
Review Article
Austin J Nutr Metab. 2019; 6(2): 1068.
Bioactive Compounds from Cereal and Pulse Processing Byproducts and Their Potential Health Benefits
Saini A, Panwar D, Panesar PS* and Bera MB
Food Biotechnology Research Laboratory, Department of Food Engineering & Technology, Sant Longowal Institute of Engineering and Technology, India
*Corresponding author: Parmjit S. Panesar, Food Biotechnology Research Laboratory, Department of Food Engineering & Technology, Sant Longowal Institute of Engineering and Technology, India
Received: October 03, 2019; Accepted: November 05, 2019; Published: November 12, 2019
Abstract
An enormous amount of byproducts (bran, husk, germ meal etc.) is generated worldwide during the processing of cereals and pulses, which are a rich source of bioactive compounds. The major components include phenolic acids (ferulic, gentisic, p-hydroxybenzoic, gallic, sinapic, syringic, vanillic, caffeic, p-coumaric), dietary fibers (β-glucan), vitamins (thiamin, riboflavin, niacin, pantothenic acid) etc. These bioactive components are associated with various health benefits such as anti-inflammatory, antioxidant and anti-diabetic, anti-carcinogenic etc as well as modulation of the metabolic processes. This review provides comprehensive information about different types of bioactive compounds present in the byproducts produced from cereal and pulse processing industries and their health benefits.
Keywords: Bioactive Compounds; Cereal and Pulse Byproducts; Health Benefits
Introduction
Bioactive compounds are the phytoconstituents that are a part of food chain and are responsible for numerous beneficial changes in the human health such as antioxidant properties, anti-cancer, antiinflammation, anti-allergenic, anti-atherogenic and anti-proliferative agents, depending upon the pathway and their bioavailability in the body. These compounds are found in plant, animal, marine and their byproducts, which are associated with various factors such as bioactivity, chemical structure, and dose etc [1]. Also, these compounds are known for their preventive action from many diseases.
In context to this, cereal and pulses are the major sources of energy and protein in the diet. Also, these bioresources contain a wide range of different bioactive compounds, vitamins, fibres and minerals. Most of the existing studies have focused on the bioactive compounds in fruits, vegetables and in their byproducts. However, some of the bioactive compounds are unique in nature and only present in cereals but cannot be obtained from fruits and vegetables [2].
Cereals (wheat, oat, rice, corn and barley) and pulses generate valuable byproducts during dry, wet milling, malting and pearling such as aleurone, pericap, germ, coat, husk, germ meal, shorts, spent grain, testa, hyaline and part of the endosperm which are a good source of potentially marketable bioactive compounds. These byproducts are intensely utilized in first valorization by extracting the bioactive compounds such as polyphenols, vitamins, flavonoids, dietary fibres etc. After that, it proceeds for the another valorization where it is generally associated to biorefinery concept [3]. This concept is involved to produce minimum waste and maximum utilization of different bioresources during processing. since, these cereal and pulse byproducts are the major sources for the production of value-added products such as functional foods, nutraceuticals and food additives and further it can be utilized in production of biofuels and energy etc rather than downgraded by using as a cattle feed or/and dispose in the environment directly [4] as shown in Figure 1. This review is focused on the bioactive compounds from the byproducts which are produced during milling and processing of cereal and pulses along with their health benefits.
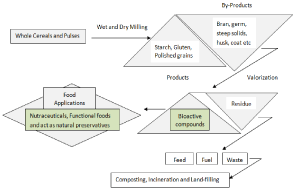
Figure 1: Schematic representation of bioactive compounds from cereal and
pulse byproducts.
Major Categories of Bioactive Compounds Present in Cereals and Pulses
Bioactive compounds are often produced in small quantities while their chemical structures, properties and functions vary widely. These compounds are synthesized by some primary metabolites (e.g. amino acids) or from intermediates obtained by primary metabolism in specialized cell types and only during a particular growth stage, or under specific conditions, make their extraction and purification quite difficult [5]. Commercially, the useful bioactive compounds (secondary metabolites) are terpenoids, polyphenols, vitamins, alkaloids etc. [6], which are utilized in the production of functional foods and pharmaceuticals.
Phenolic compounds
Phenolic compounds are important for their antioxidant properties and protection from degenerative diseases like cancer and heart diseases [7]. Phenolic acids are a group of the derivatives of benzoic and cinnamic acid such as capsaicin, ellagic, salicylic, tannic, vanillin, gallic, syringic, p-coumaric, o-coumaric, m-coumaric, caffeic, ferulic, sinapic, chlorogenic acids which occur in both forms (free and bound) [8]. Flavonoids are the compounds that consist of two aromatic rings joined by a three-carbon link. Also, flavonoids belong to different sub-classes such as anthocyanins, flavonols, flavanones, flavones and flavonols. Furthermore, lignans are a group of the dietary phytoestrogen compounds that are present in the cereals such as wheat, oat, corn and rye [9]. These polyphenolic bioactive compounds include lariciresinol, sacoisolariciresinol, pinoresinol, matairesinol and syringaresinol [8]. Another important group is alkylresorcinols (5-n-alkylresorcinols) which are 1, 3-dihydroxybenzene derivatives with an alkyl chain at position 5 of the benzene ring, indicate the amphiphilic properties.
Carotenoids
Carotenoids are a class of more than 600 naturally occurring tetraterpenoid organic pigments synthesized by plants, algae, and photosynthetic bacteria which occur commonly in transform. These molecules are the source of colour like yellow, orange, and red [10] and have received attention because of their role as pro-vitamins and antioxidants. These compounds are classified into two classes, carotene (β-carotene, γ-carotene, α-carotene and β-cryptoxanthin) and xanthophylls (lutein, astaxanthin and zeaxanthin). Carotenoids are present in whole-grain cereals [11] whereas fruits and vegetables are the principal sources of carotenoids and its deficiency can cause many diseases such as night blindness, keratomalacia, xerophthalmia, corneal ulceration, scarring and at last irreversible blindness [12].
Phytic and oxalic acid
Phytic acid (myoinositol hexaphosphate; IP6) is the major source of phosphorus and its salts known as the phytates which can result in various health beneficial effects such as anticarcinogen and decreasing the risk of heart diseases or diabetes [13,14]. Phytic acid is an important seed constituent, which is present in all nuts, cereals (aleurone layer), legumes and oil seeds about 1-3% [15].
Oxalic acid [(COOH)2] is present in many plants which occurs in the cell sap of the plant in the form of K or Ca salts. Legumes are the source of minerals but due to the phytates and oxalates, their bioavailability was observed lower than other food [16].
Phytosterols
Phytosterols are the combination of plant sterols and stanols, which are present in small quantities. Their structures are similar to cholesterol, differing only in the side chain group and/or presence or absence of the double bond. The most common phytosterols are β-sitosterol, stigmasterol and campesterol. These compounds are present in different legumes such as kidney bean, pea, chickpea and butter bean. Cereal products contain significant amount of plant sterols than vegetables. The level of these compounds varies in different cereals and in various parts of the kernel [17]. Various studies have shown that sterols and stanols are beneficial in preventing the colon cancer and decrease the cholesterol level [18].
Saponins
The ‘saponin’ term is derived from the Latin word sapo, means ‘soap’, because saponin molecules produce soap-like foams when shaken with water. These molecules are known as non-volatile, surface active compounds that are composed of lipid-soluble aglycone consisting of either a sterol or triterpenoid which are attached to water-soluble sugar residues differing in type and amount of sugars. Saponins have been reported in lupin, lentil, pea, black gram and beans while major sources of saponins are soybean and chickpea in the human diet [19-23]. Various studies suggest that saponins possess different properties such as anti-carcinogenic, hypocholesterolemic and immune-stimulatory [24,25].
Bioactive Compounds of Cereal Byproducts
Cereals mainly contain three parts such as endosperm, germ and bran. The main cereals are rice, oats, maize, barley, rye, sorghum, wheat, millet which consist of different phytochemicals including polyphenols, flavonoids, anthocyanin, carotenoids etc. Whole grains and their byproducts consist of dietary fibers (lignin/lignans, cellulose, β-glucans, and arabinoxylans), alkylresorcinols, phytic acid, avenanthramides, cinnamic acid, inositols, ferulic acid, γ-oryzanols, phenolic compounds, tocols and betaine and phytosterols which are present in small quantity as shown in Table 1. These polyphenolic bioactive compounds include lariciresinol, sacoisolariciresinol, pinoresinol, matairesinol and syringaresinol [8]. Generally, two main phenolic acids present in cereals are ferulic and p-coumaric acids [26]. Also, other phenolic acids and flavonoids are present in black, red and brown rice bran i.e. syringic acid, protocatechuic acid, cinnamic acid, myrecitin, catechin, luteolin, quercetin and apigenin [27]. Generally, concentration of flavonoids in cereals is less, except for barley that has the measurable amount and wide range of flavonoids present in sorghum [28].
(Phytochemicals Categories)
Compounds
Concentration
Sources
References
Vitamins
Thiamin
Riboflavin
Niacin
Pantothenic acid
Pyridoxine
Folate
Vitamin E
γ-tocopherol
α-tocopherolH
γ-tocotrienol
0.65 mg/100g
0.51 mg/100g
28 mg/100g
3.15 mg/100g
1 mg/100g
0.23 mg/100g
10.76-28.43 mg/g DM
13.12-67.30 mg/g DM
16.91-53.09 mg/g DM
Wheat Bran
Coloured rice bran
[39,40]
Total carotenoids
57.9 µg/g
32.0 µg/g
4.2 µg/g
Corn germ meal
Corn bran
Wheat bran
[41]
Lignan
Betaine
Phytosterols
Choline
4.75 mg/100g
868 mg/100g
158 mg/100g
172 mg/100g
Wheat bran
[39]
Total carotenoids
Caotenoid
β-carotene
9.11 µg/g
0.74 µg/g
101 µg/100 g
Wheat germ
Wheat bran
Whole rice bran
[42,43]
Total anthocyanins
294.62 mg Cy3-
GE/100 g DM
77.87 mg Cy3-
GE/100 g DM
10.72 mg Cy3-
GE/100 g DM
Black rice bran
Red rice bran
Brown rice bran
[27]
Total Flavonoids content
262.4 to 681.6 mg/100 g
Cereal milling byproducts (Bran, germ, shorts, husk, germ meal)
[41]
358 ± 7.2 mg CE/100g dw
Defatted Rice bran
[44]
3000-4300 µg/g
Wheat bran
[45]
40.15-823.88 mg QE/100 g DM
Rice bran fractions
[27]
Phytosterols
4.73-2020 µg/g
Wheat Bran
[39]
Alkylresorcinol
489-1429 µg/g
Wheat bran
[46]
Dietary Fibre
Soluble dietary fibres
β-glucan
33.4-63.0% dm
344-2050 µg/g
Wheat bran
Barley Bran
[47,48]
Phenolic compounds
ferulic, gentisic, p-hydroxybenzoic, gallic, sinapic, protocatechuic, syringic, vanillic acids, and vanillin, Caffeic, p-coumaric
-
Rice bran biomass
[49]
Phenolic acid
Ferulic acid
1376-1918 µg/g
Wheat bran
[50]
Polyphenols
Total phenolics
Total flavonoids
Caffeic acid
Ferulic acid
p-hydroxybenzoic acid
p-coumaric acid
14.90 ± 0.70 mg GAE/g
3.08 ± 0.17 mg CE/g
3.68 ± 0.14 mg/100g
33.64 ± 1.01 mg/100g
12.55 ± 0.93 mg/100g
265.4 ± 2.4 mg/100g
Rice Husk
[51]
Total monomeric phenolics
3454.4 ± 98.7 μg/g dw
Defatted Rice bran
[44]
Total phenolic content
1861.9 mg GAE/100 g
844.7 mg GAE/100 g
698.2 mg GAE/100 g
1925 mg GAE/100 g
Wheat germ
Wheat bran
Wheat shorts
Corn bran
[41]
10.55 mg GAE/g.
Defatted wheat germ
[52]
4206.16 µg/g.
Wheat bran
[53]
5698.8 to 6820.3 mg Ferulic acid/kg dw.
Different wheat bran fractions
[54]
769.7±17.2 mg GAE/100g dw
Defatted Rice bran
[44]
269.85 to 1214.7 mg GAE/100 g DM.
Rice bran fractions
[27]
GAE=Gallic Acid Equivalents
Table 1: Some selected studies of bioactive compounds from cereal byproducts.
Alkylresorcinols (5-n-alkylresorcinols) are plant-derived phenolic lipids that are synthesized by higher plant families and found in rye, wheat, wheat-rye and barley while they are absent in oats and edible parts of rice [29]. Moreover, avenanthramides are substituted cinnamic acid amides of anthranilic acids and these specific polyphenols are reported in oats which have anti-atherogenic, anti-inflammatory and anti-oxidant properties [30,31].
Dietary fibre like beta-glucan is the linear polymer which consists of glucose molecules connected by 70% of β-(1-4) and 30% of β-(1-3) linkages and found in the aleurone layer cells, endosperm and bran of grains [8,32]. Skendi et al. [33] and Marconi et al. [34] reported the range of β-glucan in cereal bran, which contains 5-24% (barley), 3-7% (oat) and 1% (wheat). Carotenoids are also present in whole-grain cereals such as alpha-carotene, beta-cryptoxanthin, beta-carotene, lutein and zeaxanthin [35].
Bioactive compounds are specific for some cereals in quantity such as beta-glucans in barley and oats, alkylresorcinol in rye and γ-oryzanol in rice etc. The outer layer of grains contains higher level of bioactive compounds [36]. Generally, bran and germ are removed during cereal processing. However, these byproducts are an important source of bioactive compounds and play a vital role in performing health benefits [8]. Products which have no bran and germ in their composition are often supplemented with such substances which are biologically active [37,38]. Furthermore, research is required to identify the unknown bioactive compounds from waste and their utilization in value added products, their various properties and functions in the body.
Bioactive Compounds in Pulse Byproducts
India is the largest producer of pulses (around 25%) in the world and according to the United Nations general assembly, year 2016 has been declared as the international year of pulses [55]. Pulses are the important source of valuable nutrients such as carbohydrates, proteins and low in calories. It also supplies bioactive compounds, which have health benefits, and it may be negative and positive depending on the pathways, which perform their specific function in the body. The major pulses comprise of lentil (Lens culnaris), pigeon pea (Cajanus cajan), black gram (Vigna mungo), lupin (Lupinus spp.), kidney bean (Phaseolus vulgaris), green gram (Vigna radiate), chickpea (Cicer arietinum), moth bean (Vigna acontifolia), fieldpea (Pisum sativum), lima bean (Vigna lunatus) and rice bean (Vigna umbellata) and their waste, contains many bioactive components such as phytosterols, phytates, phenolic compounds, phytosterols, saponins and lectins (Table 2). Pulses are the foods which have a very low glycaemic index (GI: 28-52) and numerous studies have indicated that low GI foods in diet, decrease the risk factors for coronary heart diseases and benefits in dietary treatment [56,57]. Recent research is mainly focused on the development of value added products using pulses and replacement of energy intense products, which are the responsible for many negative health effects such as obesity etc [58]. Moreover, polyphenols of the pulses contribute to the colour, sensory characteristics as well as numerous biological properties [59]. These compounds are mainly present in seed coat of the pulses, which is generally removed during milling. During processing of the black gram, 25% of the black gram is removed as the by-product, which is rich source of polyphenols [60]. Authors are continuing their research in the direction towards the identification and quantification of bioactive compounds in pulses for their beneficial health effects. Choung et al. [61] reported the concentration of phenolic compounds in seed coats of kidney beans such as petunidin 3-glucoside, cyaniding 3,5-diglucoside, delphinidin 3-glucoside, pelargonidin 3-glucoside and cyaniding 3-glucoside are 0-0.17, 0-0.04, 0-2.61, 0-0.59 and 0-0.12 mg/g dry basis respectively. Another study has been investigated the research on the defatted lentil samples and reported that total phenolic content in free form (1.37-5.53 mg GAE/g), esterified form (2.32-21.54 mg GAE/g) and insoluble form (2.55-17.51 mg GAE/g) [62].
Categories (Phytochemicals)
Concentration
Sources
References
Total phenols
[59,63]
Free phenolics
1.75 ± 0.02 mgCatE/g
Soybean hull
1.50 ± 0.05 mgCAE/g
Chickpea hull
Bound phenolics
0.33 ± 0.04 mgCatE/g
Soybean hull
0.11 ± 0.02 mgCAE/g
Chickpea hull
Total flavonoids
Free flavonoids
0.73 ± 0.02 mgCatE/g
Soybean hull
0.42 ± 0.02 mgCatE/g
Chickpea hull
Bound flavonoids
1.21 ± 0.07 mgCatE/g
Soybean hull
0.94 ± 0.03 mgCatE/g
Chickpea hull
Total flavonoid content
1730 mg QE/100g
Black cowpeas seed coat
[64,65]
31.68-85.17 mg CE/g
Peanut seed coat
104 mg CE/100g
Pea seed coat
2651 mg CE/ 100g
Pigeon pea seed coat
Carotenoids
0.415 mg/100g
Black gram seed coat
[60]
Anthocyanin
87 mg/100g
Black gram seed coat
[66]
Total Phenolic content
146 to 5798 mg/100 g DW
Bean Coat
[64,67]
0.2 to 32.6 mg CE/g DW
Chickpea seed coat
C-Glycosylated flavonoids
[68]
Vitexin
536.54 ± 7.94 µg/g
Black gram Husk
29.99 ± 2.36 µg/g
Black gram Germ
202.89 ± 5.57 µg/g
Black gram Aleurone
91.46 ± 1.39 µg/g
Black gram Plumule
Isovitexin
1221 ± 15.47 µg/g
Black gram Husk
71.94 ± 4.34 µg/g
Black gram Germ
518.61 ± 10.13 µg/g
Black gram Aleurone
214.51 ± 5.64 µg/g
Black gram Plumule
GAE= Gallic Acid Equivalents; RE= Rutin8 Equivalents; CE= Catechin Equivalents; QE=Quercetin Equivalents.
Table 2: Some selected studies of bioactive compounds from pulse byproducts.
Health Benefits of Cereal and Pulse Byproducts
The byproducts are obtained after processing of cereal and pulse, which are rich source of various bioactive compounds. These compounds are allocated in free, bound and soluble-cojugated form in whole cereal grains. Numerous studies have been done for recovering the bioactive compounds from byproducts and their antioxidant properties which have high potential for providing various health benefits, e.g. anti-inflammation and anti-tumor activity by black rice bran. Although the incorporation of byproducts directly in different food products acts as the functional ingredient e.g. the wheat bran of four varieties have been added at different levels of concentration (0%, 12.5%, 25%, 37.5%) into the wheat flour and prepared snacks showed the improved oxygen radical absorption capacity due to addition of wheat bran [69]. The main reason for having interest in these functional compounds (polyphenols) is due to their synergistic antioxidative action, which protect from different diseases e.g. neurodegenerative diseases. Another study was conducted in which different test foods samples such as high soluble fibre (barley alone), medium soluble fibre (mixture of barley and brown rice/whole wheat) and low soluble fiber (brown rice/whole wheat) were taken by the hypercholesterolemic men. The results concluded that total and LDL-cholesterols level were decreased significantly in high-soluble fiber diet (barley β-glucans) as compard to other diets [70].
The various pharmacological effects of rice bran derived products involve in decrement of total cholesterol, triglycerides, lowdensity lipoprotein cholesterol, aortic ester accumulation, hepatic thiobarbituric acid and increased in the high density lipoprotein cholesterol [71,72]. On the other hand, numerous epidemiological and intervention studies have concluded that whole cereals (germ and bran layer) are attributed for their combined effects which are beneficial for human health e.g. epidemiological data has recommended the consumption of dietary fibre (mixture of soluble and insoluble) which lowers the risk of cancer especially colon cancer [73,74]. Furthermore, phenolic lipids like alkylresorcinols (5-n-alkylresorcinols) which perform many functions by improving intestinal peristalsis, reduce the glucose absorption to blood and controlling the cholesterol transfer into blood [75,76]. Moreover, these compounds show the antibacterial and antifungal activities [77].
According to different studies, pulse consumption in diet, reduces the risk of cancer because of the presence of antinutrient content, fibre, antioxidant and micronutrients [78,79] Moreover, coronary heart diseases are related to the elevation in the triglycerides and cholesterol level in body and by consumption of pulses in diet, it has reduced the risk factors which are correlated with coronary heart diseases [80].
Many studies have indicated that regular use of pulses is associated with the weight management [81-83]. These bioactive compounds have potential health benefits such as anti-microbial, anti-inflammatory, anti-allergic, antioxidant and anti-diabetic, antiulcer, anti-carcinogenic, anti-atherogenic and anti-thrombotic which prevent from many infectious, cardiovascular, degenerative diseases and promote the vascular health [84-87]. These health effects are observed by consumption of pulses four times or more in a week compared with less than once a week, was associated with 11% lower risk of cardiovascular disease and 22% lower risk of CHD [88]. Therefore, bioactive compounds have various health benefits can be potentially used in nutraceutical, cosmetic, food, pharmaceutical applications.
Summary
Cereal and pulse processing industry is an important part of food industry, which generates the huge volume of byproducts throughout the year. These byproducts are generally considered as wastes and are either dumped or utilized as cattle feed. However, for their better utilisation, these can be used for recovering the bioactive compounds or can be directly used as natural preservatives in different food systems to increase their functional properties. Further, lot of research is being carried out in processing of food industry byproducts for production of functional foods either by using these byproducts as whole or using extract of bioactive compounds as replacer of synthetic additives.
References
- Abdelkarim G. “What is a bioactive compounds? A combined definition for a preliminary consensus”. International Journal of Nutrition and Food Sciences. 2014; 3: 174-179.
- Jones JM, Reicks M, Adams J, Fulcher G, Marquart L. “Becoming Proactive With the Whole-Grains Message. Nutrition Today. 2004; 39: 10-17.
- Carciochi RA, Galván D’Alessandro L, Vauchel P, Rodriguez MM, Nolasco SM, Dimitrov K. “Valorization of Agrifood By-Products by Extracting Valuable Bioactive Compounds Using Green Processes”. In: Grumezescu AM, Holban AM (Eds.), Ingredients Extraction by Physicochemical Methods in Food. Academic Press. 2017; 191-228.
- Rombaut N, Tixier S, Bily A, Chemat F. “Green extraction processes of natural products as tools for biorefinery.” Biofuel S, Bioproducts and Biorefining. 2014; 8: 530-544.
- Verpoorte R, Memelink J. “Engineering secondary metabolite production in plants.” Current Opinion in Biotechnology. 2012; 13: 181-187.
- Yang B, Jiang Y, Shi J, Ashraf M. “Extraction and pharmacological properties of bioactive compounds from longan (Dimocarpus longan Lour.) fruit-A review.” Food Research International. 2010; 44: 1837-1842.
- Harborne JB, Williams CA. “Advances in flavonoid research since 1992.” Phytochemistry. 2000; 55: 481-504.
- Gani SM, Wani F, Masoodi A, Hameed G. “Whole-grain cereals bioactive compounds and their health benefits: A Review.” Journal of Food Processing & Technology. 2012; 3: 146-156.
- Thompson LU, Robb P, Serraino M, Cheung F. “Mammalian lignin production from various foods.” Nutrition and Cancer. 1991; 16: 43-52.
- Britton G, Liaaen-Jensen S, Pfander H. Carotenoids Handbook, Basel. Birkhäuser Verlag. 2004.
- Adom KK, Sorrells ME, Liu RH. “Phytochemicals and antioxidant activity of milled fractions of different wheat varieties.” J Agric Food Chem. 2005; 53: 2297-2306.
- Vucenik, Shamsuddin AM. “Protection against cancer by dietary IP6 and inositol.” Nutr Cancer. 2006; 55: 109-125.
- Minihane M, Rimbach G. “Iron absorption and the iron binding and antioxidant properties of phytic acid.” International Journal of Food Science and Technology. 2002; 37: 741-748.
- Graf E, Eaton JW. “Antioxidant functions of phytic acid.” Free Radic Biol Med. 1990; 8: 61-69.
- Sandberg S. “Bioavailability of minerals in legumes.” Br J Nutr. 2002; 88: 281-285.
- Normén L, Brants HA, Voorrips LE, Andersson HA, van den Brandt PA, Goldbohm RA. “Plant sterol intake and colorectal cancer risk in the Netherlands Cohort Study on Diet and Cancer.” American Journal of Clinical Nutrition. 2001; 74: 141-148.
- Liu RH. “Whole grain phytochemicals and health.” Journal of Cereal Science. 2007; 46: 209-219.
- Oakenfull D. “Saponins in food-A review.” Food Chemistry. 1981; 19-40.
- Woldemichael GM, Montenegro G, Timmer-mann BN. “Triterpenoidal Lupin Saponins from the Chilean Legume Lupinus oreophilus Phil.” Phytochemistry. 2003; 63: 853-857.
- Ruiz RG, Price KR, Arthur AE, Rose ME, Rhodes MJC, Fenwick RG. “Effect of Soaking and Cooking on the Saponin Content and Composition of Chickpeas (Cicer arietinum) and Lentils (Lens culinaris).” Journal of Agricultural and Food Chemistry. 1996; 44: 1526-1530.
- El-Adawy TA. “Nutritional Composition and Antinutritional Factors of Chickpeas (Cicer arietinum L.) Under- going Different Cooking Methods and Germination.” Plant Food for Human Nutrition. 2002; 57: 83-97.
- Shi J, Arunasalam K, Yeung D, Kakuda Y, Mittal G, Jiang Y. “Saponins from Edible Legumes: Chemistry, Processing, and Health Benefits.” J Med Food. 2004; 7: 67-78.
- Kerem Z, German-Shashoua H, Yarden O. “Microwave-assisted extraction of bioactive saponins from chickpea (Cicer arietinum L).” Journal of the Science of Food and Agriculture. 2005; 85: 406-412.
- Rao V, Gurfinkel DM. Dietary saponins and human health. In W. Oleszek & A. Marston (Eds.), Saponins in food, feedstuff’s and medicinal plants. Dordrecht: Kluwer Academic Publishers. 2000; 255-270.
- Holtekjølen K, Kinitz C, Knutsen SH. “Flavanol and bound phenolic acid contents in different barley varieties.” Journal of Agricultural and Food Chemistry. 2006; 54: 2253-2260.
- Ghasemzadeh, Karbalaii MT, Jaafar HZE, Rahmat A. “Phytochemical constituents, antioxidant activity, and antiproliferative properties of black, red, and brown rice bran” Chemistry Central Journal. 2018; 12: 1-13.
- Mc Murough, Baert T. “Identification of proanthocyanidins in Beer and their direct Measurement with a Dual Electrode Electrochemical Detector.” Journal-Institute of Brewing”. 1994; 100: 409-416.
- Ross B, Kamal-Eldin A, Lundin EA, Zhang JX, Hallmans G, Aman P. “Cereal alkylresorcinols are absorbed by humans.” J Nutr. 2003; 133: 2222-2224.
- Sunnerheim BK, Bryngelsson S, Fagerlund A, Engman L, Andersson RE, Dimberg LH. “Avenanthramides in oats (Avena sativa L.) and structureantioxidant activity relationships.” J Agric Food Chem. 2003; 51: 594-600.
- Peterson DM, Hahn MJ, Emmons CL. “Oat avenanthramides exhibit antioxidant activities in vitro.” Food Chemistry. 2002; 79: 473-478.
- Bartlomiej S, Justyna RK, Ewa N. “Bioactive compounds in cereal grainsoccurrence, structure, technological significance and nutritional benefits-a review.” Food Science and Technology International. 2002; 18: 59-568.
- Skendi C, Biliaderis G, Lazaridou AA, Izydorczyk MS. “Structure and rheological properties of water soluble b-glucans from oat cultivars of Avena sativa and Avena bysantina.” Journal of Cereal Science. 2003; 38: 15-31.
- Marconi E, Graziano M, Cubadda RR. “Composition and utilization of barley pearling by-products for making functional pastas rich in dietary fiber and b-glucans.” Cereal Chemistry. 2000; 77: 133-139.
- Adom K, Sorrells ME, Liu RH. “Phytochemical Profiles and Antioxidant Activity of Wheat Varieties.” J AgricFood Chem. 2003; 51: 7825-7834.
- Sommer. “Vitamin A deficiency and clinical disease: an historical overview.” The Journal of Nutrition. 2008; 38: 1835-1839.
- Heiniö RL, Liukkonen KH, Myllymäki O, Pihlava JM, Adlercreutz H, Heinonen SM, et al. “Quantities of phenolic compounds and their impacts on the perceived flavour attributes of rye grain.” Journal of Cereal Science. 2008; 47: 566-575.
- Jones M, Reicks M, Adams J, Fulcher G, Weaver G, Kanter M, et al. “The importance of promoting a whole grain foods message.” Journal of the American College of Nutrition. 2002; 21: 293-297.
- Edge JJ, Marquart L. “A new life for whole grains.” Journal of the American Dietetic Association. 2005; 105: 1856-1860.
- Fardet A. “New hypotheses for the health-protective mechanisms of wholegrain cereals: What is beyond fibre?” Nutrition Research Reviews. 2010; 23: 65-134.
- Huang YP, Lai HM. “Bioactive compounds and antioxidative activity of colored rice bran.” Journal of Food and Drug Analysis. 2016; 24: 564-574.
- Smuda SS, Mohsen SM, Olsen K, Aly MH. “Bioactive compounds and antioxidant activities of some cereal milling by-products” Journal of Food Science and Technology. 2018; 55: 1134-1142.
- Ndolo VU, Beta T. “Distribution of carotenoids in endosperm, germ, and aleurone fractions of cereal grain kernels.” Food Chem. 2013; 139: 663-671.
- Al-Okbi SY, Hussein AMS, Hamed IM, Mohamed DA, Helal AM. “Chemical, rheological, sensorial and functional properties of gelatinized corn-rice bran flour composite corn flakes and tortilla chips.” Journal of Food Processing and Preservation. 2014; 3: 83-89.
- Zhao G, Zhang R, Dong L, Huang F, Liu L, Deng Y, et al. “A Comparison of the Chemical Composition, In Vitro Bioaccessibility and Antioxidant Activity of Phenolic Compounds from Rice Bran and Its Dietary Fibres.” Molecules. 2018; 23: E202.
- Brewer R, Kubola J, Siriamornpun S, Herald TJ, Shi YC, “Wheat bran particle size influence on phytochemical extractability and antioxidant properties.” Food Chem. 2014; 152: 483-490.
- Luthria DL, Lu, Y, John KM. “Bioactive phytochemicals in wheat: extraction, analysis, processing, and functional properties.” Journal of Functional Foods. 2005; 18: 910-925.
- Curti E, Carini E, Bonacini G, Tribuzio G, Vittadini E. “Effect of the addition of bran fractions on bread properties.” Journal of Cereal Science. 2013; 57: 325-332.
- Sainvitu P, Nott K, Richard G, Blecker C, Jérôme C, Wathelet JP, et al. “Structure, properties and obtention routes of flaxseed lignin secoisolariciresinol: a review.” Biotechnology, Agronomy, Society, Environment. 2012; 16: 115-124.
- Pourali F, Asghari S, Yoshida H. “Production of phenolic compounds from rice bran biomass under subcritical water conditions.” Chemical Engineering Journal. 2010; 160: 259-266.
- Brouns F, Hemery Y, Price R, Anson NM. “Wheat aleurone: separation, composition, health aspects, and potential food use.” Critical Reviews in Food Science and Nutrition. 2012; 52: 553-568.
- Gao Y, Guo X, Liu Y, Fang Z, Zhang M, Zhang R, et al. “A full utilization of rice husk to evaluate phytochemical bioactivities and prepare cellulose nanocrystals.” Scientific reports. 2018; 8: 10482.
- Liu F, Chen Z, Shao J, Wang C, Zhan C. “Effect of fermentation on the peptide content, phenolics and antioxidant activity of defatted wheat germ.” Food Bioscience. 2017; 20: 141-148.
- Zhao HM, Guo XN, Zhu KX. “Impact of solid state fermentation on nutritional, physical and flavor properties of wheat bran.” Food Chem. 2017; 217: 28-36.
- Ciccoritti R, Taddei F, Nicoletti I, Gazza L, Corradini D, D’Egidio MG, et al. “Use of bran fractions and debranned kernels for the development of pasta with high nutritional and healthy potential.” Food chemistry. 2017; 225: 77-86.
- Oyeyinka SA, Singh S, Amonsou EO. “Physicochemical properties of starches extracted from bambara groundnut landraces.” Starch Stӓrke. 2017; 69: 3-4.
- Rizkalla SW, Bellisle F, Slama G. Health benefits of low glycaemic index foods, such as pulses, in diabetic patients and healthy individuals. Br J Nutr. 2002; 88: S255-S262.
- Atkinson FS, Foster-Powell K, Brand-Miller JC. “International Tables of Glycemic Index and Glycemic Load Values.” Diabetes Care. 2008; 31: 2281- 2283.
- Rebello J, Greenway FL, Finley JW. “A review of the nutritional value of legumes and their effects on obesity and its related co-morbidities.” Obesity Reviews. 2014; 15: 392-407.
- Balasundram N, Sundaram K, Samman S. “Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses.” Food Chemistry. 2006; 99: 191-203.
- Girish TK, Pratape VM, Rao UJSP. “Nutrient distribution, phenolic acid composition, antioxidant and alpha-glucosidase inhibitory potentials of black gram (Vigna mungo L.) and its milled by-products.” Food Research International. 2012; 46: 370-377.
- Choung G, Choi BR, An YN, Chu YH, Cho YS. “Anthocyanidin profile of Korean cultivated kidney bean (Phaseolus vulgaris L.).” Journal of Agricultural and Food Chemistry. 2003; 51: 7040-7043.
- Alshikh N, de Camargo AC, Shahidi F. Phenolics of selected lentil cultivars: Antioxidant activities and inhibition of low-density lipoprotein and DNA damage. Journal of functional foods. 2015; 18: 1022-1038.
- Niño-Medina G, Muy-Rangel D, Urías-Orona V. “Chickpea (Cicer arietinum) and Soybean (Glycine max) Hulls: Byproducts with Potential Use as a Source of High Value-Added Food Products.” Waste Biomass Valorization. 2017; 8: 1199-1203.
- Gan RY, Deng ZQ, Yan AX, Shah NP, Lui WY, Chan CL, Corke H. “Pigmented edible bean coats as natural sources of polyphenols with antioxidant and antibacterial effects.” LWT-Food Science and Technology. 2006; 73: 168- 177.
- Attree R, Du B, Xu B. “Distribution of phenolic compounds in seed coat and cotyledon, and their contribution to antioxidant capacities of red and black seed coat peanuts (Arachis hypogaea L.).” Industrial Crops and Products. 2015; 67: 448-456.
- Scalbert CM, Morand C, Manach C, Remesy C, Jimenez L. Dietary polyphenols and the prevention of diseases. CRC Crit Rev Food Sci Nutr. 2005; 45: 287-306.
- Segev HB, Kapulnik Y, I. Shomer, M. Oren Shamir, Galili S. “Determination of polyphenols, flavonoids, and antioxidant capacity in colored chickpea (Cicer arietinum L.).” J Food Sci. 2010; 75: S115-S119.
- Girish TK, Kumar KA, Rao UJSP. “C-Glycosylated flavonoids from black gram husk: Protection against DNA and erythrocytes from oxidative damage and their cytotoxic effect on HeLa cells.” Toxicol Rep. 2016; 3: 652-663.
- Fleischman EF, Kowalski RJ, Morris CF, Nguyen T, Li C, Ganjyal G, et al. “Physical, textural, and antioxidant properties of extruded waxy wheat flour snack supplemented with several varieties of bran.” Journal of food science and Technology. 2016; 81: E2726-2733.
- Smith CE, Tucker KL. “Health benefits of cereal fibre: a review of clinical trials.” Nutrition Research Reviews. 2011; 24: 1-23.
- Ha TY, Han S, Kim SR, Kim IH, Lee HY, Kim HK. “Bioactive components in rice bran oil improve lipid profiles in rats fed a high-cholesterol diet.” Nutrition Research. 2005; 25: 597-606.
- Wilson TA, Nicolosi RJ, Woolfrey B, Kritchevsky D. “Rice bran oil and oryzanol reduce plasma lipid and lipoprotein cholesterol concentrations and aortic cholesterol ester accumulation to a greater extent than ferulic acid in hypercholesterolemic hamsters.” J Nut Biochem. 2007; 18: 105-112.
- Hirayama T. “Diet and cancer” Nutrition and Cancer. 1: 67-81.
- Block G, Patterson B, Subar A. “Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence.” Nutr Cancer. 1992; 18: 1-29.
- Zieli A, Ki H, Koz A, Owska H. “Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions.” J Agric Food Chem. 2000; 48: 2008-2016.
- Adam A, Crespy V, Levrat-Verny MA, Leenhardt F, Leuillet M, Demigné C, Rémésy C. “The bioavailability of ferulic acid is governed primarily by the food matrix rather than its metabolism in intestine and liver in rats,” J Nutr. 2002; 132: 1962-1968.
- Ross B, Chen Y, Frank J, Swanson JE, Parker RS, Kozubek A, Lundh T, Vessby B, et al. “Cereal alkylresorcinols elevate gamma-tocopherol levels in rats and inhibit gammatocopherol metabolism in vitro.” The Journal of Nutrition. 2004; 34: 506-510.
- Avune D, De Stefani E, Ronco A, Boffetta P, Deneo-Pellegrini H, Acosta G, et al. “Legume intake and the risk of cancer: a multisite case-control study in Uruguay.” Cancer Causes & Control. 2009; 20: 1605-1615.
- Mudryj N, Yu N, Aukema HM. “Nutritional and health benefits of pulses.” Appl Physiol Nutr Metabol. 2014; 39: 1197-1204.
- Ha V, Sievenpiper JL, De Souza RJ, Jayalath VH, Mirrahimi A, Agarwal AA, et al. “Effect of dietary pulse intake on established therapeutic lipid targets for cardiovascular risk reduction: a systematic review and meta-analysis of randomized controlled trials.” CMAJ. 2014; 186: E252-E262.
- Mc Crory, Hamaker BR, Lovejoy JC, Eichelsdoerfer PE. “Pulse consumption, satiety, and weight management.” Advances in Nutrition. 2010; 1: 17-30.
- Papanikolaou Y, Fulgoni VL. “Bean consumption is associated with greater nutrient intake, reduced systolic blood pressure, lower body weight, and a smaller waist circumference in adults: results from the National Health and Nutrition Examination Survey 1999-2002.” J Am Coll Nutr. 2008; 27: 569-576.
- Singh B, Singh JP, Shevkani K, Singh N, Kaur A. “Bioactive constituents in pulses and their health benefits.” J Food Sci Technol. 2017; 54: 858-870.
- Daglia M. “Polyphenols as antimicrobial agents.” Curr Opin Biotechnol. 2012; 23: 174-181.
- Jin L, Ozga JA, Lopes-Lutz D, Schieber A, Reinecke DM. “Characterization of proanthocyanidins in pea (Pisum sativum L.), lentil (Lens culinaris L.), and faba bean (Vicia faba L.) seeds.” Food Research International. 2012; 46: 528- 535.
- Ozcan T, Akpinar-Bayizit A, Yilmaz-Ersan L, Delikanli B. “Phenolics in human health.” International Journal of Chemical Engineering and Applications. 2014; 5: 393.
- He JA, Giusti MM. “Anthocyanins: natural colorants with health-promoting properties.” Annu Rev Food Sci Technol. 2010; 163-186.
- Bazzano LA, He J, Ogden LG, Loria C, Vupputuri S, Myers L, et al. “Legume consumption and risk of coronary heart disease in US men and women: NHANES I Epidemiologic Follow-up Study.” Arch Intern Med. 2001; 161: 2573-2578.