
Research Article
Austin J Nutr Metab. 2021; 8(2): 1106.
Threshold of Daily Energy and Protein Requirements to Prevent Weight Loss in Patients with Alzheimer’s Disease in Japan
Nakamura T*
Department of Food Sciences and Human Nutrition, Ryukoku University, Japan
*Corresponding author: Nakamura T, Department of Food Sciences and Human Nutrition, Ryukoku University, 1-5 Yokotani, Seta Oe-cho, Otsu, Shiga 520-2194, Japan
Received: March 03, 2021; Accepted: April 03, 2021; Published: April 10, 2021
Abstract
Background and Aims: Patients with Alzheimer’s Disease (AD) frequently develop weight loss. However, little is known about the energy and protein thresholds that cause weight loss. The purpose of this study was to determine the threshold of daily energy and protein requirements to prevent weight loss in patients with AD.
Methods: We included 75 Japanese long-term care hospital patients with probable AD (22 men and 53 women, aged 65–101 years) in an interventional study. After a one-week survey using weighed food records weighed food records, the relationship between the obtained energy and protein intake and weight loss after three months was examined. Multiple regression analysis was used to examine the daily determinants of weight loss. Subsequently, receiver operating characteristic curves were used to examine the threshold for discriminating weight loss.
Results: Sixty-one (81.3%) patients were malnourished or at risk of malnutrition. Twenty patients (26.7%) had >5% weight loss. The significant associations with weight loss were Mini Nutritional Assessment (MNA) point, energy intake, and protein intake; with a MNA point at cutoff of 17.25, an energy intake at cutoff of 29.93kcal/kg, and a protein intake at cutoff of 1.122g/kg.
Conclusion: To prevent weight loss in AD patients, it is important to prevent malnutrition and administer more than 30kcal/kg energy intake and more than 1.1g/kg protein intake. Future studies with a larger sample size are needed to determine the threshold of daily energy and protein requirements to prevent weight loss.
Keywords: Alzheimer’s disease; Energy requirement; Protein requirement; Weight loss
Abbreviations
ABW: Actual Body Weight; AD: Alzheimer’s Disease; AUC: Area Under the Curve; BMI: Body Mass Index; MMSE: Mini Mental State Examination; MNA: Mini Nutritional Assessment; ROC: Receiver Operating Characteristic; WFRs: Weighted Food Records
Introduction
The number of patients with Alzheimer’s Disease (AD) was 30,000 in 1999 in Japan [1] and by 2014, this number had increased 18-fold to 530,000 [2]. Furthermore, it is estimated that the number will reach approximately 5.5-6.5 million by 2040 [3] and has, thus, become a major problem in Japan.
Among the several kinds of problems, one major nutritional management problem in patients with AD is unintended weight loss [4,5]. Weight loss frequently occurs even in the early stages of AD [6]. This weight loss is so problematic because it may be associated with severe complications, such as alteration of the immune system, muscular atrophy, and loss of independence [6]. It may even be an indicator of mortality among patients with AD [7].
There are many causes of weight loss in the elderly and in persons with AD [8,9]. It has been reported that patients with AD have difficulty beginning a meal, experience a loss of appetite, and a slight risk of developing dysphagia in the early stages of the disease [10,11]. Subsequently, as the disease progresses, dysphagia becomes severe in patients with AD [12,13]. Moreover, the olfactory function related to appetite is altered in AD [14]. Since meals become problematic and unappealing, food intake reduces.
Weight loss occurs when energy expenditure exceeds energy intake. However, the amount of energy and protein required to prevent weight loss in patients with AD remains unclear [15]. Little is known about the energy and protein thresholds that cause weight loss. It is also unclear how much energy and protein units per body weight are needed for patients with AD. A recent systematic review showed that the findings regarding association between unintended weight loss and energy and protein intake are not consistent [16].
To prevent weight loss in patients with AD, their daily energy and protein requirements need to be fulfilled. High-quality nutritional data are thus essential to correctly determine the threshold of daily energy and protein requirements. In our previous study, we examined the daily energy and protein intake of patients with AD using Weighed Food Records (WFRs) to measure food intake with good accuracy in long-term care [17]. Using these data, we determined the threshold of daily energy and protein requirements to prevent weight loss in patients with AD. In our knowledge, this is the first study to determine the threshold of daily energy and protein requirements to prevent weight loss in patients with AD in a nursing care hospital in Japan.
Methods
The patients
We selected patients with AD from a previous clinical study that was carried out to evaluate the validity and reliability of the visual estimation method in patients with AD. Details of the methods used in that study have been reported previously [17]. In summary, this intervention study examined the data of patients who had been hospitalized at the Mitate Hospital (Tagawa City, Fukuoka). Patients were diagnosed with probable AD by a psychiatrist, according to the Diagnosis and Statistical Manual of Mental Disorders, Revised Third Edition [18]. Other patients, with different types of neurological disorders, pressure sores, or impairment of renal, hepatic, or cardiac function, and those with an active inflammatory reaction based on a serum C reactive protein >0.06mg/dL were excluded from this study. A total of 82 patients (24 men and 58 women), who had been consuming hospital meals as their sole source of nutrition, were recruited for this study in December 2012. The study began in February 2013 and ended three months later, in April 2013 [17]. Subsequently, patients receiving drip infusion or artificial nutrition, or those with changes in hospitalization or meal status were excluded from the study. The remaining 75 patients (22 men and 53 women) were included in the analysis. The patients’ hospital stay ranged from 1 month to 6 years. This study was approved by the Ethics Committee of Mitate Hospital (2012.12), and written informed consent was obtained from the patients’ family members.
Physical and biochemical parameters
Physical measurements and blood biochemistry tests were performed on the first day of this study, body height and weight were measured, and Body Mass Index (BMI) was determined. At the conclusion of the 3-month study, body weight was remeasured, and weight loss was calculated by subtracting the body weight at 3 months from the body weight measurement on the first day. The criteria proposed in 2018, by the world’s first Global Leadership Initiative on Malnutrition [19] state that weight loss should be >5% within 6 months to be considered as highly significant. Therefore, we assessed the threshold of daily energy and protein requirements to prevent a weight loss of >5% over a 3-month period for patients with AD.
Geriatric assessment
Nutritional status was evaluated by a charge nurse using the Mini Nutritional Assessment (MNA) [20], BMI, and serum albumin level [21]. Serum albumin, hemoglobin, and C-reactive protein levels were measured as the biological parameters. The Mini Mental State Examination (MMSE) [22] was used to assess cognitive function, while the Barthel Index (BI) [23] was used to evaluate activities of daily living. All parameters were measured at the end of the study period.
The parameters were defined as follows: Patients with an MMSE score of ≤21 were strongly suspected to have dementia, those with a score between 22 and 26 were suspected to have mild dementia, and those with a score between 27 and 30 were considered not to have dementia [22]. Regarding BI, patients with a score of ≤40 were considered to require considerable overall assistance, while those with a score ≥60 were considered to require only slight assistance [23]. Concerning MNA, patients with a rating of <17 were considered to have malnutrition, those with a rating between 17 and 23.5 were suspected to have malnutrition, and those with a rating between 24 and 30 were considered to have an excellent nutritional status [20].
Nutritional parameters
The methodological details are described elsewhere [17]. Briefly, a dietary survey using WFMs was conducted during the first seven days of this study. Food consumption for each patient was calculated by subtracting the weight of the plate waste from the weight of the served food. The same dietary survey was conducted, for one week each, in March and April, and the patients whose diets changed significantly were excluded from the analysis. Based on food consumption, 49 components, including energy, protein, fat, carbohydrate, minerals, vitamins, and dietary fiber, were calculated. To evaluate food and nutrient intake, energy and protein intake per kilogram were calculated according to the Actual Body Weight (ABW) of each patient.
Statistical analysis
The category data were expressed as numbers (%) of patients and subjected to chi-squared test. Continuous data were described as mean and Standard Deviation (SD) and were subjected to the Mann-Whitney U test. Multivariable linear regression analysis was performed to determine factors independently related to weight loss. Since there was a strong correlation between energy intake and protein intake, they were analyzed separately. To determine the cutoff point of the MNA score, the daily energy and protein requirements to prevent weight loss (>5%) in patients with AD, we used the Receiver Operating Characteristic (ROC) curve method. All P-values were 2-sided, with 0.05 as the threshold for significance. All statistical analyses were performed using SPSS (IBM SPSS Statistics for Windows, Version 27.0, IBM Corp., Armonk, NY, USA).
Results and Discussion
Table 1 shows the characteristics of the study patients. The mean age of the patients was 83.7 ± 7.2 years (65-101 years) and 22 (29.3%) of the patients were men. A total of 61 (81.3%) patients were malnourished or at risk of malnourishment according to MNA. In all, 20 (26.7%) had >5% weight loss. The >5% group had significantly lower values for weight loss, MNA, energy/ABW, and protein/ABW than the ≤5% group, but others showed no significant difference.
All
(n=75)Weight loss
=5%
(n=55; 73.3%)>5%
(n=20; 26.7%)Age (y)
83.7 ± 7.2
84.2 ± 7.1
82.3 ± 7.4
Sex (male), n (%)
22 (29.3)
16 (29.1)
6 (30.0)
Length of disease duration (y)
5.7 ± 3.6
5.3 ± 3.1
6.9 ± 4.4
Length of stay (y)
1.8 ± 1.6
1.8 ± 1.5
1.8 ± 1.8
Height (cm)
149 ± 10
149 ± 10
150 ± 10
Actual body weight (kg)
42.6 ± 9.3
41.5 ± 8.9
45.5 ± 10.1
Body mass index (kg/m2)
19.0 ± 3.5
21.8 ± 3.1
18.2 ± 3.2
Weight loss in 3 months (kg)
2.5 ± 6.3
-0.14 ± 1.6
5.1 ± 2.6**
Staff assisting with eating, n (%)
27 (36.0)
18 (32.7)
9 (45.0)
Geriatric assessments, Median (interquartile range)
Mini nutritional assessment
20 (17-22)
20 (18-23)
17 (14-21)**
Mini mental state examination
11 (4-17)
11 (5-15)
11 (2-19)
Barthel-Index
25 (10-60)
25 (10-55)
28 (5-65)
Biochemical and dietary values
Serum Albumin (g/dL)
3.5 ± 0.4
3.5 ± 0.4
3.4 ± 0.4
Hemoglobin (g/dL)
11.2 ± 1.5
11.4 ± 1.4
10.7 ± 1.6
Food consumption rate (%)
91.8 ± 10.0
89.6 ± 13.4
86.8 ± 13.7
Amount of energy provided (kcal)
1535 ± 290
1537 ± 302
1527 ± 247
Amount of energy consumed (kcal)
1301 ± 320
1320 ± 327
1219 ± 267
Energy/Actual body weight (kcal)
30.6 ± 7.3
32.4 ± 7.5
27.5 ± 7.3**
Amount of protein provided (g)
57.9 ± 9.6
58.3 ± 9.7
56.7 ± 9.4
Amount of protein consumed (g)
49.2 ± 10.9
50.2 ± 11.0
45.9 ± 9.6
Protein/Actual body weight (g)
1.2 ± 0.3
1.2 ± 0.3
1.0 ± 0.3**
Values are given as mean ± SD or median (interquartile range). *P<0.05, **P<0.01 Difference between groups.
Table 1: Characteristics of study patients with Alzheimer’s disease according to weight loss.
Tables 2 and 3 show the results of the multivariate linear regression analyses. Low MNA scores, energy/ABW, and protein/ ABW were independently associated with weight loss.
Bata Coefficient
Standard error
P value
Age
-0.09
0.04
0.053
Sex
-0.22
0.69
0.747
Body mass index
0.11
0.14
0.425
Length of disease duration
0.15
0.1
0.147
Length of stay
-0.11
0.22
0.626
Staff assisting with eating
-1.4
0.99
0.162
Mini nutritional assessment
-0.47
0.12
<0.001
Barthel-Index
0.03
0.02
0.096
Mini mental state examination
0.01
0.06
0.876
Energy/Actual body weight
-0.1
0.05
0.03
Table 2: Multivariable liner regression analysis of energy and other independent variables associated with weight loss in patients with Alzheimer’s disease (n=75), Adjusted R²: 0.293.
Bata Coefficient
Standard error
P value
Age
-0.08
0.04
0.074
Sex
-0.2
0.7
0.78
Body mass index
0.1
0.14
0.469
Length of disease duration
0.15
0.1
0.15
Length of stay
-0.13
0.22
0.557
Staff assisting with eating
-1.35
0.99
0.179
Mini nutritional assessment
-0.48
0.12
<0.001
Barthel-Index
0.03
0.02
0.155
Mini mental state examination
0.02
0.06
0.692
Protein/Actual body weight
-2.42
1.18
0.045
Table 3: Multivariable liner regression analysis of protein and independent variables associated with weight loss in patients with Alzheimer’s disease (n=75), Adjusted R²: 0.286.
The MNA score at a cutoff of 17.25 had a sensitivity of 76.8%, a specificity of 65.0% with an Area Under the Curve (AUC) of 0.749; energy intake at a cutoff of 29.93 (kcal/kg) had a sensitivity of 67.3%, a specificity of 67.3%, and AUC of 0.701; and protein intake at a cutoff of 1.12g/kg had a sensitivity of 65.5% and a specificity of 65.0% with an AUC of 0.731, to prevent a >5% weight loss (Figure 1).
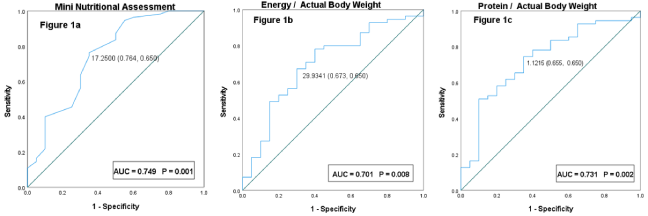
Figure 1: Receiver operating characteristic curves of weight loss (>5%) for a) Mini Nutritional Assessment; b) energy/actual body weight; c) protein/actual body
weight. ROC: Receiver Operating Characteristic Curve; AUC: Area Under The Curve.
We found significant associations between weight loss and MNA score, energy intake, and protein intake, with cutoffs for MNA score, energy requirement, and protein requirement at 17, 30 kcal/kg, and 1.1g/kg, respectively, rounding the decimal point to the nearest whole number.
Hence, the daily energy and protein requirements per ABW needed to prevent weight loss from the cutoff values of 30kcal/kg and 1.1g/kg. The European Society for Clinical Nutrition and Metabolism guidelines regarding nutrition in dementia recommend screening for malnutrition and close monitoring of body weight and oral nutritional supplements to improve nutritional status; however, the energy and protein requirements are not indicated [5]. A review to determine energy requirements and to evaluate energy expenditure in cases of neurological diseases concluded that energy expenditure changes according to the restriction of physical activity due to the disorder [24]. Moreover, a recent review also did not give a clear threshold and the reason for variation was similar to that in the previous study mentioned here [25]. A one-year study showed that patients with AD required 35kcal/kg of body weight to maintain their weight [26]. One study in a Dutch geriatric practice recommended that ≥400kcal/day be added to >1500kcal/day and a ≥1.2g/kg protein intake [27]. Based on these previous studies, we consider that our recommended 30kcal/ kg and 1.1g/kg are reasonable values to prevent weight loss.
The guidelines of the Japanese Society for Parenteral and Enteral Nutrition do not indicate the recommended amount of energy and protein for patients with dementia [28]. These guidelines only recommend an energy intake of between 20 and 30 kcal/kg/day and a protein intake of between 1.0 and 1.2 g/kg for elderly people. The daily energy requirement of 30kcal/kg and protein requirement of 1.2g/kg are the upper limits of the requirements for the elderly. Patients with AD have a higher rate of malnutrition than the general elderly population [4,5]. This also suggests that the requirements derived from this study are reasonable.
As the threshold of weight loss, the MNA is more accurate than the energy or protein requirements. Moreover, the 17 points were also in line with the MNA criteria for malnutrition [20]. Unfortunately, due to the inadequacy of data, there was a bias in the MNA scores. At the end of the study, we assessed the MNA of patients with AD; the MNA items included weight loss. Therefore, it is natural for these factors to be correlated.
However, only 3 out of the 24 items on the MNA were for weight loss, suggesting that other items may have influenced the results. The MNA asks about digestive symptoms and mental stress. These factors may interfere with nutrient absorption [29]. The MNA also asks if the patient can walk on his/her own, but if the patient is bedridden, the assimilation of nutrients may be inhibited [30]. In short, if they are malnourished, they may not be able to take in the nutrients contained in the food they eat. These effects of nutritional status on weight loss were thought to be equal to or greater than that of food intake.
The present study has several limitations. First, the study sample size was small, which reduced the power of the study and increased the margin of error. Second, this study was a single-center trial conducted in a long-term nursing care hospital. Third, no neurological disorders other than AD were identified. Therefore, the results of this study are not readily generalizable in terms of external validity to support widespread changes in practice and should be interpreted with caution.
One strength of this study was the measurement of WFMs as a means of calculating the amount of energy and protein consumed. This requires considerable time and effort.
Conclusion
The present study showed the cutoff values for energy and protein requirements per actual body weight to prevent weight loss to be 30 kcal/kg and 1.1 g/kg. However, since nutritional status affects weight loss more than daily energy and protein requirements, it is necessary to monitor the weight of patients with AD and reevaluate daily energy and protein requirements to prevent weight loss. Future studies with a larger sample size are needed to determine more accurate thresholds of daily energy and protein requirements to prevent weight loss.
Acknowledgements
The author would like to thank all the study patients, nursing staff, and dietitians. We also wish to thank M.D Masakatu Yoshida and M.D Yoshiaki Honda for their assistance during this research, and R.D Nobuko Amano for her data collection using WFRs. We would like to thank Editage (www.editage.jp) for English language editing.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The Diagnosis of Dementia due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimers Dement. 2011; 7: 263-269.
- White Paper & Reports. Annual Health, Labour and Welfare Report. 2016.
- MHLW Grants System. 2014.
- Poehlman ET, Dvorak RV. Energy Expenditure, Energy Intake, and Weight Loss in Alzheimer Disease. Am J Clin Nutr. 2000; 71: 650S-655S.
- Volkert D, Chourdakis M, Faxen-Irving G, Frühwald T, Landi F, Suominen MH, et al. ESPEN Guidelines on Nutrition in Dementia. Clin Nutr. 2015; 34: 1052-1073.
- Gillette GS, Abellan VKG, Alix E, Andrieu S, Belmin J, Bonnefoy M, et al. IANA (International Academy on Nutrition and Aging Expert Group): Weight Loss and Alzheimer’s Disease. J Nutr Health Aging. 2007; 11: 38-48.
- de Sousa OV, Mendes J, Amaral TF. Nutritional and Functional Indicators and Their Association With Mortality Among Older Adults with Alzheimer’s Disease. Am J Alzheimers Dis Other Demen. 2020; 35: 1533317520907168.
- Venturelli M, Cè E, Limonta E, Muti E, Scarsini R, Brasioli A, et al. Possible Predictors of Involuntary Weight Loss in Patients with Alzheimer’s Disease. PLoS One. 2016; 11: e0157384.
- Edahiro A, Hirano H, Yamada R, Watanabe Y. Comparative Study of Eating Behavior in Elderly Patients with Alzheimer’s Disease and Vascular Dementia: A First Report. Comparison of Disturbed Eating Behavior. Nihon Ronen Igakkai Zasshi. 2013; 50: 651-660.
- Edahiro A, Hirano H, Yamada R, Chiba Y, Watanabe Y, Tonogi M, et al. Factors Affecting Independence in Eating among Elderly with Alzheimer’s Disease. Geriatr Gerontol Int. 2012; 12: 481-490.
- Humbert A, McLaren DG, Kosmatka K, Fitzgerald M, Johnson S, Porcaro E, et al. Early Deficits in Cortical Control of Swallowing in Alzheimer’s Disease. J Alzheimers Dis. 2010; 19: 1185-1197.
- Goes VF, Mello-Carpes PB, de Oliveira LO, Hack J, Magro M, Bonini JS. Evaluation of Dysphagia Risk, Nutritional Status and Caloric Intake in Elderly Patients with Alzheimer’s. Rev Lat Am Enfermagem. 2014; 22: 317-324.
- Jung HJ, Shin IS, Lee JE. Olfactory Function in Mild Cognitive Impairment and Alzheimer’s Disease: A Meta-Analysis. Laryngoscope. 2019; 129: 362- 369.
- Kai K, Hashimoto M, Amano K, Tanaka H, Fukuhara R, Ikeda M. Relationship Between Eating Disturbance and Dementia Severity in Patients with Alzheimer’s Disease. PLoS One. 2015; 10: e0133666.
- Müller MJ, Geisler C. From the Past to Future: From Energy Expenditure to Energy Intake to Energy Expenditure. Eur J Clin Nutr. 2017; 71: 678.
- Doorduijn AS, van de Rest O, van der Flier WM, Visser M, de van der Schueren MAE. Energy and Protein Intake of Alzheimer’s Disease Patients Compared to Cognitively Normal Controls: Systematic Review. J Am Med Dir Assoc. 2019; 20: 14-21.
- Amano N, Nakamura T. Accuracy of the Visual Estimation Method as a Predictor of Food Intake in Alzheimer’s Patients Provided with Different Types of Food. Clin Nutr ESPEN. 2018; 23: 122-128.
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical Diagnosis of Alzheimer’s Disease: Report of the NINCDS-ADRDA Work Group under the Auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984; 34: 939-944.
- Jensen GL, Cederholm T, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM Criteria for the Diagnosis of Malnutrition: A Consensus Report from the Global Clinical Nutrition Community. JPEN J Parenter Enteral Nutr. 2019; 43: 32-40.
- Huhmann MB, Perez V, Alexander DD, Thomas DR. A Self-Completed Nutrition Screening Tool for Community-Dwelling Older Adults with High Reliability: A Comparison Study. J Nutr Health Aging. 2013; 17: 339-344.
- Fuhrman MP, Charney P, Mueller CM. Hepatic Proteins and Nutrition Assessment. J Am Diet Assoc. 2004; 104: 1258-1264.
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J Psychiatr Res. 1975; 12: 189-198.
- Mahoney FI, Barthel DW. Functional Evaluation: The Barthel index. Md State Med J. 1965; 14: 61-65.
- Anasako Y, Akamatsu R. A Systematic Review of the Reliability and Validity of the Visual Estimation Method to Measure Plate Waste in Food Service Facilities. Jpn J Nutr Diet. 2014; 72: 181-192.
- Çekici H, Acar Tek N. Determining Energy Requirement and Evaluating Energy Expenditure in Neurological Diseases. Nutr Neurosci. 2020; 23: 543- 553.
- Spindler AA, Renvall MJ, Nichols JF, Ramsdell JW. Nutritional Status of Patients with Alzheimer’s Disease: a 1-Year Study. J Am Diet Assoc. 1996; 96: 1013-1018.
- van Asselt DZ, van Bokhorst-de van der Schueren MA, van der Cammen TJ, Disselhorst LG, Janse A, Lonterman-Monasch S, et al. Assessment and Treatment of Malnutrition in Dutch Geriatric Practice: Consensus Through a Modified Delphi Study. Age Ageing. 2012; 41: 399-404.
- Japanese Society for Parenteral & Eternal Nutrition. Japanese Society for Parenteral & Eternal Nutrition Guideline 3rd Edition. Tokyo: Shorinsha Press. 2013; 388.
- van der Heide F. Acquired Causes of Intestinal Malabsorption. Best Pract Res Clin Gastroenterol. 2016; 30: 213-224.
- Matsakas A, Patel K. Intracellular Signalling Pathways Regulating the Adaptation of Skeletal Muscle to Exercise and Nutritional Changes. Histol Histopathol. 2009; 24: 209-222.