
Review Article
Austin J Nutr Metab. 2021; 8(2): 1107.
Zinc Supplements in COVID-19 Pathogenesis-Current Perspectives
Majeed M1,2, Chavez M2, Nagabhushanam K2 and Mundkur L1*
¹Sami-Sabinsa Group Limited, Bangalore 560 058, Karnataka, India
²Sabinsa Corporation, East Windsor, NJ 08520, USA
*Corresponding author: Lakshmi Mundkur, Sami-Sabinsa Group Limited, Peenya Industrial Area, Bangalore 560 058, Karnataka, India
Received: March 16, 2021; Accepted: April 26, 2021; Published: May 03, 2021
Abstract
Zinc is an indispensable trace element required for several critical functions of the human body. Deficiencies of micronutrients can impair immune function and increase susceptibility to infectious disease. It is noteworthy that higher susceptibility to the SARS-CoV-2 viral infection is seen in individuals with micronutrient deficiencies and poorer overall nutrition. Research in the last two decades suggests that one-third of the global population may be deficient in zinc, which affects the health and well-being of individuals of all ages and gender. Zinc deficiency is now considered one of the factors associated with susceptibility to infection and the detrimental progression of COVID-19. The trace element is essential for immunocompetence and antiviral activity, rendering zinc supplements highly popular and widely consumed. Zinc supplements are required in small doses daily, and their absorption is affected by food rich in fiber and phytase. The organic forms of zinc such as picolinate, citrate, acetate, gluconate, and the monomethionine complexes are better absorbed and have biological effects at lower doses than inorganic salts. Considering the present global scenario, choosing the right zinc supplement is essential for maintaining good health. In the present review, we reexamine the role of zinc in immunity and antiviral activity and a comparative account of different forms of zinc supplements.
Keywords: Zinc; SARS-CoV-2; Zinc deficiency; Antiviral immunity; Supplements
Introduction
Nutritional deficiency contributes to poor health and susceptibility to infection. The deficiency in micronutrients are not easily recognized as their manifestation is not very distinct and hence may not get noticed. Correcting the micronutriient deficiencies may be helpful in supporting the immune function and resist frequent infection, especially in the vulnerable population.
Zinc is only second to iron as an important trace element in the human body. It is abundantly distributed throughout body tissues and is vital for growth and development, gene expression, and immune functions [1,2]. Zinc is a structural component of nearly 2000 transcription factors and a required cofactor for more than 300 enzymes, which help in digestion, metabolism, and neuronal functions [2]. Numerous studies have shown that zinc is essential for maintaining a strong immune function, blood sugar levels and keeping skin, hair, eyes, and heart healthy [3]. Daily intake is required for maintaining the levels to support the essential biological functions as only 20-40% mineral is absorbed by the enterocytes in the gut, while the residual zinc is excreted [4]. Zinc may be stored in skeletal muscle and bone and a very small fraction (10-20 μM) is found circulating in the blood [5]. The prevalence of zinc deficiency is estimated to be 17-20% globally, predominant in African and Asian countries [6]. Zinc deficiency is commonly observed in the geriatric population, vegans/vegetarians, and individuals with chronic disease such as immunosuppression, Chronic Obstructive Pulmonary Disease (COPD), asthma, cardiovascular diseases, autoimmune diseases, kidney diseases, obesity, diabetes, liver disorders, inflammatory bowel disease and cancer, who are also known to be at high risk for SARS-CoV-2 infection [7,8]. Zinc is vital for a proper immune response as its deficiency results in defective lymphocyte responses, lymphopenia, and thymic atrophy [9]. In the present scenario of the pandemic viral infection, robust immunity is a major concern. In this review, we focus on the role of zinc in immunity and antiviral response, and the importance of zinc supplements in prophylaxis and treatment of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) infections.
Zinc in Respiratory Health: SARS-CoV-2 Infection
The world is now facing a serious pandemic, caused by the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), also known as the novel Coronavirus Disease 2019 (COVID-19). There is an urgent need for pharmacological, traditional, and complementary medicine approaches and nutritional intervention to aid in prevention, treatment, and recovery from the infection. SARSCoV- 2 is an enveloped beta coronavirus with a positive-sense singlestranded RNA genome [10]. It is transmitted via direct contact, respiratory secretions and remains stable on surfaces for days [11,12]. High morbidity has been observed among the elderly, especially those with prevailing chronic diseases [13]. The pathogenesis of COVID-19 is yet to be fully understood, but the multifactorial pathology results in a systemic hyperinflammatory response, cytokine storm, and an associated thromboembolic complication in severe cases [14,15].
Zinc, as a trace element, has potent antiviral and immunomodulatory properties [16]. It is used as a cofactor by different cellular proteins with immunomodulatory and antioxidant actions [17]. Zinc is essential for the development and activation of T-lymphocytes, which are the leading cells for defense against viral infections [18]. The antiviral property of zinc has been studied extensively in hepatitis C virus, coronavirus, human immunodeficiency virus and others [19].
Zinc is considered a potential supportive treatment due to its immune-modulatory and antiviral effect [16]. Hydroxychloroquine, a zinc ionophore, has been increasingly used as antiviral therapy for COVID-19 patients [21,22]. Further, zinc is known to improve antiviral immunity and diminish the risk of hyper-inflammation. The anti-oxidative effect of zinc could reduce lung damage and curtail secondary infections [22,23]. A significant number of COVID-19 patients were found to be zinc deficient compared to healthy individuals in a recent study. Severity and complications due to infection, were higher in zinc deficient patients, who had prolonged hospital stay associated with increased mortality [24].
Mechanism of Antiviral Activity of Zinc
Viral entry
The entry of infectious agents is prevented by the ciliary cells in mucosal layers. The coronavirus infection damages the ciliated epithelium and ciliary dyskinesia, thus impairing the mucociliary clearance [25]. Zinc could increase the ciliary beat frequency, the number, and the length of bronchial cilia, thus improving the elimination of virus particles and prevention of secondary bacterial infections [25]. Disruptions in the respiratory epithelial integrity facilitate the entry of the virus. Decreasing zinc level was found to increase the epithelial leakage in the respiratory tract in an ex vivo model of COPD, while lung integrity could be improved by zinc supplementation [26,27]. Zinc supplementation was shown to increase proteolysis of E-cadherin/beta-catenin and increase the expression of tight junction proteins like Claudin-1 and ZO-1, which improved lung integrity in a murine model of acute lung injury [27,28]. Further, zinc had an inhibitory effect on LFA-1/ICAM-1 interaction, which reduced leukocyte recruitment and inflammation in the respiratory tract, while high zinc levels improved the tolerance of the lung towards damage induced by mechanical ventilation [29,30].
SARS-CoV-2 infects cells expressing the surface receptors Angiotensin-Converting Enzyme 2 (ACE-2). Zinc binds to the active center of the ACE-2 active center and is thus essential for its enzymatic activity. The expression of ACE-2 expression is regulated by Sirt-1, which is downregulated by zinc. Thus, zinc is likely to have an indirect effect on ACE-2 expression and thus viral entry into the cell [31].
Viral replication
The antiviral effects of zinc have been demonstrated against several human viruses. Increasing the intracellular zinc concentration with zinc-ionophores like pyrithione was reported to impair viral replication in vitro [32,33]. Recent studies have shown the efficacy of chloroquine, a zinc ionophore, as an antiviral treatment for COVID-19 (20). Positive stranded RNA viruses use RNA-dependent polymerase for replication. Increased intracellular zinc ion concentrations was found to inhibit the viral replication by directly inhibiting the RNA polymerase activity in Vero cells [34]. Zinc could also interfere with the synthesis and assembly of viral proteins [35]. It was suggested that zinc plays a vital role in preventing viral fusion with the host membrane, decrease the viral polymerase function, impair protein translation and processing, block viral particle release, and destabilize the viral envelope in different viral models [34,36,37]. Zinc was also reported to act in a synergistic manner with standard antiviral therapy [38]. Overexpression of metallothionines was also reported to inhibit replicating few viruses such as flaviviruses and encephalitis virus. Metallothionines are hypothesized to sequester Zn2+ away from the viral proteins by acting as zinc chaperones and facilitating antiviral signaling [39].
Zinc and Immune Response
Zinc is crucial for the proper folding and activity of various cellular enzymes and transcription factors. It is a component of the thymic hormone and thus mediates the normal development and function of innate immune response cells, neutrophils, and natural killer cells [38]. Zinc deficiency suppresses human immunity by affecting T-helper cells and the balance of the helper T cell (Th1 and Th2) functions, antibody response, activity of natural killer cells and macrophages [40,41]. Supplementation with zinc could activate the interleukin -2 expression and normalize the cellular immune response in elderly individuals and reduce mortality from infections [42,43]. Apart from improving immune response, zinc is known to play an important role in maintaining immune tolerance. It induces regulatory cell differentiation while dampening the proinflammatory Th17 and Th9 differentiation [44-46]. Thus, zinc supplements improve the T cell function, thereby strengthening the cell-mediated immunity [47]. Zinc also enhances the phagocytosis, intracellular killing, and cytokine production in macrophages [47]. In elderly population, zinc supplementation is believed to help to manage immune senescence [48]. Zinc deficiency is associated with higher susceptibility to infections, which could be reversed with supplementation. Hepatitis and human papilloma virus-infected individuals showed an enhanced response to antiviral therapies when supplemented with zinc [49,50].
Zinc and antiviral immunity
Zinc was shown to induce the production of antiviral interferons (IFN-a and IFN-γ) in leukocytes and reduce the release of proinflammatory cytokine, TNF-a [51]. Zinc supplementation in the elderly restored the production of IFN-a [52]. Imbalance in the immune response is a hallmark of SARS-CoV-2 infection. Heightened cytokine release increases reactive oxygen and nitrogen species (ROS and RNS), and hyperactive immune cells in the lungs complicate the disease, leading to lung tissue destruction, systemic inflammation, and organ failure [53]. This leads to the development of Acute Respiratory Distress Syndrome (ARDS) in patients, accompanied by fluid accumulation in the lungs, interstitial edema with severely limited oxygen exchange [54]. Elevated levels of proinflammatory mediators, increased ROS levels reversible by zinc supplementation [27,55]. COVID-19 patients show increased expression T cell exhaustion markers like Tim-3 and PD-1 and neutrophilia and lymphopenia, associated with poor prognosis of these patients [56,57]. Zinc is required for the development and function of lymphocytes and its supplementation can reverse lymphopenia [58,59]. In the recovery phase of COVID-19 patients an increase of CD14+ monocytes and NK cells could be correlated with clinical improvement [60]. These CD14+ cells require sufficient intracellular zinc levels for phagocytic activity and inflammatory response [9], while zinc supplementation increased the cytotoxicity of NK cells and cytotoxic T cells toward their target cells [61]. These studies suggest that zinc balances immune response by influencing several cellular pathways.
Zinc supplementation in viral infections
Several studies showed reduced symptom severity, frequency, and duration of common cold after zinc supplementation. Higher susceptibility to infections associated with zinc deficiency could be reversed with supplementation. Further, zinc supplementation enhanced the response to antiviral therapies in hepatitis and human papilloma virus-infected individuals [49,50]. In patients with human immunodeficiency virus (HIV) infections, zinc supplementation was found to increase the peripheral CD4+ T cells [62]. An increase in zinc deficiency with age increases the susceptibility of older individuals to viral infections. Elderly subjects supplemented with 45 mg elemental zinc/day for a year, demonstrated a remarkable reduction in the incidence of infection and plasma oxidative stress markers [58]. Zinc supplementation enhanced the NK cell cytotoxicity in both healthy and zinc deficient elderly individuals [63,64], and increased the peripheral CD4+ T cells in HIV patients [42]. A systematic metaanalysis showed that zinc consumed as gluconate lozenges reduce the first signs of cold duration and severity [65,66]. Few clinical studies in the last two decades are listed in Table 1.
Supplement
Dose/Duration
Disease
Effect
Reference
Zinc gluconate
10 mg of elemental zinc per day for 60 days
Acute Respiratory Infections
Decreased episodes
[125]
30 mg of elemental zinc for 12 months.
Cystic fibrosis (children)
Reduced duration of antibiotics
[126]
(5/11.5 mg) lozenges, every 2-3 h/d.
Common cold
Reduced duration of illness
[127]
Zinc acetate
20 mg/d for 5 days
Lower RTI (children)
Increased recovery rates (boys)
[113]
Zinc bis-glycinate
30 mg/d of elemental zinc for 7 days
Lower RTI (children)
Decreased duration of lower RTI
[128]
Zinc sulphate
15 mg/d for 7 months
Common cold
Decreased incidence
[129]
60–90 mg/d for 12 months
Ventilation associated pneumonia
Decreased incidence
[130]
20 mg/d of elemental of zinc for 2 weeks
Lower RTI (children)
Reduced morbidity
[131]
Zinc Oxide
5 mg/d for 12 months
Upper RTI (children)
Decreased incidence
[132]
RTI: Respiratory Tract Infection; mg/d: Milligram Per Day.
Table 1: Zinc supplementation in respiratory viral infections.
Zinc absorption and homeostasis
The cellular homeostasis of zinc is mediated by two protein families of zinc transporters and metallothionein [67]. Zinc absorption occurs mostly in the small intestine by a carrier-mediated mechanism [68]. Zinc ions released from food during digestion, are transported across the cell membrane into the portal circulation by specific transport proteins. Zinc is delivered to tissues through systemic circulation as a complex bound to albumin or metallothionein [69]. Elimination of zinc from the body is mediated mainly through the gastrointestinal tract. The balance between total zinc absorption and endogenous intestinal excretion is the primary means of maintaining zinc homeostasis in animals [69]. Phytic acid and fibers in diet, bind to zinc in the gastrointestinal tract and limit its bio-availability, while proteins have a positive influence on absorption [70]. High dietary calcium intake [71], high dosage of iron [72], and cadmium level [73] are also reported to limit the bioavailability of zinc. A complex of zinc with ligands, chelators, amino acids, and organic acids, increases its solubility and bioavailability [74].
Zinc deficiency
Zinc deficiency occurs most frequently in vegetarians, the elderly, and individuals with chronic gut diseases, which cause malabsorption [75]. Inherited diseases like acrodermatitis enteropathica and cystic fibrosis, as well as a high intake of copper, iron, or phytic acid, cause reduced absorption of zinc [76]. Currently, almost 17% of the global population suffers from zinc deficiency [77], and most importantly, it is responsible for 4% of global child morbidity and mortality [78]. Zinc deficiency is represented by growth retardation, loss of appetite, and impaired immune function. In severe deficiency cases, hair loss, diarrhea, delayed sexual maturation, impotence, hypogonadism in males, eye and skin lesions have also been reported [79-81]. Thymic atrophy, lymphopenia, and defective lymphocyte responses, resulting in a compromised immune system, are common due to zinc deficiency [82]. Inadequate zinc intake also causes significant etiological changes like adolescent nutritional dwarfism, diarrhea, pneumonia, disturbed neurological performances, and abnormal fetal development [83]. Zinc deficiency has also been correlated with acute viral hepatitis, liver cirrhosis, reduced testosterone and progesterone levels, and other reproductive abnormalities [76]. Other symptoms of zinc deficiency are weight loss, difficulty in wound healing, taste deviations, and mental fatigue [84-86]. In the absence of biomarkers to establish physiological zinc status, the early stages of zinc insufficiency are rarely recognized [87]. Clinical symptoms of zinc deficiency can be present even in the absence of abnormal laboratory indices [79]. Thus, it is always essential to determine the plasma levels of zinc, which is also not very straightforward as free zinc levels are very low in serum or plasma. Generally, clinical factors such as digestive diseases and zinc deficiency symptoms are considered when determining the need for zinc supplementation.
Sources and daily recommended dose of zinc
Zinc is found in several plant and animal products. Some of the major food products include oysters, red meat, poultry, seafood, fortified breakfast cereals, beans, nuts, whole grains, and dairy products [88]. The average daily Recommended Daily Allowance (RDA) of zinc as defined by the US Institute of Medicine/Food and Nutrition board in the 2001 Dietary Reference Intakes (DRIs) is 11mg/day for men and 8mg/day for women [89]. The requirement is higher for vegetarians as zinc is not readily available from a vegetarian diet [79]. The pharmacological dose of zinc is equivalent to 40mg/day of elemental zinc, the tolerable upper intake level for zinc in adults. Zinc supplements are recommended to avoid nutrient imbalances and to manage diseases, wherein zinc may be used as an adjunct therapy [90].
Comparative bioavailability and efficacy of zinc supplements
Zinc is supplemented in humans as sulfate, acetate, gluconate, picolinate, histidine and methionine salts [91]. Very few studies involve a direct comparison of the bioavailability of different forms of zinc in humans. The important fact is that the form of zinc needs to become dissociated into zinc ions, which then bind to ligands (proteins) for transport [92]. The organic sources of zinc such as complexes of the metal with amino acids or organic acids, have been reported to meet the requirement at lower doses and have a better effect than inorganic salts [93-95].
In a randomized, double-masked, 3-way crossover study, watersoluble zinc salts gluconate, sulfate, and acetate were given as a supplement in 15 healthy adults. This study showed that zinc citrate and gluconate had comparable absorption, which was significantly higher than zinc oxide [96]. The absorption of zinc from zinc methionine, zinc sulfate, and zinc polysorbate either in a water solution or added to a standard meal was compared in nine adults in another study. The plasma levels of zinc were significantly higher with zinc methionine and polyascorbate than zinc sulfate. Supplementation with meal reduced the absorption of all the forms of zinc [91,97]. In one study, the comparative absorption of zinc after oral administration of zinc picolinate, zinc citrate and zinc gluconate were studied in 15 healthy human volunteers in a double-blind four-period crossover design. Zinc levels significantly increased in hair, urine and erythrocytes at the end of 4 weeks following oral supplementation of zinc picolinate but not with the citrate and gluconate forms suggesting picolinate form may have better bioavailability [98]. Zinc chelated with methionine was also found to have better bioavailability compared to zinc oxide and zinc polysaccharides in beagle dogs [97]. The higher bioavailability of zinc methionine over other zinc sources is attributed to the stable methionine complex, which is preferentially transported into tissues compared to other amino acids. It is reported to be a more potent antioxidant than vitamin E, vitamin C, β-carotene, and 4-6 times more effective than other zinc salts such as oxide and sulfate, citrate, gluconate, and picolinate [99,100]. Compared to polyascorbate and sulfate, zinc methionine has 16% better absorption capability (Figure 2) [91]. These studies suggest that organic source of zinc is better absorbed compared to zinc salts.
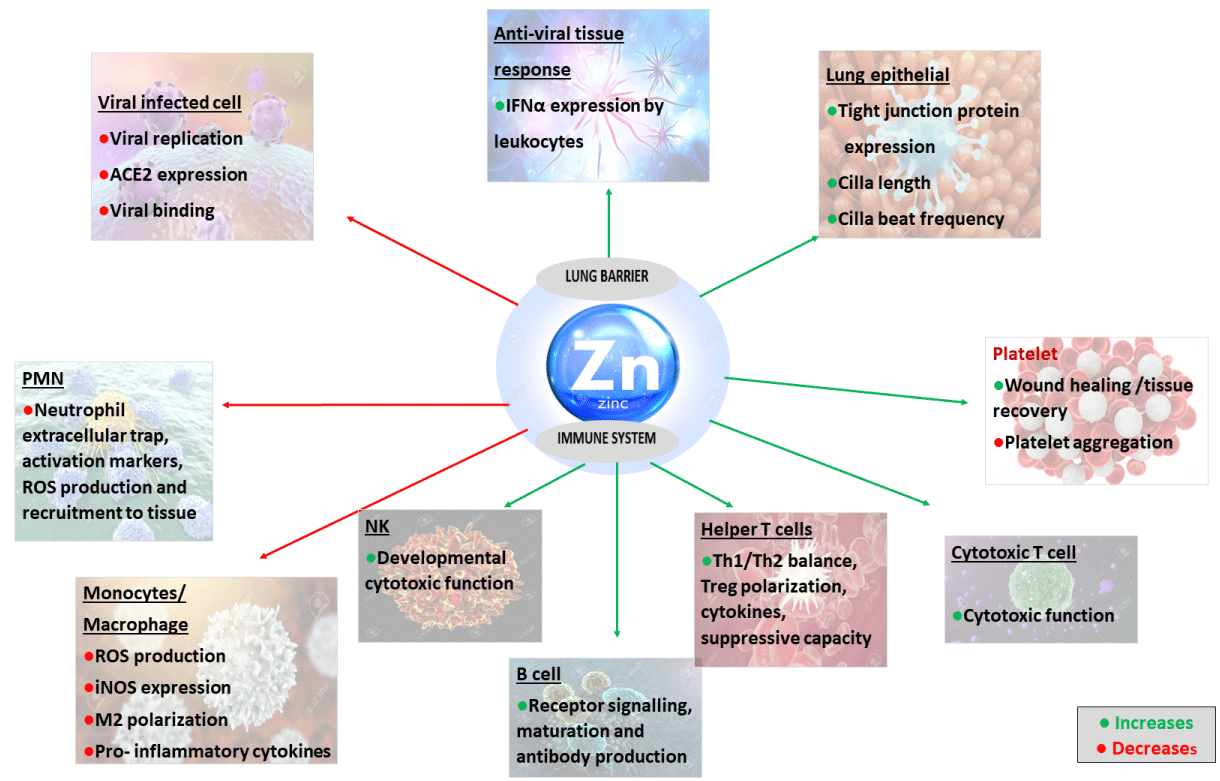
Figure 1: Role of Zinc in immune response and antiviral activity: Zinc supplementation may prevent viral entry, suppress viral replication and improve the antiviral
immune response.
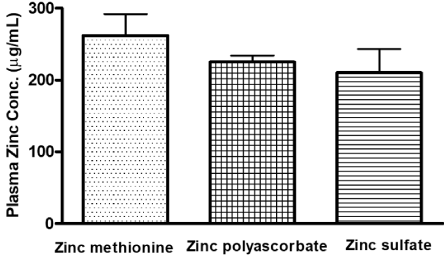
Figure 2: Comparative bioavailability study of various Zinc salts [91].
Corroborating these studies, the efficacy of organic zinc was also reported to be better than zinc salts. Shrimp fed diets with organic zinc supplementation (methionine, lysine and glycine chelates) produced significantly higher growth, survival and immune parameters than ZnSO4 treatment [101]. Zinc nicotinate, an organic source, was significantly better than zinc carbonate salt in improving growth performance, hematology, serum biochemical constituents oxidative stress, and immunity in rats [102]. Similar results were observed in sheep supplemented with zinc methionine, which showed improved growth, energy balance, and gastrointestinal development [103].
Clinical Studies with Zinc Supplements
Zinc citrate
Zinc citrate has been widely studied in the improvement of oral health. In a crossover clinical study, zinc citrate dentifrice showed a 24-52% reduction in anaerobic bacteria and streptococci compared to control formulation after 14 days. The zinc citrate dentifrice could reduce biofilm formation and also significantly reduce anaerobic bacteria and streptococci, five hours post brushing compared to control [104]. In another clinical study, the use of zinc citrate dentifrice for 6 months showed a statistically significant (50.2%) reduction in severe plaque and severe gingivitis (66.7%) reduction in over the control dentifrice [105].
Zinc gluconate
Zinc gluconate supplementation for three months showed efficacy in reducing acne in a multicenter randomized, double-blind trial in comparison with minocycline [106]. In a randomized, double-blind, placebo-controlled trial, of zinc gluconate supplementation in elderly subjects for 12 months, the incidence of infections, TNF-a levels and plasma oxidative stress markers were significantly lower with zinc gluconate supplementation than placebo, suggesting its efficacy in immune modulation [107]. In another study, zinc gluconate supplementation for eight weeks significantly reduced the levels of hs CRP and IL-6 in serum compared to placebo, suggesting a favorable effect on obesity-related inflammation in young adults [108]. Zinc gluconate supplementation improved nutritional status and clinical outcome in patients with ulcerative colitis, reinforcing zinc’s role as an important dietary component in disease control [109]. Zinc gluconate administration prior to allergen exposure significantly decreased the neutrophil infiltration and TNF-a release into the airways in mice. Zinc supplementation reduced airway hyperresponsiveness and serum IgE levels, although Th2 cytokine expression was not affected [110]. Dietary supplementation with zinc gluconate for three months effectively reduced respiratory morbidity in preschool children, suggesting that zinc gluconate supplements can positively influence response to infection and build immunity [111].
Zinc acetate
Zinc acetate was evaluated for the reduction in symptoms of cold and respiratory infections in clinical studies. Compared to placebo, zinc acetate supplemented individuals had a shorter mean overall duration of cold symptoms, cough, nasal discharge, and overall severity [112]. In a controlled trial, children in the age group of 2-24 months were treated with zinc acetate either alone or in combination with vitamin A. Recovery from illness severity and fever significantly better in zinc acetate treated boys compared to placebo control [113]. In another study, zinc acetate and chlorhexidine diacetate mouth rinse showed long-term efficacy against intra-oral halitosis than placebo mouth rinse [114].
Zinc picolinate
Zinc picolinate is an organic supplement wherein zinc atom is attached to a picolinic acid molecule. While the supplement is believed to increase bioavailability, scientific literature to support the claim is limited. In a clinical study, patients with COPD were supplemented with zinc picolinate for eight weeks, significantly increasing mean antioxidant (superoxide dismutase) and zinc levels. However, no significant change in levels of forced expiratory volume in one second (FEV1) and the ratio of FEV1 and Forced Vital Capacity (FVC), FEV1/FVC (%) parameters was observed after zinc supplementation [115].
Zinc monomethionine
Zinc methionine is a complex of zinc with DL- or L-methionine. The amino acid methionine is one of the essential amino acids for humans and a free radical scavenger due to the presence of sulfur atom. It is involved in the production of S-adenosyl methionine, L cysteine, and glutathione, which are involved in maintaining the cellular redox state. Dietary methionine and cysteine are important to ensure the health of the intestine and immune function. Unlike other zinc supplements, the methionine form does not remove iron from cells, causing anemia or increasing lead absorption [116].
Zinc methionine acts as a potent free radical scavenger and inhibitor of oxidative stress and cellular injury. Zinc methionine was shown to resist binding with dietary fiber and phytate, which usually inhibits zinc absorption. Compared to other salts, zinc methionine showed higher inhibition of superoxides (Figure 3a) and hydroxyl free radicals (Figure 3b) [99]. The antioxidant activity of zinc methionine was reported to be comparable to vitamins E, C, and β-carotene, and significantly more than other zinc salts.
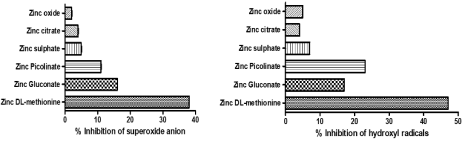
Figure 3: Inhibition of a) superoxide anion and b) hydroxyl radicals by Zinc methionine in comparison to other salts at 50μM concentration [99].
Zinc methionine supplemented to 48 patients orally for three months showed improvement in the global acne count. 80-100 % improvement was observed in 78% (38/48) patients. A significant reduction in pustules, papules and closed comedones was reported in the study [117]. Zinc methionine as a dietary supplement in laying hens showed positive effects on the zinc status of liver, duodenum, jejunum, intestinal morphology, and metallothionein mRNA expression [118]. A 24 week, randomized controlled study on 27 client-owned dogs with chronic Canine Atopic Dermatitis (CAD) receiving zinc methionine showed a significant decrease in Canine Atopic Dermatitis Lesion Index (CADLI) and pruritus Visual Analog Scale (VAS) [119]. Better bioavailability of Cu-and Zn- in gut absorption value, plasma, and liver tissue was observed in female sheep with Cu- and Zn-methionine supplementation compared to Cu- and Zn-sulphate [120]. In a recent clinical study, a phytomineral supplement containing zeaxanthin, lutein, piperine, extracts of bilberry and saffron, in combination with zinc monomethionine, maintained eye health and stalled further progression of early-stage dryness in patients with age-related macular degeneration [121].
Current status and future perspectives
Zinc has proven antiviral and immune-boosting activities and is considered as a prophylactic or adjunct therapy for SARS-CoV-2 infection. Several clinical trials using zinc as either a prophylactic or adjunct therapy are being carried out in different parts of the world, highlighting this mineral’s relevance for COVID-19. Several researchers have speculated the role of zinc in COVID-19 pathogenesis and prophylaxis. Table 2 lists the publications related to the role of zinc in COVID-19.
S.No
Title
Reference
1
Zinc for the prevention and treatment of SARS-CoV-2 and other acute viral respiratory infections: a rapid review.
[133]
2
Zinc supplementation for males during COVID-19: Is it beneficial.
[134]
3
Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients.
[122]
4
Targeting zinc metalloenzymes in coronavirus disease 2019.
[135]
5
Treatment of SARS-CoV-2 with high dose oral zinc salts: A report on four patients.
[123]
6
Treatment with Zinc is Associated with Reduced In-Hospital Mortality Among COVID-19 Patients: A Multi-Center Cohort Study
[136]
7
Association Between Low Zinc Levels and Severity of Acute Respiratory Distress Syndrome by New Coronavirus (SARS-CoV-2).
[137]
8
Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker.
[138]
9
Zinc against COVID-19? Symptom surveillance and deficiency risk groups.
[139]
10
COVID-19: Poor outcomes in patients with zinc deficiency.
[124]
11
Investigate Oral Zinc as a Prophylactic Treatment for Those at Risk for COVID-19.
[140]
12
Zinc and COVID-19: Basis of Current Clinical Trials.
[141]
13
Randomised controlled trial for high-dose intravenous zinc as adjunctive therapy in SARS-CoV-2 (COVID-19) positive critically ill patients: trial protocol.
[142]
14
Can Zn Be a Critical Element in COVID-19 Treatment?
[143]
15
Possible role of zinc (Zn) as an adjunctive therapy in gastrointestinal symptoms of COVID-19 infectious disease.
[144]
16
Do Zinc Supplements Enhance the Clinical Efficacy of Hydroxychloroquine?: a Randomized, Multicenter Trial.
[145]
Table 2: Role of Zinc in COVID-19.
So far, one clinical study has shown better outcome in patients supplemented with zinc and one case study reports beneficial effects of zinc supplementation for COVID-19 progression [122,123]. Complications associated with COVID-19 infection were found to be higher in patients with lower levels of zinc compared to healthy individuals [124]. Although we may not yet understand the optimum level of zinc supplementation for COVID-19 infection, it is well established that zinc levels are important to develop resistance against the infection and positively influence the immune system. Thus, it is imperative to choose the right supplement with a proven safety record. Organic source of zinc appears to be better absorbed compared to zinc salts. Zinc as gluconate, citrate, and methionine chelates are extensively studied. Surprisingly, although Zinc picolinate is widely used as a supplement with greater bioavailability claims, there are very few scientific studies to back this claim. The methionine form of Zinc (Zinc monomethionine) may be the preferred supplement due to its superior bioavailability. Methionine is involved in the synthesis of several essential hormones and growth factors and is the methyl donor in biological reactions in the form of S-adenosyl methionine. Another advantage could be that its least affected by diet composition and does not affect the iron absorption. Robust immune system and resistance to infection are the need of the hour and zinc supplements may be the answer to tackle the pandemic positively. These strategies will definitely help humanity in facing the future emergence of pandemic infections.
Conclusions
The current pandemic of SARS-CoV-2 infection has prompted researchers to look for essential nutrient supplements with antiviral properties and induce an effective immune response. Although randomized controlled studies on the effect of zinc supplements on SARS-CoV2 infection are minimal, several trials are being planned and few are ongoing. Evidence from literature, strongly suggests that zinc supplementation may be highly beneficial in reducing the severity and morbidity associated with the infection. Zinc supplements are cost effective and are simple options to respond to oxidative stress, uncontrolled inflammation and infection caused by the virus. Choosing the right supplement for the population at risk may be highly helpful for tackling the pandemic more effectively.
Acknowledgment
The authors acknowledge the scientific writing team of Sabinsa corporation and Sami Labs Limited for their help in preparing the manuscript.
Conflicts of Interest
All the authors are affiliated with Sami-Sabinsa Group Limited or Sabinsa Corporation.
References
- WHO/FAO. Vitamin and mineral requirements in human nutrition: report of a joint FAO/WHO expert consultation. 2 edition. Bangkok, Thailand: Geneva: FAO/WHO. 1998; 230-245.
- King JC. Zinc: an essential but elusive nutrient. Am J Clin Nutr. 2011; 94: 679S-684S.
- Roohani N, Hurrell R, Kelishadi R, Schulin R. Zinc and its importance for human health: An integrative review. J Res Med Sci. 2013; 18: 144-157.
- Mammadova-Bach E, Braun A. Zinc Homeostasis in Platelet-Related Diseases. International Journal of Molecular Sciences. 2019; 20: 5258.
- Kambe T, Tsuji T, Hashimoto A, Itsumura N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol Rev. 2015; 95: 749-784.
- White JV, Guenter P, Jensen G, Malone A, Schofield M. Consensus Statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: Characteristics Recommended for the Identification and Documentation of Adult Malnutrition (Undernutrition). Journal of the Academy of Nutrition and Dietetics. 2012; 112: 730-738.
- Updated rapid risk assessment from ECDC on the novel coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2020; 25.
- Wessels I, Rink L. Micronutrients in autoimmune diseases: possible therapeutic benefits of zinc and vitamin D. J Nutr Biochem. 2020; 77: 108240.
- Wessels I, Maywald M, Rink L. Zinc as a Gatekeeper of Immune Function. Nutrients. 2017; 9: 1286.
- Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020; 7: 11.
- Zhou Y, Zeng Z, Xu Y, Ying J, Wang B, Majeed M, et al. Application of Bacillus coagulans in Animal Husbandry and Its Underlying Mechanisms. Animals (Basel). 2020; 10: 454.
- van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020; 382: 1564-1567.
- Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020.
- Galimberti S, Baldini C, Barate C, Ricci F, Balducci S, Grassi S, et al. The CoV-2 outbreak: how hematologists could help to fight Covid-19. Pharmacological research. 2020; 157: 104866.
- Yazdanpanah F, Hamblin MR, Rezaei N. The immune system and COVID-19: Friend or foe? Life sciences. 2020; 256: 117900.
- Zhang L, Liu Y. Potential interventions for novel coronavirus in China: A systematic review. Journal of medical virology. 2020; 92: 479-490.
- Iddir M, Brito A, Dingeo G, Fernandez Del Campo SS, Samouda H, La Frano MR, et al. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients. 2020; 12: 1562.
- Wintergerst ES, Maggini S, Hornig DH. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab. 2006; 50: 85-94.
- Barocas JA, So-Armah K, Cheng DM, Lioznov D, Baum M, Gallagher K, et al. Zinc deficiency and advanced liver fibrosis among HIV and hepatitis C co-infected anti-retroviral naïve persons with alcohol use in Russia. PloS one. 2019; 14: e0218852.
- Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019- nCoV) in vitro. Cell research. 2020; 30: 269-271.
- Xue J, Moyer A, Peng B, Wu J, Hannafon BN, Ding WQ. Chloroquine is a zinc ionophore. PloS one. 2014; 9: e109180.
- Wessels I, Rolles B, Rink L. The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis. Frontiers in Immunology. 2020; 11: 1712.
- Rahman MT, Idid SZ. Can Zn Be a Critical Element in COVID-19 Treatment? Biological trace element research. 2020: 1-9.
- Jothimani D, Kailasam E, Danielraj S, Nallathambi B, Ramachandran H, Sekar P, et al. COVID-19: Poor outcomes in patients with zinc deficiency. Int J Infect Dis. 2020; 100: 343-349.
- Chilvers MA, McKean M, Rutman A, Myint BS, Silverman M, O’Callaghan C. The effects of coronavirus on human nasal ciliated respiratory epithelium. Eur Respir J. 2001; 18: 965-970.
- Roscioli E, Jersmann HP, Lester S, Badiei A, Fon A, Zalewski P, et al. Zinc deficiency as a codeterminant for airway epithelial barrier dysfunction in an ex vivo model of COPD. International journal of chronic obstructive pulmonary disease. 2017; 12: 3503-3510.
- Wessels I, Pupke JT, von Trotha KT, Gombert A, Himmelsbach A, Fischer HJ, et al. Zinc supplementation ameliorates lung injury by reducing neutrophil recruitment and activity. Thorax. 2020; 75: 253-261.
- Bao S, Knoell DL. Zinc modulates cytokine-induced lung epithelial cell barrier permeability. American journal of physiology Lung cellular and molecular physiology. 2006; 291: L1132-L1141.
- Novick SG, Godfrey JC, Pollack RL, Wilder HR. Zinc-induced suppression of inflammation in the respiratory tract, caused by infection with human rhinovirus and other irritants. Medical hypotheses. 1997; 49: 347-357.
- Boudreault F, Pinilla-Vera M, Englert JA, Kho AT, Isabelle C, Arciniegas AJ, et al. Zinc deficiency primes the lung for ventilator-induced injury. JCI insight. 2017; 2: e86507.
- Rosenkranz E, Metz CH, Maywald M, Hilgers RD, Weßels I, Senff T, et al. Zinc supplementation induces regulatory T cells by inhibition of Sirt- 1 deacetylase in mixed lymphocyte cultures. Molecular nutrition & food research. 2016; 60: 661-6371.
- Uchide N, Ohyama K, Bessho T, Yuan B, Yamakawa T. Effect of antioxidants on apoptosis induced by influenza virus infection: inhibition of viral gene replication and transcription with pyrrolidine dithiocarbamate. Antiviral Res. 2002; 56: 207-217.
- Krenn BM, Gaudernak E, Holzer B, Lanke K, Van Kuppeveld FJ, Seipelt J. Antiviral activity of the zinc ionophores pyrithione and hinokitiol against picornavirus infections. J Virol. 2009; 83: 58-64.
- te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010; 6: e1001176.
- Read SA, Obeid S, Ahlenstiel C, Ahlenstiel G. The Role of Zinc in Antiviral Immunity. Adv Nutr. 2019; 10: 696-710.
- Suara RO, Crowe JE. Effect of zinc salts on respiratory syncytial virus replication. Antimicrobial agents and chemotherapy. 2004; 48: 783-790.
- Cai H, Zhang Y, Ma Y, Sun J, Liang X, Li J. Zinc binding activity of human metapneumovirus M2-1 protein is indispensable for viral replication and pathogenesis in vivo. J Virol. 2015; 89: 6391-6405.
- Overbeck S, Rink L, Haase H. Modulating the immune response by oral zinc supplementation: a single approach for multiple diseases. Archivum immunologiae et therapiae experimentalis. 2008; 56: 15-30.
- Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011; 472: 481-485.
- Prasad AS. Effects of zinc deficiency on Th1 and Th2 cytokine shifts. The Journal of infectious diseases. 2000; 182: S62-S68.
- Haase H, Rink L. The immune system and the impact of zinc during aging. Immun Ageing. 2009; 6: 9.
- Haase H, Rink L. The immune system and the impact of zinc during aging. Immunity & Ageing. 2009; 6: 9.
- Gammoh NZ, Rink L. Zinc in Infection and Inflammation. Nutrients. 2017; 9: 624.
- Rosenkranz E, Maywald M, Hilgers RD, Brieger A, Clarner T, Kipp M, et al. Induction of regulatory T cells in Th1-/Th17-driven experimental autoimmune encephalomyelitis by zinc administration. J Nutr Biochem. 2016; 29: 116- 123.
- Kitabayashi C, Fukada T, Kanamoto M, Ohashi W, Hojyo S, Atsumi T, et al. Zinc suppresses Th17 development via inhibition of STAT3 activation. Int Immunol. 2010; 22: 375-386.
- Maywald M, Wang F, Rink L. Zinc supplementation plays a crucial role in T helper 9 differentiation in allogeneic immune reactions and non-activated T cells. J Trace Elem Med Biol. 2018; 50: 482-488.
- Nagulendran K, Ramalingam M, Vava Mohideen H. Preventive role of Cyperus rotundus rhizomes extract on age associated changes in glucose and lipids. Pharmacologyonline. 2007; 2.
- Boukaiba N, Flament C, Acher S, Chappuis P, Piau A, Fusselier M, et al. A physiological amount of zinc supplementation: effects on nutritional, lipid, and thymic status in an elderly population. Am J Clin Nutr. 1993; 57: 566- 572.
- Bae SN, Lee KH, Kim JH, Lee SJ, Park LO. Zinc induces apoptosis on cervical carcinoma cells by p53-dependent and -independent pathway. Biochem Biophys Res Commun. 2017; 484: 218-223.
- Himoto T, Hosomi N, Nakai S, Deguchi A, Kinekawa F, Matsuki M, et al. Efficacy of zinc administration in patients with hepatitis C virus-related chronic liver disease. Scand J Gastroenterol. 2007; 42: 1078-1087.
- Ibs KH, Rink L. Zinc-altered immune function. J Nutr. 2003;133: 1452s-1456s.
- Cakman I, Kirchner H, Rink L. Zinc supplementation reconstitutes the production of interferon-alpha by leukocytes from elderly persons. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 1997; 17: 469-472.
- Cheng ZJ, Shan J. 2019 Novel coronavirus: where we are and what we know. Infection. 2020; 48: 155-163.
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England). 2020; 395: 1033-1034.
- Wessels I, Haase H, Engelhardt G, Rink L, Uciechowski P. Zinc deficiency induces production of the proinflammatory cytokines IL-1β and TNFa in promyeloid cells via epigenetic and redox-dependent mechanisms. J Nutr Biochem. 2013; 24: 289-297.
- Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front Immunol. 2020; 11: 827.
- Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;5 5: 102763.
- Hönscheid A, Rink L, Haase H. T-lymphocytes: a target for stimulatory and inhibitory effects of zinc ions. Endocrine, metabolic & immune disorders drug targets. 2009; 9: 132-144.
- Kaltenberg J, Plum LM, Ober-Blöbaum JL, Hönscheid A, Rink L, Haase H. Zinc signals promote IL-2-dependent proliferation of T cells. European journal of immunology. 2010; 40: 1496-1503.
- Wen W, Su W, Tang H, Le W, Zhang X, Zheng Y, et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell discovery. 2020 ;6: 31.
- Rolles B, Maywald M, Rink L. Influence of zinc deficiency and supplementation on NK cell cytotoxicity. Journal of Functional Foods. 2018; 48: 322-328.
- Asdamongkol N, Phanachet P, Sungkanuparph S. Low plasma zinc levels and immunological responses to zinc supplementation in HIV-infected patients with immunological discordance after antiretroviral therapy. Jpn J Infect Dis. 2013; 66: 469-474.
- Ravaglia G, Forti P, Maioli F, Bastagli L, Facchini A, Mariani E, et al. Effect of micronutrient status on natural killer cell immune function in healthy freeliving subjects aged >/=90 y. Am J Clin Nutr. 2000; 71: 590-598.
- Mocchegiani E, Muzzioli M, Giacconi R, Cipriano C, Gasparini N, Franceschi C, et al. Metallothioneins/PARP-1/IL-6 interplay on natural killer cell activity in elderly: parallelism with nonagenarians and old infected humans. Effect of zinc supply. Mech Ageing Dev. 2003; 124: 459-468.
- Singh M, Das RR. Clinical potential of zinc in prophylaxis of the common cold. Expert Rev Respir Med. 2011; 5: 301-303.
- Singh M, Das RR. Zinc for the common cold. The Cochrane database of systematic reviews. 2011: CD001364.
- Bonaventura P, Benedetti G, Albarède F, Miossec P. Zinc and its role in immunity and inflammation. Autoimmunity Reviews. 2015; 14: 277-285.
- Cousins RJ. Absorption, transport, and hepatic metabolism of copper and zinc: special reference to metallothionein and ceruloplasmin. Physiol Rev. 1985; 65: 238-309.
- Hambidge M, Krebs NF. Interrelationships of key variables of human zinc homeostasis: relevance to dietary zinc requirements. Annu Rev Nutr. 2001; 21: 429-452.
- Hambidge KM, Miller LV, Krebs NF. Physiological requirements for zinc. Int J Vitam Nutr Res. 2011; 81: 72-78.
- Wood RJ, Zheng JJ. High dietary calcium intakes reduce zinc absorption and balance in humans. Am J Clin Nutr. 1997; 65: 1803-1809.
- Whittaker P. Iron and zinc interactions in humans. Am J Clin Nutr. 1998; 68: 442S-446S.
- Brzóska MM, Moniuszko-Jakoniuk J. Interactions between cadmium and zinc in the organism. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2001; 39: 967-980.
- Brnic M, Wegmüller R, Zeder C, Senti G, Hurrell RF. Influence of phytase, EDTA, and polyphenols on zinc absorption in adults from porridges fortified with zinc sulfate or zinc oxide. J Nutr. 2014; 144: 1467-1473.
- Krebs NF. Update on zinc deficiency and excess in clinical pediatric practice. Ann Nutr Metab. 2013; 62: 19-29.
- Chasapis CT, Ntoupa P-SA, Spiliopoulou CA, Stefanidou ME. Recent aspects of the effects of zinc on human health. Archives of Toxicology. 2020; 94: 1443-1460.
- White JV, Guenter P, Jensen G, Malone A, Schofield M, Academy of N, et al. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012; 112: 730-738.
- Penny ME. Zinc Supplementation in Public Health. Annals of Nutrition and Metabolism. 2013; 62: 31-42.
- Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol. 2006; 20: 3-18.
- Prasad AS. Zinc deficiency: its characterization and treatment. Met Ions Biol Syst. 2004; 41: 103-137.
- Wang LC, Busbey S. Images in clinical medicine. Acquired acrodermatitis enteropathica. N Engl J Med. 2005; 352: 1121.
- Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998; 68: 447S-463S.
- Bhowmik D, Bhattacharjee C, Kumar S. A potential medicinal importance of zinc in human health and chronic disease. Int J Pharm Biomed Sci. 2010; 1: 5-11.
- Ploysangam A, Falciglia GA, Brehm BJ. Effect of marginal zinc deficiency on human growth and development. J Trop Pediatr. 1997; 43: 192-198.
- Nishi Y. Zinc and growth. J Am Coll Nutr. 1996; 15: 340-344.
- Heyneman CA. Zinc deficiency and taste disorders. Ann Pharmacother. 1996; 30: 186-187.
- King JC, Brown KH, Gibson RS, Krebs NF, Lowe NM, Siekmann JH, et al. Biomarkers of Nutrition for Development (BOND)-Zinc Review. The Journal of Nutrition. 2016; 146: 858S-885S.
- NUTTAB. 2010.
- Ruz M, Carrasco F, Rojas P, Basfi-fer K, Hernández MC, Pérez A. Nutritional Effects of Zinc on Metabolic Syndrome and Type 2 Diabetes: Mechanisms and Main Findings in Human Studies. Biological Trace Element Research. 2019; 188: 177-188.
- Santos HO, Teixeira FJ, Schoenfeld BJ. Dietary vs. pharmacological doses of zinc: A clinical review. Clin Nutr. 2020; 39: 1345-1353.
- Rosado JL, Muñoz E, López P, Allen LH. Absorption of zinc sulfate, methionine, and polyascorbate in the presence and absence of a plantbased rural mexican diet. Nutrition Research. 1993; 13: 1141-1151.
- Tubek S. Selected zinc metabolism parameters in premenopausal and postmenopausal women with moderate and severe primary arterial hypertension. Biol Trace Elem Res. 2007; 116: 249-256.
- Moghaddam HN, Jahanian R. Immunological Responses of Broiler Chicks Can Be Modulated by Dietary Supplementation of Zinc-methionine in Place of Inorganic Zinc Sources. Asian-Australas J Anim Sci. 2009; 22: 396-403.
- Swiatkiewicz S, Arczewska-Wlosek A, Józefiak D. The efficacy of organic minerals in poultry nutrition: review and implications of recent studies. World’s Poultry Science Journal. 2014; 70: 475-486.
- Nagalakshmi D, Sridhar K, Parashuramulu S. Replacement of inorganic zinc with lower levels of organic zinc (zinc nicotinate) on performance, hematological and serum biochemical constituents, antioxidants status, and immune responses in rats. Vet World. 2015; 8: 1156-1162.
- Wegmuller R, Tay F, Zeder C, Brnic M, Hurrell RF. Zinc absorption by young adults from supplemental zinc citrate is comparable with that from zinc gluconate and higher than from zinc oxide. J Nutr. 2014; 144: 132-136.
- Lowe JA, Wiseman J. A comparison of the bioavailability of three dietary zinc sources using four different physiologic parameters in dogs. J Nutr. 1998; 128: 2809S-2811S.
- Barrie SA, Wright JV, Pizzorno JE, Kutter E, Barron PC. Comparative absorption of zinc picolinate, zinc citrate and zinc gluconate in humans. Agents and Actions. 1987; 21: 223-228.
- Bagchi D, Bagchi M, Stohs SJ. Comparative in vitro oxygen radical scavenging ability of zinc methionine and selected zinc salts and antioxidants. General pharmacology. 1997; 28: 85-91.
- Bagchi D, Vuchetich PJ, Bagchi M, Tran MX, Krohn RL, Ray SD, et al. Protective effects of zinc salts on TPA-induced hepatic and brain lipid peroxidation, glutathione depletion, DNA damage and peritoneal macrophage activation in mice. General pharmacology. 1998; 30: 43-50.
- Lin S, Lin X, Yang Y, Li F, Luo L. Comparison of chelated zinc and zinc sulfate as zinc sources for growth and immune response of shrimp (Litopenaeus vannamei). Aquaculture. 2013; 406-407: 79-84.
- Nagalakshmi D, Sridhar K, Parashuramulu S. Replacement of inorganic zinc with lower levels of organic zinc (zinc nicotinate) on performance, hematological and serum biochemical constituents, antioxidants status, and immune responses in rats. Veterinary world. 2015; 8: 1156-1162.
- Jafarpour N, Khorvash M, Rahmani HR, Pezeshki A, Hosseini Ghaffari M. Dose–responses of zinc–methionine supplements on growth, blood metabolites and gastrointestinal development in sheep. Journal of Animal Physiology and Animal Nutrition. 2015; 99: 668-765.
- Sreenivasan PK, Furgang D, Markowitz K, McKiernan M, Tischio-Bereski D, Devizio W, et al. Clinical anti-microbial efficacy of a new zinc citrate dentifrice. Clinical Oral Investigations. 2008; 13: 195-202.
- Williams C, McBride S, Mostler K, Petrone DM, Simone AJ, Crawford R, et al. Efficacy of a dentifrice containing zinc citrate for the control of plaque and gingivitis: a 6-month clinical study in adults. Compendium of continuing education in dentistry (Jamesburg, NJ: 1995). 1998; 19: 4-15.
- Dreno B, Moyse D, Alirezai M, Amblard P, Auffret N, Beylot C, et al. Multicenter randomized comparative double-blind controlled clinical trial of the safety and efficacy of zinc gluconate versus minocycline hydrochloride in the treatment of inflammatory acne vulgaris. Dermatology (Basel, Switzerland). 2001; 203: 135-140.
- Prasad AS, Beck FW, Bao B, Fitzgerald JT, Snell DC, Steinberg JD, et al. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr. 2007; 85: 837-844.
- Kim J, Ahn J. Effect of zinc supplementation on inflammatory markers and adipokines in young obese women. Biol Trace Elem Res. 2014; 157: 101- 106.
- de Moura MSB, Soares NRM, Barros SÉ L, de Pinho FA, Silva TMC, Bráz DC, et al. Zinc gluconate supplementation impacts the clinical improvement in patients with ulcerative colitis. Biometals: an international journal on the role of metal ions in biology, biochemistry, and medicine. 2020; 33: 15-27.
- Morgan CI, Ledford JR, Zhou P, Page K. Zinc supplementation alters airway inflammation and airway hyperresponsiveness to a common allergen. Journal of inflammation (London, England). 2011; 8: 36.
- Sazawal S, Black RE, Jalla S, Mazumdar S, Sinha A, Bhan MK. Zinc Supplementation Reduces the Incidence of Acute Lower Respiratory Infections in Infants and Preschool Children: A Double-blind, Controlled Trial. Pediatrics. 1998; 102: 1-5.
- Prasad AS, Fitzgerald JT, Bao B, Beck FW, Chandrasekar PH. Duration of symptoms and plasma cytokine levels in patients with the common cold treated with zinc acetate. A randomized, double-blind, placebo-controlled trial. Annals of internal medicine. 2000; 133: 245-252.
- Mahalanabis D, Lahiri M, Paul D, Gupta S, Gupta A, Wahed MA, et al. Randomized, double-blind, placebo-controlled clinical trial of the efficacy of treatment with zinc or vitamin A in infants and young children with severe acute lower respiratory infection. Am J Clin Nutr. 2004; 79: 430-436.
- Erovic Ademovski S, Mårtensson C, Persson GR, Renvert S. The longterm effect of a zinc acetate and chlorhexidine diacetate containing mouth rinse on intra-oral halitosis-A randomized clinical trial. Journal of clinical periodontology. 2017; 44: 1010-1019.
- Kirkil G, Hamdi Muz M, Seçkin D, Sahin K, Küçük O. Antioxidant effect of zinc picolinate in patients with chronic obstructive pulmonary disease. Respiratory medicine. 2008; 102: 840-844.
- Chien XX, Zafra-Stone S, Bagchi M, Bagchi D. Bioavailability, antioxidant and immune-enhancing properties of zinc methionine. BioFactors (Oxford, England). 2006; 27: 231-244.
- Sardana K, Garg VK. An observational study of methionine-bound zinc with antioxidants for mild to moderate acne vulgaris. Dermatologic therapy. 2010; 23: 411-418.
- Li L, Li H, Zhou W, Feng J, Zou X. Effects of zinc methionine supplementation on laying performance, zinc status, intestinal morphology, and expressions of zinc transporters’ mRNA in laying hens. Journal of the Science of Food and Agriculture. 2019; 99: 6582-6588.
- McFadden RA, Heinrich NA, Haarstad AC, Tomlinson DJ. A doubleblinded, randomized, controlled, crossover evaluation of a zinc methionine supplement as an adjunctive treatment for canine atopic dermatitis. Veterinary dermatology. 2017; 28: 569-e138.
- Pal DT, Gowda NK, Prasad CS, Amarnath R, Bharadwaj U, Suresh Babu G, et al. Effect of copper- and zinc-methionine supplementation on bioavailability, mineral status and tissue concentrations of copper and zinc in ewes. J Trace Elem Med Biol. 2010; 24: 89-94.
- Majeed M, Majeed S, Nagabhushanam K. An Open-Label Pilot Study on Macumax Supplementation for Dry-Type Age-Related Macular Degeneration. Journal of medicinal food. 2020.
- Carlucci PM, Ahuja T, Petrilli C, Rajagopalan H, Jones S, Rahimian J. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. J Med Microbiol. 2020; 69: 1228-1234.
- Finzi E. Treatment of SARS-CoV-2 with high dose oral zinc salts: A report on four patients. Int J Infect Dis. 2020; 99: 307-309.
- Jothimani D, Kailasam E, Danielraj S, Nallathambi B, Ramachandran H, Sekar P, et al. COVID-19: Poor outcomes in patients with zinc deficiency. Int J Infect Dis. 2020; 100: 343-349.
- Shah UH, Abu-Shaheen AK, Malik MA, Alam S, Riaz M, Al-Tannir MA. The efficacy of zinc supplementation in young children with acute lower respiratory infections: a randomized double-blind controlled trial. Clinical nutrition. 2013; 32: 193-199.
- Abdulhamid I, Beck FW, Millard S, Chen X, Prasad A. Effect of zinc supplementation on respiratory tract infections in children with cystic fibrosis. Pediatric pulmonology. 2008; 43: 281-287.
- Turner RB, Cetnarowski WE. Effect of treatment with zinc gluconate or zinc acetate on experimental and natural colds. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2000; 31: 1202-1208.
- Rerksuppaphol S, Rerksuppaphol L. A randomized controlled trial of zinc supplementation in the treatment of acute respiratory tract infection in Thai children. Pediatric reports. 2019; 11: 7954.
- Kurugöl Z, Akilli M, Bayram N, Koturoglu G. The prophylactic and therapeutic effectiveness of zinc sulphate on common cold in children. Acta paediatrica (Oslo, Norway: 1992). 2006; 95: 1175-1181.
- Hasanzadeh Kiabi F, Alipour A, Darvishi-Khezri H, Aliasgharian A, Emami Zeydi A. Zinc Supplementation in Adult Mechanically Ventilated Trauma Patients is Associated with Decreased Occurrence of Ventilator-associated Pneumonia: A Secondary Analysis of a Prospective, Observational Study. Indian journal of critical care medicine: peer-reviewed, official publication of Indian Society of Critical Care Medicine. 2017; 21: 34-39.
- Malik A, Taneja DK, Devasenapathy N, Rajeshwari K. Zinc supplementation for prevention of acute respiratory infections in infants: a randomized controlled trial. Indian pediatrics. 2014; 51: 780-784.
- Martinez-Estevez NS, Alvarez-Guevara AN, Rodriguez-Martinez CE. Effects of zinc supplementation in the prevention of respiratory tract infections and diarrheal disease in Colombian children: A 12-month randomised controlled trial. Allergologia et immunopathologia. 2016; 44: 368-375.
- Arentz S, Hunter J, Yang G, Goldenberg J, Beardsley J, Myers SP, et al. Zinc for the prevention and treatment of SARS-CoV-2 and other acute viral respiratory infections: a rapid review. Adv Integr Med. 2020; 7: 252-260.
- Ateya AM, Sabri NA. Zinc supplementation for males during COVID-19: Is it beneficial? Med Hypotheses. 2021; 146: 110403.
- Doboszewska U, Wlaz P, Nowak G, Mlyniec K. Targeting zinc metalloenzymes in coronavirus disease 2019. Br J Pharmacol. 2020; 177: 4887-4898.
- Frontera JA, Rahimian JO, Yaghi S, Liu M, Lewis A, de Havenon A, et al. Treatment with Zinc is Associated with Reduced In-Hospital Mortality Among COVID-19 Patients: A Multi-Center Cohort Study. Res Sq. 2020.
- Goncalves TJM, Goncalves S, Guarnieri A, Risegato RC, Guimaraes MP, de Freitas DC, et al. Association Between Low Zinc Levels and Severity of Acute Respiratory Distress Syndrome by New Coronavirus (SARS-CoV-2). Nutr Clin Pract. 2020.
- Heller RA, Sun Q, Hackler J, Seelig J, Seibert L, Cherkezov A, et al. Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker. Redox Biol. 2021; 38: 101764.
- Joachimiak MP. Zinc against COVID-19? Symptom surveillance and deficiency risk groups. PLoS Negl Trop Dis. 2021; 15: e0008895.
- McPherson SW, Keunen JE, Bird AC, Chew EY, van Kuijk FJ. Investigate Oral Zinc as a Prophylactic Treatment for Those at Risk for COVID-19. Am J Ophthalmol. 2020; 216: A5-A6.
- Pal A, Squitti R, Picozza M, Pawar A, Rongioletti M, Dutta AK, et al. Zinc and COVID-19: Basis of Current Clinical Trials. Biol Trace Elem Res. 2020.
- Perera M, El Khoury J, Chinni V, Bolton D, Qu L, Johnson P, et al. Randomised controlled trial for high-dose intravenous zinc as adjunctive therapy in SARS-CoV-2 (COVID-19) positive critically ill patients: trial protocol. BMJ Open. 2020; 10: e040580.
- Rahman MT, Idid SZ. Can Zn Be a Critical Element in COVID-19 Treatment? Biol Trace Elem Res. 2021; 199: 550-558.
- Abbasinazari M. Possible role of zinc (Zn) as an adjunctive therapy in gastrointestinal symptoms of COVID-19 infectious disease. Gastroenterol Hepatol Bed Bench. 2020; 13: 417-418.
- Abd-Elsalam S, Soliman S, Esmail ES, Khalaf M, Mostafa EF, Medhat MA, et al. Do Zinc Supplements Enhance the Clinical Efficacy of Hydroxychloroquine?: a Randomized, Multicenter Trial. Biol Trace Elem Res. 2020.