value may reduce aw, moisture content and whiteness of CGF flour. In addition, incorporation of UABP flour and FABP flour could increase the particle size and reduce whiteness of CGF flour, due to reduction of surface area that permits more reflection of light. Sulieman et al. explained that the decrease in whiteness may be attributed to an increase of dietary fibers, protein and some colored pigments that present in the UABP flour and FABP flour added, in addition to some changes in color that happened in the FABP flour during the fermentation process [23]. The reduction in lightness with increase protein and fiber contents was also observed in a research study by Tharise et al. for composite gluten-free flour [24].
Sample
aw
Moisture (g/100 g)
L*
a*
b*
∆ E
UABP flour
0.09 ± 0.00a
3.58 ± 0.11a
65.68 ± 0.13a
2.30 ± 0.06b
8.68 ± 0.09a
28.14 ± 0.10b
FABP flour
0.05 ± 0.00a
2.23 ± 0.05b
52.37 ± 0.11b
2.54 ± 0.03a
6.35 ± 0.07b
43.04 ± 0.14a
Control
0.57 ± 0.02a
11.85 ± 0.04a
95.83 ± 0.12a
0.29 ± 0.01g
3.86 ± 0.02g
2.86 ± 0.11g
UABP flour F1
0.53 ± 0.01ab
11.42 ± 0.03c
85.81 ± 0.61c
0.96 ± 0.04e
5.98 ± 0.13c
7.94 ± 0.61e
F2
0.51 ± 0.01b
11.33 ± 0.01d
81.78 ± 0.42e
1.26 ± 0.02c
7.06 ± 0.03b
12.09 ± 0.41c
F3
0.50 ± 0.03b
11.09 ± 0.04e
79.22 ± 0.10f
1.44 ± 0.03a
7.56 ± 0.17a
14.68 ± 0.08a
FABP flour F1
0.54 ± 0.01ab
11.65 ± 0.03b
87.16 ± 0.35b
0.83 ± 0.03f
4.57 ± 0.14f
6.30 ± 0.34f
F2
0.53 ± 0.02ab
11.37 ± 0.03cd
82.89 ± 0.20d
1.13± 0.02d
5.21± 0.08e
10.58 ± 0.21d
F3
0.50 ± 0.02b
11.04 ± 0.08e
79.80 ± 0.26f
1.33 ± 0.01b
5.70 ± 0.06d
13.70 ± 0.26b
Mean ± standard deviation (n=3). Mean values within a column followed by a different letter are significantly different (p ˂0.05). UABP flour=Unfermented Agaricus bisporus Polysaccharide flour; FABP flour=Fermented Agaricus bisporus Polysaccharide flour.
Table 1: Water activity (aw), moisture content and color of fermented and unfermented Agaricus bisporus polysaccharide flours, and composite gluten-free flours fortified with fermented and unfermented polysaccharide flours.
Syneresis
Syneresis or loss of water is a retrogradation of flour starch gel during cold storage and negatively affects the quality of baked products. Figure 1a,b (Supplementary Data) presents the syneresis percentages of UABP flour, FABP flour and CGF flour formulation gels stored at 4°C for 1 to 6 days. UABP flour exhibited lower syneresis values (ranging from 64.17 to 67.93%) than FABP flour (ranging from 68.99 to 71.43%) on all days of storage (except in 6th day of storage) (Supplementary Data, Figure 1a), probably due to the strong and stable water absorption and holding capacity of the UABP flour gel system, as well as the release of water soluble nutrients, such as Soluble Dietary Fibers (SDFs) and organic acids from FABP flour during the refrigerated storage time. High intracellular and intermolecular hydrogen bonding and the ability of the gel to hold water reduce syneresis during storage. [25]. Conversely, SPF had the highest syneresis percentage on the 2nd and 3rd days of storage, which may have been due to its higher amylose content (data not shown). For the CGF flour gels (Supplementary Data, Figure 1b), the control (4.16 to 10.57%), UABP flour F1 (5.26 to 10.70%) and UABP flour F2 (6.76 to 10.75%) gels had higher syneresis percentages compared to FABP flour formulation gels, especially on the 2nd, 3rd and 4th days of storage. Moreover, among the FABP flour formulation gels, the FABP flour F3 showed the lowest syneresis percentage, which ranged from 1.34% to 7.87% on all storage days (Supplementary Data, Figure 1b). We observed that the syneresis percentage of the gels was lower when FABP flour was added to the CGF flour, whereas incorporation of UABP flour led to more syneresis on some storage days; most of the samples exhibited the highest syneresis percentage on the 3rd and 4th days of storage. A possible explanation for this observation is that a lower starch and higher protein and Insoluble Dietary Fiber (IDF) content was present when UABP flour was added, and more SDFs and organic acids was contributed by FABP flour. In addition, incorporation of FABP flour led to the formation of a strong and stable gel during the refrigerated storage time, a result of interaction between glutinous rice flour starch and FABP flour constituents, whereas the opposite trend was seen with incorporation of UABP flour. Akesowan and Taweesakulvatchara reported that hydrocolloids and proteins in the flour starch gel could interact with water molecules and reduce the syneresis [26].
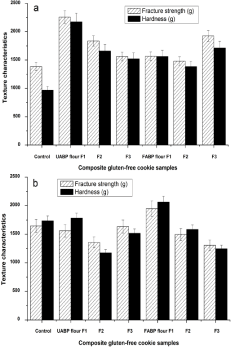
Figure 1: Textural characteristics of Composite Gluten-Free (CGF) cookies
fortified with fermented and unfermented Agaricus Bisporus Polysaccharide
(FABP and UABP) flours and stored at 25°C for 4 months (a) and 6 months
(b).
Water activity and moisture content of stored CGF cookies
Water activity (aw) and moisture content are the most important determinants of food products shelf-life, and water activity below 0.6 with lower moisture content and temperature prevent microbial growth [27,28]. Effect of storage for 4 and 6 months at 25°C on aw and moisture content of CGF cookies is provided in Table 2. During the storage period, both aw and moisture content increased, and both CGF cookie formulations had higher values of aw and moisture compared to the control, but within permissible limits. In addition, aw and moisture content of CGF cookies increased with increasing levels of UABP flour and FABP flour added (Table 2). The increase in aw and moisture could be due to hygroscopic nature of the dried sample, storage environment and type of packaging material. Furthermore, the presence of fibers in the product can lead to an increase in moisture absorption during the storage. These results are in agreement with a previous study by Jan et al., who confirmed that aw and moisture content of gluten-free cookies stored for 4 months at room temperature in laminated pouches and metalized polyester polyethylene increased, but in safe limits [27]. Moreover, Jensen and Risbo stated that within the range of 0.30-0.60 aw, lipids are most stable to oxidation, and under 0.75 aw is considered acceptable in preventing microbial growth [29]. Overall, the aw less than 0.50 and low moisture content could not get to microbial growth, suggesting the potential safety of the cookies during storage time.
Sample
aw
Moisture (g/100 g)
4 months
6 months
4 months
6 months
Control
0.37 ± 0.00e
0.40 ± 0.00c
4.85 ± 0.11f
5.17 ± 0.01e
UABP flour F1
0.39 ± 0.01d
0.41 ± 0.00b
5.51 ± 0.01e
5.75 ± 0.04d
F2
0.40 ± 0.01c
0.42 ± 0.00a
6.03 ± 0.02b
6.10 ± 0.02b
F3
0.39 ± 0.00d
0.39 ± 0.00d
5.90 ± 0.02c
5.91 ± 0.03c
FABP flour F1
0.41 ± 0.00b
0.41 ± 0.00b
5.73 ± 0.07d
5.86 ± 0.26c
F2
0.40 ± 0.00c
0.41 ± 0.01b
5.92 ± 0.01c
6.06 ± 0.01b
F3
0.42 ± 0.00a
0.42 ± 0.00a
6.16 ± 0.02a
6.17 ± 0.01a
Mean ± standard deviation (n=3). Mean values within a column followed by a different letter are significantly different (p ˂ 0.05). UABP flour=Unfermented Agaricus bisporus polysaccharide flour; FABP flour=Fermented Agaricus bisporus polysaccharide flour. aw=Water activity.
Table 2: Water activity and moisture content of composite gluten-free cookies fortified with fermented and unfermented Agaricus bisporus polysaccharide flours and stored at 25°C for 4 and 6 months.
Texture characteristics of stored CGF cookies
Texture characteristics of CGF cookies stored for 4 and 6 months at 25°C are shown in Figure 1a and b. All CGF cookie formulations had higher fracture strength and hardness compared to the control during 4 months of storage (Figure 1a). In addition, UABP flour F1 and FABP flour F3 revealed the highest fracture strength and hardness among CGF cookie formulations, but FABP flour formulations had soft texture than UABP flour formulations (Figure 1a). During 6 months of storage (Figure 1b), all CGF cookie formulations showed lower fracture strength and hardness compared to the control, except FABP flour F1. Moreover, UABP flour F2 and FABP flour F3 were the lowest fracture strength and hardness among all the CGF cookie samples, which is due to more gaining in moisture content. After 4 months of storage, the aw and moisture content increased, and break force decreased in both CGF cookie formulations. Nagi et al. reported that the hardness of cookies decreased by moisture absorption during 3 months of storage period. Prolonged exposure of the cookies to ambient storage conditions led to water absorption from the atmosphere into the product’ matrix and changed the textural characteristics [30,31]. On the other hand, some stored CGF cookies had higher fracture strength and hardness than fresh CGF cookies (data not shown), especially UABP flour F1, FABP flour F1 and FABP flour F3.
1H NMR spectroscopy of stored CGF cookies
Amount and state of water play an important role in the fresh and stored baked product properties; and understanding of water dynamics and molecular interactions between water and food components can be studied by the application of a Nuclear Magnetic Resonance (NMR), such as a CPMG pulse sequence. Therefore, in this study, CPMG pulse sequence was used to determine the water mobility of SPF, UABP flour, FABP flour, and fresh and stored CGF cookies at 25°C for 4 months. A longer T2 relaxation time represents a higher degree of molecular freedom. As shown in Figure 2a-c, in all samples (except stored UABP flour F3), T2 relaxation time distribution curves showed three CPMG proton populations as follows: P1 is denoted as bound water (intra-starch granule water), P2 as mobilized water (water interaction with starch, protein and sucrose) and P3 with a long T2 relaxation time as margarine (a polar phase). These are in consistent with previous studies of Hao et al. and Serial et al. for biscuits and biscuit doughs fortified with fibers, respectively [32,33]. For flour samples (Figure 2a), FABP flour was wider in all proton populations (P1, P2 and P3) than that of UABP flour and SPF. Furthermore, P2 and P3 of UABP flour and FABP flour overlapped and all flour samples showed higher water immobility and lower water mobility. This could be attributed to higher connection and interaction between macromolecules (e.g. protein and carbohydrate) and water in flour samples. In fresh CGF cookies (Figure 2b), all samples contained P1 (T21=0.01-1.75 ms), P2 (T22=2.01-10.72 ms) and P3 (T23=10.72-533.67 ms) with different T2 relaxation time, except the control and UABP flour F1 exhibited P2 (T22=0.33-7.05 ms) and P3 (T23=14.17- 403.70 ms). It is clear that the protons shifted to the lower relaxation time with increasing addition of FABP flour and UABP flour to CGF cookies, possibly due to increased in protein and dietary fiber contents. Furthermore, UABP flour F2, UABP flour F3, and FABP flour F3 were the highest fat protons, a result of their longest T2 relaxation time. According to Ruan and Chen, higher protein and carbohydrate levels in foods decreased water mobility, whereas lower levels of these macromolecules increased the mobility [12]. With regard to stored CGF cookies (Figure 2c), UABP flour F3 showed additional fat proton (P4) with highest relaxation time of 403.70-932.60 ms (T24), while FABP flour F3 exhibited strong linked proton (P1) at 0.01-1.32 ms. Moreover, there was an increase in T22 (P2) of the other stored CGF cookie samples, possibly due to some protons migrating to more mobile domains. On the other hand, during 4 months of storage, CGF cookies might be absorbed some water, which affects their textural characteristics (e.g hardness and fracture strength). Therefore, Nagi et al. reported that the hardness of cookies decreased by moisture absorption during 3 months of storage period. In this study, an increase in T22 was ascribed to the weakening of the starch and protein network due to a hygroscopic nature of dried sample [30]. Changes occurring in abundances of P1, P2, and P3 may be related to molecular dynamics of water interacting with biopolymers of baked sample [34]. Cookie is a complex product and difficult to interpret the NMR relaxation signal due to of its multiexponential behavior, and attribute the relaxation components to solid phase, water phase and fat phase. But, in this study, the later phase (e.g. P3) was more intensity and visible in most of CGF cookie samples.
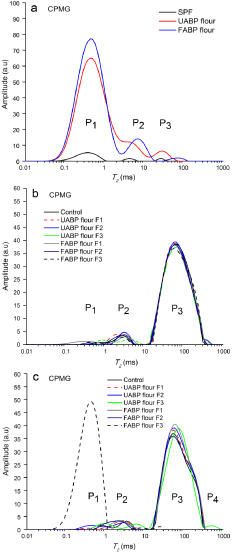
Figure 2: Relaxation T2 distribution of Sweet Potato Flour (SPF), UABP
flour, FABP flour and CGF cookies fortified with FABP flour and UABP
flour (a =flours, b=fresh CGF cookies and c=stored CGF cookies at 25˚C
for 4 months; T2=Spin-spin relaxation time, and P1, P2 and P3=CPMG proton
populations).
FT-IR spectroscopy of CGF cookies
To identify the FABP flour, UABP flour (Supplementary Data, Figure 2) and CGF cookies fractions and functional groups, the characteristic absorption of the samples was determined by FTIR in the range of 4000-500 cm-1. As presented in Figure 3a and b, the broad stretching bands at 3420.02 cm-1 (Figure 3a) and 3416.32 cm-1 (Figure 3b) were attributed to O-H groups with stretching vibration. The weak absorption peaks at 2938.12 cm-1 (FABP flour, a) and 2925.57 cm-1 (FABP flour F1 and FABP flour F2, b), and asymmetrical peaks at 2925.57 and 2854.25 cm-1 (other CGF cookies, b) were indicative of the presence of C-H stretching vibration; the band at 1417.19 cm-1 (Figure 4a) and 1417.36 cm-1 (Figure 3b) corresponds to C-H bending vibration. The existence of a 2854.25 cm-1 peak in the control, UABP flour formulations and FABP flour F3 (Figure 3b) was attributed to the amylose-lipid complex that forms during the baking process, and the lower intensity of this peak in UABP flour formulations result of proteins combined with starch molecules. According to Lian et al., the presence of a peak at ~2852 cm-1 in starch could be indicated to the protein or lipid bound to the starch component. In addition, two absorption peaks at 1745.98 in all CGF cookie samples except FABP flour F1 and FABP flour F2 and at 1643.60-1651.58 cm-1 in FABP flour (Figure 3a) and all CGF cookies (Figure 3b) were characteristic of C=O (carbonyl) stretching vibration [35]. A strong peak at ~1651.58 cm-1 ascribed to amide I, which can be assigned to protein structure in FABP flour added to the CGF cookies, as mentioned by Radzki et al. in the previous study for polysaccharide extracted from oyster mushroom [36]. Adebiyi et al. explained that the fermentation and malting processes reduced the carbonyl bands, associating with a decrease in the total lipids that present in the samples [37]. Furthermore, three bands at ~1156.11, ~1049.88 and 927.37 cm-1 were found in FABP flour and CGF cookies (Figure 3a and b), which are assigned to glycosidic linkages of polysaccharides. These bands are interpreted as follows: ~1156.11 cm-1 for C-O-C bond stretching, and ~1049.88 and 927.37 cm-1 for C-O-H bending of β-glucan. These values are in agreement with a previous result by Shah et al. for structural characteristics of β-glucan from barley and oat [38]. In general, UABP flour formulations and FABP flour formulations were distinguishable in some peaks and intensities, but both of them composed of glycosidic linkages of β-glucans.
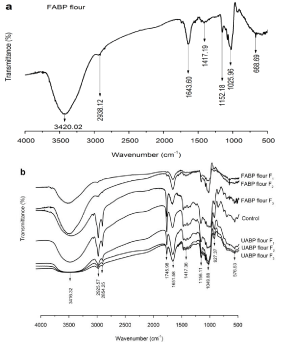
Figure 3: FTIR spectra of Fermented Agaricus Bisporus Polysaccharide
(FABP) flour (a) and Composite Gluten-Free (CGF) cookies (b).
Volatile compounds of CGF cookies
The volatile profile determined by SPME-GC/MS (Table 3) revealed 13 and 24 compounds in FABP flour and CGF cookies, respectively: 5 aldehydes, 6 alcohols, 4 ketones, 2 furans, 2 acids, and 5 alkanes, mainly ascribed to fermentation and fortification processes, as well as thermal reactions. Hexanal and Nonanal were the most abundant aldehydes in FABP flour and CGF cookies, and higher in CGF cookie formulations than the control. Among the alcohol compounds, 2-furanmethanol and 1-methoxy-2-propanol had the highest percentages peak area in CGF cookies, but these alcohols were absent in FABP flour and UABP flour (data not shown). Pasqualone et al. stated that several lipid oxidation-derived saturated and unsaturated aldehydes and alcohols, such as hexanal, nonanal and 1-hexanol were found at higher levels in purple cookies [39]. The amount of furan and furan derivatives of CGF cookies, such as 2-furanmethanol and 2-furancarboxaldehyde (furfural) generation occur in the Maillard reaction during the baking process. The presence of skim milk powder with fructose and other sugars in cookies dough formulation contributes in the formation of some pyranones with a pleasant sweet aroma in the final product. In addition, 3-hydroxy-2-butanone, 2-pentyl furan, and tetradecane were detected in FABP flour (Table 3) and UABP flour (data not shown). Release of phenolic compounds from FABP flour, as well as intensive acidification that occurred during fermentation process led to an increase in flavor compounds. High levels of 2(5H)-furanone, dihydro-2(3H)-furanone and acetic acid, and low levels of hexanoic acid were observed in CGF cookies. According to Mildner-Szkudlarz et al., 2-furanmethanol, furfural and dihydro-2(3H)-furanone are aroma compounds of baking process identified in fresh cookies [40]. Furthermore, alkanes exhibited the highest percentages peak area in both FABP flour and CGF cookies among all volatile compounds, but the control had higher decane, undecane and dodecane than FABP flour formulations (Table 3). In the study of volatile compounds for gluten-free breads, decane and undecane showed the highest percentages peak area in Agaricus Bisporus Polysaccharide (ABP) flour and inulin Formulation (F1) and inulin Formulation (F2) [41,42]. Overall, aroma of heat-treated food is mainly related to the added ingredients and the Maillard reaction, besides interaction between the Maillard reaction and lipid degradation components.
Volatile compounds
RT (min)
FABP flour
Control
UABP flour
Peak area (%)
FABP flour
F1
F2
F3
F1
F2
F3
Aldehydes
Hexanal*
7.39
2.98
11.28
14.74
9.49
21.16
19.83
13.32
13.26
Heptanal
10.23
nd
0.37
0.61
0.59
1.34
0.57
0.72
nd
Octanal
13.17
nd
0.17
0.4
0.35
0.45
0.34
0.31
0.22
Nonanal*
15.53
0.37
2.08
3.39
2.89
3.62
2.65
1.84
1.86
Benzaldehyde
17.74
0.3
0.14
0.33
0.44
1.05
0.55
0.65
nd
Alcohols
1-Methoxy-2-Propanol*
8.63
nd
1.69
2.32
2.83
1.53
2.25
1.8
1.03
2- Ethyl- 1- Hexanol
17.25
1.78
0.11
nd
nd
nd
nd
nd
nd
1- Pentanol
12.21
0.24
nd
nd
nd
nd
nd
0.53
0.69
1- Octen- 3- ol
16.85
0.57
nd
nd
nd
nd
nd
nd
nd
2- Furanmethanol*
19.64
nd
14.16
9.78
8.4
4.79
7.81
6.12
3.07
Benzyl alcohol
22.27
0.52
nd
nd
nd
0.88
nd
nd
nd
Ketones
3-Hydroxy-2-butanone
13.27
1.61
nd
nd
nd
nd
nd
nd
nd
6-Methyl-5-hepten-2-one
14.33
nd
nd
0.21
0.27
0.3
nd
nd
nd
Dihydro-2(3H) Furanone
19.28
nd
3.51
1.84
1.18
0.69
1.43
0.9
0.51
2(5H)- Furanone*
20.88
nd
1.93
2.66
2.7
1.98
2.99
1.45
0.96
Furans
2- Pentylfuran
11.95
1.96
nd
nd
nd
nd
nd
nd
nd
2-Furancarboxaldehyde
16.76
nd
1.24
0.66
0.64
0.64
0.38
0.51
0.41
Acids
Acetic acid
16.53
nd
1
0.46
0.51
0.97
0.8
4.84
9.01
Hexanoic acid
21.88
nd
0.22
0.1
0.08
0.23
0.41
0.91
0.37
Alkanes
2,2,4,6,6-pentamethyl- Heptane
4.84
5.3
2.46
2.52
2.28
7.76
13.6
7.29
4.19
Decane*
5.75
17.52
10.17
11.46
10.96
4.55
4.08
2.65
2.86
Undecane*
8.51
25.72
23.68
26.08
26.18
13.18
12.01
7.96
10.18
Dodecane*
10.78
nd
2.32
2.76
2.28
3.3
2.74
1.69
nd
Tetradecane
11.18
2.62
nd
nd
nd
nd
nd
nd
Nd
Peak area values represent the average of two determinations; *Major volatile compounds (peak area ˃ 1.50 %); RT=Retention Time; nd=not detected.
Table 3: Volatile compounds of Fermented Agaricus bisporus Polysaccharide (FABP) flour and composite gluten-free cookies fortified with FABP flour and UABP flour.
Composite Gluten-Free (CGF) cookies were prepared using Sweet Potato Flour (SPF)/Glutinous Rice Flour (GRF) enriched with FABP flour and UABP flour and stored at 25°C for 4 and 6 months. Addition of both mushroom polysaccharide flours to CGF cookies exhibited good physicochemical properties. In addition, texture, aw, moisture, 1H NMR spectroscopy, FTIR spectroscopy and volatile compounds of CGF cookies were influenced by incorporation levels of FABP flour and UABP flour, besides storage time. The stored CGF cookie formulations (for 6 months) had higher aw and moisture content (within permissible limits), and lower fracture strength and hardness, with water migration to more mobile domains (for 4 months) compared to the control, using CPMG pulse sequence analysis. CGF cookies were ascribed to the relaxation components of solid phase, water phase and fat phase; and the later phase was more intensity and visible in the present study.
Acknowledgement
We would like to thank the Priority Academic Program Development of Jiangsu Province, Higher Education Institutes, Wuxi, China. We also extend our sincere gratitude to all co-authors for their contributions to this work, as well as the staff and students of the Research Center of Convenient Food and Quality Control.
This study was funded by Ministry of Education of the People’s Republic of China (Grant No. 214122).
References
- Bower SL. What is celiac disease?. In: Celiac disease: a guide to living with gluten intolerance. Bower SL, Sharrett MK, Plogste S. (eds). Demos Medical Publishing, New York. 2006; 1-9.
- Zhang D, Mu T, Sun H. Comparative study of the effect of starches from five different sources on the rheological properties of gluten-free model doughs. Carbohydr Polym. 2017; 176: 345-355.
- Gallagher E. Gluten-free food science and technology, John Wiley & Sons (eds). Blackwell Publishing, Ltd., Wiley-Blackwell, Lowa. 2009; 978-1-405- 15915-9.
- Arshad MU, Anjum FM, Zahoor T. Nutritional assessment of cookies supplemented with defatted wheat germ. Food Chem. 2007; 102: 123-128.
- Mamat H, Abu-Hardan MO, Hill SE. Physicochemical properties of commercial semi-sweet biscuit. Food Chem. 2010; 121: 1029-1038.
- Ho J, Konerding M, Gaumann A, Groth M, Liu WK. Fungal polysaccharopeptide inhibits tumor angiogenesis and tumor growth in mice. Life Sci. 2004; 75: 1343-1356.
- Jeurink P, Noguera C, Savelkoul H, Wichers H. Immunomodulatory capacity of fungal proteins on the cytokine production of human peripheral blood mononuclear cells. Int. Immunopharmacol. 2008; 8: 1124-1133.
- Jeong SC, Koyyalamudi SR, Jeong YT, Song CH, Pang G. Macrophage immunomodulating and antitumor activities of polysaccharides isolated from Agaricus bisporus white button mushrooms. J Medic Food. 2012; 15: 58-65.
- Wang L, Liu HM, Qin GY. Structure characterization and antioxidant activity of polysaccharides from Chinese quince seed meal. Food Chem. 2017; 234: 314-322.
- Sicard, D, Legras JL. Bread, beer and wine: Yeast domestication in the Saccharomyces sensu strict complex. Comptes Rendus Biol. 2011; 334: 229-236.
- Landete JM. A review of food-grade vectors in lactic acid bacteria: from the laboratory to their application. Critic Rev. Biotechnol. 2017; 37: 296-308.
- Ruan RR, Chen PL. Water in foods and biological materials: A Nuclear Magnetic Resonance Approach. 1st ed. Technomic Publishing Company, Inc, Lancaster, Pennsylvania. 1998.
- Sulieman AA, Zhu KX, Peng W, Shoaib M, Hassan HA, Zhou H-M. Compositional, functional and pasting properties of composite flour fortified with button mushroom (Agaricus bisporus) powder and inulin. J. Food Nutri. Res. 2017; 5: 614-621.
- Fan L, Zhang S, Yu L, Ma L. Evaluation of antioxidant property and quality of bread containing Auricularia auricula polysaccharide flour. Food Chem. 2007; 101: 1158-1163.
- Sai Manohar R, Haridas Rao P. Effect of emulsifiers, fat level and type on the rheological characteristics of biscuit dough and quality of biscuits. J Sci Food Agri. 1999; 79: 1223-1231.
- AOAC. Official Methods of Analysis of AOAC. 18th ed. Association of Official Analytical Chemists, Washington, DC, USA. 2006.
- Kuar L, Singh N, Sodhi SN. Some properties of potatoes and their starches II. Morphological, thermal and rheological properties of starches. Food Chem. 2002; 79: 183-192.
- Kaur M, Sandhu KS, Arora AP, Sharma A. Gluten free biscuits prepared from buckwheat flour by incorporation of various gums: Physicochemical and sensory properties. LWT - Food Sci Technol. 2015; 62: 628-632.
- Meiboom S, Gill D. Modified spin-echo method for measuring nuclear relaxation times. Rev. Scient. Instrum. 1958; 29: 688-691.
- Carr HY, Purcell EM. Effects of diffusion on free precession in nuclear magnetic resonance experiments. Physical Review. 1954; 94: 630-638.
- Jouraiphy A, Amir S, Winterton PEl, Gharous M, Revel JC, Hafidi M. Structural study of the fulvic fraction during composting of activated sludge–plant matter: Elemental analysis, FTIR and 13C NMR. Bioresource Technology. 2008; 99: 1066-1072.
- Aponte M, Boscaino F, Sorrentino A, Coppola R, Masi P, Romano A. Volatile compounds and bacterial community dynamics of chestnut-flour based sourdoughs. Food Chem. 2013; 141: 2394-2404.
- Sulieman AA, Zhu KX, Peng W, Hassan HA, Obadi M, Ahmed MI, et al. Elect of Agaricus bisporus polysaccharide flour and inulin on the antioxidant and structural properties of gluten-free breads. Food Measure. 2019.
- Tharise N, Jolianti E, Norminah M. Evaluation of physico-chemical and functional properties of composite flour from cassava, rice, potato, soybean and xanthan gum as alternative of wheat flour. Int Food Res J. 2014; 21: 1641-1649.
- Phimolsiripol Y, Siripatrawan U, Henry CJK. Pasting behavior, textural properties and freeze-thaw stability of wheat flour-crude malva nut (Scaphium scaphigerum) gum system. J Food Eng. 2011; 105: 557-562.
- Akesowan A, Taweesakulvatchara S. Freeze-thaw stability of native starches as modified by konjac flour and soy protein isolate. J Appl Sci Res. 2009; 5: 2477-2481.
- Jan R, Saxena DC, Singh S. Effect of storage conditions and packaging materials on the quality attributes of gluten-free extrudates and cookies made from germinated chenopodium (Chenopodium album) flour. J Food Measure. 2017; 11: 1071-1080.
- Ellin DM. Microbial Food spoilage-losses and control strategies. Food Research Institute, University of Wisconsin-Madison, Madison. 2007.
- Jensen PN, Risbo J. Oxidative stability of snack and cereal products in relation to moisture sorption. Food Chem. 2007; 103: 717-724.
- Nagi HPS, Kaur J, Dar BN, Sharma S. Effect of storage period and packaging on the shelf life of cereal bran incorporated biscuits. Amer J Food Technol. 2012; 7: 301-310.
- Giannou V, Lebesi D, Tzia C. Packaging and shelf life prediction of bakery products. In: bakery products science and technology. Zhou W, Hui YH, De Leyn I, Pagani MA, Rosell CM, Selman JD, Therdthai N (eds). John Wiley & Sons, Ltd., UK. 2014; 354-371.
- Hao FY, Lu LX, Ge CF. Effect of fat content on water sorption properties of biscuits studied by nuclear magnetic resonance. J Food Nutri Res. 2014; 2: 814-818.
- Serial MR, Blanco Canalis MS, Carpinella M, Valentinuzzi MC, León AE, Ribotta PD, et al. Influence of the incorporation of fibers in biscuit dough on proton mobility characterized by time domain NMR. Food Chem. 2016; 192: 950-957.
- Bosmans GM, Lagrain B, Ooms N, Fierens E, Delcour JA. Biopolymer interactions, water dynamics, and bread crumb firming. J Agric Food Chem. 2013; 61: 4646-4654.
- Lian X, Wang C, Zhang K, Li L. The retrogradation properties of glutinous rice and buckwheat starches as observed with FT-IR, 13C NMR and DSC. Int J Biol Macromol. 2014; 64: 288-293.
- Radzki W, Ziaja-Sołtys M, Nowak J, Rzymowska J, Topolska J, Sławinska A, et al. Effect of processing on the content and biological activity of polysaccharides from Pleurotus ostreatus mushroom. LWT - Food Sci. Technol. 2016; 66: 27-33.
- Adebiyi JA, Obadina AO, Mulaba-Bafubiandi AF, Adebo OA, Kayitesi E. Effect of fermentation and malting on the microstructure and selected physicochemical properties of pearl millet (Pennisetum glaucum) flour and biscuit. J Cereal Sci. 2016; 70: 132-139.
- Shah A, Gani A, Masoodi FA, Wani SM, Ashwar BA. Structural, rheological and nutraceutical potential of β-glucan from barley and oat. Carbohydr Diet Fiber. 2017; 10: 10-16.
- Pasqualone A, Bianco AM, Paradiso VM, Summo C, Gambacorta G, Caponio F, et al. Production and characterization of functional biscuits obtained from purple wheat. Food Chem. 2015; 180: 64-70.
- Mildner-Szkudlarz S, Zawirska-Wojtasiak R, Obuchowski W, Goslinski M. Evaluation of antioxidant activity of green tea extract and its effect on the biscuits lipid fraction oxidative stability. J Food Sci. 2009; 74: 362-370.
- Sulieman AA, Zhu KX, Peng W, Hassan HA, Obadi M, Siddeeg A, et al. Rheological and quality characteristics of composite gluten-free dough and biscuits supplemented with fermented and unfermented Agaricus bisporus polysaccharide flour. Food Chem. 2019; 271: 193-203.
- Zucco F, Borsuk Y, Arntfield SD. Plantain peel-a potential source of antioxidant dietary fibre for developing functional cookies. LWT-Food Sci Technol. 2011; 44: 2070-2076.