
Research Article
Austin J Nutr Metab. 2021; 8(3): 1112.
Efficient Production of Propionic Acid in the Fed-Batch Fermentation of Propionibacterium acidipropionici and Its Metabolic Flux Analysis
Zhang Y, Zhang K, Li X, Wang Z*, Wang Y and Su Z
State Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing, China
*Corresponding author: Ziqiang Wang, State Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
Received: July 24, 2021; Accepted: August 30, 2021; Published: September 06, 2021
Abstract
To improve the fermentation efficiency of Propionibacterium acidipropionici, a simplified metabolic network was established to provide theoretical guidance for medium optimization and process regulation. The effect of glucose and glycerol on propionic acid production and metabolic flux distribution was investigated and the combination of glucose and glycerol was optimized. The results showed that the productivity of propionic acid could be improved by enhancing the synthesis of pyruvate and its flux distribution to the oxaloacetate branch. Finally, the scaled-up fed-batch fermentation of P. acidipropionici was conducted. The concentration of propionic acid reached 51.75 ± 3.62g/L with a glucose/glycerol ratio of 4:1, an improvement of 79.25% relative to the use of glucose alone, and the corresponding productivity and yield were 0.39g/(L· h) and 0.52g/g, respectively. Therefore, the combination of glucose and glycerol significantly improved the productivity of propionic acid and provides a new strategy for industrial production.
Keywords: Propionibacterium acidipropionici; Propionic acid; Combination of glucose and glycerol; Metabolic flux analysis; Scaled-up fed-batch fermentation
Introduction
Propionic acid is a natural organic weak acid and can be widely found in animals, plants, and microorganisms. As an important platform compound, propionic acid is used across a wide range of industries in the manufacture of polymers, pesticides, perfumes, and pharmaceuticals [1]. Moreover, propionic acid and its salts (e.g., calcium propionate, zinc propionate, potassium propionate) can effectively inhibit molds, Bacillus, Aerobacter, and other microorganisms, and are widely used as excellent preservatives in grain, feed, and food processing [2].
Generally, propionic acid is produced via non-sustainable petrochemical routes, raising concerns about its long-term sustainability. Therefore, the production of propionic acid by microbial fermentation has attracted widespread attention [3]. In particular, bio-based propionic acid or its calcium salt is considered as a green, safe, and pollution-free natural food antifungal agent approved by the World Health Organization and the Food and Agriculture Organization of the United Nations [4].
Propionibacteria are gram-positive facultative anaerobic bacteria that have been granted “generally recognized as safe” status by the US Food and Drug Administration, and include Propionibacterium freudenreichii, Propionibacterium shermanii, and Propionibacterium acidipropionici [5]. They are widely used in the fermentation production of propionic acid in processes that suffer from endproduct feedback inhibition and the formation of by-products such as acetic acid and succinic acid [6,7]. To overcome these limitations, considerable effort has been expended on propionic acid production, including metabolic engineering to enhance the producer strains. This work has proven difficult because of the imbalance and blindness at the systems level [8-10]. Consequently, traditional strategies like process regulation are still crucial for industrial production [11,12]. Among these approaches to improve the fermentation efficiency, in situ product removal (ISPR) has attracted widespread attention. Various ISPR processes have been developed, such as the in situ cell retention reactor [13], plant fibrous-bed bioreactor [14-16], and PEI-Poraver immobilized bioreactor [17], which not only remove propionic acid to effectively overcome the feedback inhibition effect, but also help to achieve the semi-continuous fermentation process. However, all these approaches require increased investment in equipment, thereby further increasing product costs and reducing competitiveness with petrochemical methods.
Another effective approach to reducing the production cost of propionic acid is to identify a low-cost renewable feedstock, especially for the industrial production process. Glycerol, an abundant renewable by-product of the biodiesel industry, has been utilized in the fermentation process of P. acidipropionici [18,19]. Glycerol can promote the production of propionic acid and reduce the formation of by-products because of its high degree of reduction [20]. However, glycerol slows down cell growth because of metabolic imbalance when used as the sole carbon source, so a combination of glucose and glycerol is necessary to maintain high fermentation efficiency [21,22].
In addition, metabolic flux is one of the basic parameters of cell physiology. It can provide theoretical guidance for medium optimization and process regulation by analyzing the intracellular metabolic flux distribution of key nodes related to product synthesis. Therefore, to improve the substrate conversion rate and propionic acid fermentation efficiency, it is necessary to analyze the metabolic flux distribution in P. acidipropionici. In this study, a simplified metabolic network of propionic acid production in P. acidipropionici was established based on the existing biochemical reaction steps and metabolic model. Then the effect of glucose and glycerol on propionic acid production and metabolic flux distribution was analyzed and the optimal combination of glucose and glycerol was determined based on the actual demands of industrial production. Finally, scaledup fed-batch fermentation of P. acidipropionici was conducted to evaluate the potential industrial application of the process.
Materials and Methods
Microorganism and medium
Propionibacterium acidipropionici CGMCC 1.2230 was obtained from the China General Microbiological Culture Collection Center (Beijing, China). The stock culture was incubated in deep agar slants at 30oC, stored at 4oC, and transferred to fresh agar monthly. For long-term preservation, the stock was stored in a freezer at -80oC.
The inoculum consisted of glucose (35g/L), corn steep liquor (21g/L), (NH4)2SO4 (5g/L), KH2PO4 (4g/L), and distilled water (pH 6.8-7.0). The fermentation medium was composed of glucose (60g/L), corn steep liquor (41g/L), KH2PO4 (4.6g/L), and distilled water (pH 6.8-7.0). For medium preparation, glucose was autoclaved separately.
Corn steep liquor was purchased from Lingshanhe Plant Protein Manufacturing (Xingtai, Hebei, China). Glucose and inorganic salts were analytical grade and produced by Shanghai Macklin Biochemical (Shanghai, China).
Fermentation of P. acidipropionici and carbon source optimization
P. acidipropionici was deposited on a deep agar slant and activated previously at 30oC for 24h. One loopful of culture from the activated deep agar slant was inoculated aseptically to 50mL of inoculum and cultivated statically at 30oC for 48h. Then the culture was inoculated to 500mL fermentation medium and cultivated sequentially for 132h. During the fermentation, the pH was maintained at pH 7.0 by the manual addition of NH3·H2O. The concentrations of glucose, glycerol, propionic acid, acetic acid, and succinic acid were determined every 12h.
For carbon source optimization, the fermentation process of P. acidipropionici with glucose or glycerol as the sole carbon source was analyzed, respectively. Then the combination of glucose and glycerol was used as the mixed carbon source and the ratio of glucose to glycerol was optimized to improve the productivity of propionic acid.
Fed-batch fermentation of P. acidipropionici and scaledup
After optimization of the fermentation medium, the fed-batch fermentation process of P. acidipropionici was established. The inoculum was inoculated to the fermentation medium in a 7-L fermenter with an inoculation of 5-10 %, and was cultivated at 30oC with mixing at 50rpm. During the fermentation, no sterile air was required, and the pH was maintained at pH 7.0 by the automatic addition of NH3·H2O. 500g/L glucose was fed intermittently to maintain cell growth and product synthesis when its concentration fell below 10g/L. The total carbon source concentration was 100g/L, of which 48g/L glucose and 12g/L glycerol were in the fermentation medium; 40g/L glucose was added intermittently during the feeding process. Based on these, the 150-L fed-batch fermentation of P. acidipropionici was carried out (Sartorius stedim).
Establishment of metabolic flux balance model of propionic acid
According to the literature, the metabolic processes of P. acidipropionici are very complex, and include glycolysis, pentose phosphate pathway, Wood-Werkman pathway, succinate synthesis, lactate synthesis, and acetate synthesis, as shown in Figure 1. To facilitate analysis and calculation, the simplified ideal metabolic model of propionic acid and its related metabolites in P. acidipropionici was established based on the following principles (Su et al., 2016; Antoniewicz, 2020): (1) metabolic flux analysis was based on the pseudo-steady-state assumption that P. acidipropionici cells were in a non-growth period and the growth rate was ignored; (2) the tricarboxylic acid cycle and glyoxylate pathway were ignored; (3) the reaction carried out in a fixed proportion and the intermediate reaction without branch points was simplified into a reaction equation; and (4) in the cell growth arrest stage, the total cell maintenance energy and the consumption of ATP were not equal because of the existence of many invalid cycles. Thus, the balance of total ATP was not considered. The established metabolic network of propionic acid and its related metabolites in P. acidipropionici with glucose and glycerol as the mixed carbon source included 28 reaction equations, as shown in Table 1.
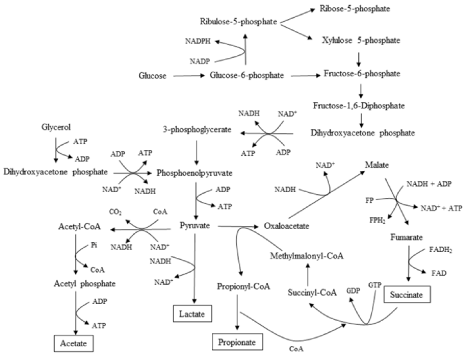
Figure 1: The metabolic pathway of propionic acid production in Propionibacterium acidipropionici.
Flux
Metabolic reactions
r1
Glucose + ATP → Glucose-6-phosphate + ADP + H+
r2
Glucose-6-phosphate → Fructose-6-phosphate
r3
Glycerol + ATP → Dihydroxyacetone phosphate + ADP+ H+
r4
Fructose-6-phosphate + ATP → Fructose-1,6-diphosphate + ADP + H+
r5
Fructose-1,6-diphosphate → Dihydroxyacetone phosphate + Glyceraldehyde-3-phosphate
r6
Glucose-6-phosphate + 2NADP + H2O → Ribulose-5-phosphate + 2NADPH +CO2
r7
Ribulose-5-phosphate→Xylulose 5-phosphate
r8
Ribulose-5-phosphate→5-phosphate ribose
r9
Xylulose 5-phosphate + 4-erythrose phosphate → Fructose-6-phosphate + Glyceraldehyde-3-phosphate
r10
Xylulose-5-phosphate + 5-phosphate-ribose → Glyceraldehyde-3-phosphate + 7-phosphate-sedoheptulose
r11
Glyceraldehyde-3-phosphate + 7-phosphate-sedoheptulose → Fructose-6-phosphate + 4-erythrosephosphate
r12
Dihydroxyacetone phosphate → Glyceraldehyde-3-phosphate
r13
Glyceraldehyde-3-phosphate + NAD+ + Pi →1,3-diphosphoglycerate + NADH+ H+
r14
1,3-diphosphoglycerate + ADP → 3-phosphoglycerate + ATP
r15
3-phosphoglycerate → 2-phosphoglycerate
r16
2-phosphoglycerate → Phosphoenolpyruvate + H2O
r17
Phosphoenolpyruvate + ADP +H+ → Pyruvate + ATP
r18
Pyruvate + NADH + H+ → Lactate + NAD+
r19
Pyruvate + CoA + NAD+→ Acetyl-CoA + CO2 +NADH
r20
Acetyl-CoA + ADP → Acetic acid + CoA + ATP
r21
Pyruvate + CO2 + ATP + H2O → Oxaloacetate + ADP + 2Pi + 2H+
r22
Oxaloacetate + NADH + H+→ L-malate + NAD+
r23
L-malic acid → Fumaric acid + H2O
r24
Fumaric acid + FADH2 → Succinic acid + FAD
r25
Succinic acid + ATP + CoA → Succinyl-CoA + Pi + ADP
r26
Succinyl-CoA → Methylmalonyl-CoA
r27
Methylmalonyl-CoA → Propionyl-CoA + CO2
r28
Propionyl-CoA + ADP → Propionic acid + CoA + ATP
Table 1: Metabolic reactions of propionic acid production by Propionibacterium acidipropionici.
According to the metabolic network and the stoichiometric balanced equation of each reaction, the relationship among the reaction rates was obtained [23,24]. It was assumed that the intermediate metabolites were in a pseudo-steady state such that their concentration change rates were zero. Based on the material balance law, the accumulation rate of metabolites can be calculated according to the following formula:
where ri(t) is the accumulation rate of intermediate metabolite i [mmol/(L·h)], rj(t) and rk(t) are the reaction rates of the j-th and k-th reactions of substance i [mmol/(L·h)], and aij and aik are the stoichiometric coefficients corresponding to each reaction. Each metabolite in the metabolic network can establish an equation based on this principle.
According to the pseudo-steady state assumption, ri(t) = 0. The m intermediate metabolites and n reactions in the metabolic network constituted m metabolic flux balance equations with a degree of freedom of F=n-m, as shown in Table 2. The overall metabolic flux distribution can be determined by measuring F reaction rates. When using glucose as the sole carbon source, the metabolic flux balance model of propionic acid established in this study included 27 reaction rates (j) and 25 equilibrium equations (k) with F = 2. When using glycerol as the sole carbon source, the metabolic flux balance model of propionic acid established in this study included 18 reaction rates (j) and 17 equilibrium equations (k) with F = 1.
Intermediate
Equations
Glucose-6-phosphate
r1-r2-r6=0
Fructose-6-phosphate
r2+r9+r11-r4=0
Fructose-1,6-diphosphate
r4-r5=0
Glyceraldehyde-3-phosphate
r5+r9+r10+r12-r11-r13=0
Ribulose-5-phosphate
r6-r7-r8=0
Xylulose 5-phosphate
r7-r9-r10=0
Ribose-5-phosphate
r8-r10=0
4-erythrose phosphate
r11-r9=0
7-phosphate-sedum heptulose
r10-r11=0
Dihydroxyacetone phosphate
r3+r5-r12=0
1,3-diphosphoglycerate
r13-r14=0
3-Phosphoglycerate
r14-r15=0
2-Phosphoglycerate
r15-r16=0
Phosphoenolpyruvate
r16-r17=0
Pyruvate
r17-r18-r19-r21=0
Acetyl-CoA
r19-r20=0
Oxaloacetate
r21-r22=0
L-malic acid
r22-r23=0
Fumaric acid
r23-r24=0
Succinic acid
r24-r25=0
Succinyl-CoA
r25-r26=0
Methylmalonyl-CoA
r26-r27=0
propionyl-CoA
r27-r28=0
CO2
r6+r19+r27-r21=0
Pi
2r21+r25-r13=0
Table 2: Equations of metabolic flux of propionic acid production by Propionibacterium acidipropionici.
During the fermentation of P. acidipropionici, the concentrations of six substances can be measured, and the corresponding reaction rates were obtained by numerical differentiation, which were the consumption rates of glucose and glycerol and the production rates of acetic acid, propionic acid, lactic acid, and succinic acid. These parameters were taken as known variables and were substituted into the metabolic balance equations to give the matrix, and the Linprog function in Matlab software was used to give the ideal metabolic flux distribution. During the calculation process, the glucose molar consumption rate was assumed to be 100mmol/(L·h).
Model validation
For the established metabolic flux balance model of propionic acid in P. acidipropionici, the degree of freedom of F was less than the number of variables determined experimentally. Therefore, the accuracy of the model can be verified by comparing the difference between the predicted result of a certain variable and its measured result. In the fermentation process with glucose as the sole carbon source, the measurable rates of propionic acid (r27) and succinic acid (r23-r24) were selected to calculate the ideal metabolic flux distribution, and the calculated acetate flux (r19) can be obtained [25,26]. The reliability of the model was verified by comparing the calculated acetate flux (r19) and the experimentally acetate flux. Similarly, the reliability of the model with glycerol as the sole carbon source can also be verified.
Cell concentration assay
To record the cell growth curve, cell concentration was measured by spectrophotometer at 600nm (OD 600nm). Before measuring, P. acidipropionici cells were centrifuged at 10,000g for 10min, and then resuspended in isovolumetric phosphate buffer (20mM, pH 7.0) to eliminate the influence of corn steep liquor and other components.
Organic acids, glucose, and glycerol assay
After centrifugation, the fermentation supernatant was used for analysis to determine the concentrations of organic acids, glucose and glycerol.
HPLC analysis of propionic acid, succinic acid, acetic acid, and lactic acid were performed using an LC-20AT system (Shimadzu, Kyoto, Japan) fitted with a Biorad HPX-87H column (5μm, 380 × 4.6 mm) and an SPD-20A UV detector operated at 215nm. The mobile phase was 5mM H2SO4 with a flow rate of 0.6mL/min at 55°C. The injection volume was 10μL. Commercially available propionic acid, succinic acid, acetic acid, and lactic acid were used as external standards.
Residual glucose and glycerol in the fermentation broth were determined using an SBA-40E biological sensor (Shandong, China) and a colorimetric method [27], respectively.
Statistical analysis and yield and productivity calculation
All experiments were carried out in triplicate or better and the mean and standard deviation (SD) values were calculated using the built-in statistical tools of Microsoft Excel. The productivity was calculated by dividing the concentration of organic acids at a certain time by the time elapsed and was expressed in units of [g/(L·h)]. The yield was calculated by dividing the mass of organic acids produced by the total amount of glucose or/and glycerol consumed and was expressed in units of [g/g].
Results and Discussion
Fermentation of P. acidipropionici and carbon source optimization
In the batch fermentation process of P. acidipropionici, cell growth was accompanied by the consumption of glucose or/and glycerol and the production of propionic acid, succinic acid, and acetic acid. When glucose was used as the sole carbon source, the concentration of propionic acid was 28.87 ± 1.27 g/L at 132h, and the corresponding productivity and yield were 0.22g/(L·h) and 0.48g/g, respectively (Figure 2A). Notably, when the concentration of propionic acid accumulated to about 25-30 g/L, the rate of cell growth and propionic acid production slowed down because of feedback inhibition. To effectively overcome this limitation, various ISPR processes were developed, which required further increases in equipment investment and was not suitable for industrial production on a temporary basis.
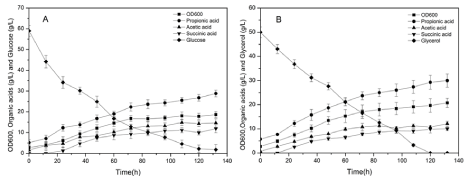
Figure 2: Time courses of OD600, glucose, glycerol, propionic acid, acetic acid, and succinic acid in Propionibacterium acidipropionici fermentation process. (A)
Glucose as the sole carbon source; (B) Glycerol as the sole carbon source.
Therefore, the fermentation of P. acidipropionici was optimized from the perspective of industrial production. First, the effect of glycerol on the production of propionic acid was analyzed. It was found that the concentration of propionic acid reached 30.52 ± 1.13 g/L at 132h, and the corresponding productivity and yield were 0.23g/(L·h) and 0.51g/g, respectively (Figure 2B). In addition, the concentrations of succinic acid and acetic acid decreased to varying degrees when glycerol was used as the carbon source. Therefore, the use of glycerol increased the production of propionic acid and reduced the production of by-products [22].
Based on these results, combinations of glucose and glycerol were investigated as mixed carbon sources. The production of propionic acid, succinic acid, and acetic acid during the fermentation were compared using glucose/glycerol ratios of 1:1, 1:2, 2:3, 3:2, and 4:1. The results showed that the optimal glucose/glycerol ratio was 4:1, providing the highest concentrations of propionic acid (36.62 ± 1.89 g/L) (Figure 3). Compared with the fermentation process using glucose as the carbon source, the productivity of propionic acid increased by 26.84%. Therefore, the combination of glucose and glycerol was considered necessary to maintain the high productivity of propionic acid.
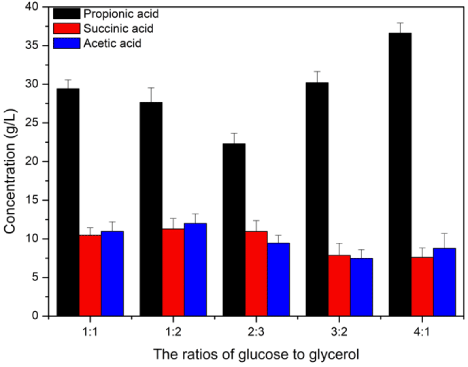
Figure 3: The production of propionic acid, succinic acid and acetic acid in Propionibacterium acidipropionici fermentation process with different ratios of glucose/
glycerol as the carbon source.
Model establishment and verification
The cellular metabolic pathway of P. acidipropionici is a complex reaction system, which is the result of the interaction of internal and external environments. To further study the metabolic process of propionic acid and its influencing mechanisms, the simplified ideal metabolic network of propionic acid was established based on the current literature, and the metabolic flux distribution in P. acidipropionici was analyzed. During the fermentation process, the production rates of propionic acid (r27) and succinic acid (r23-r24) were selected to calculate the ideal metabolic flux distribution using the Linprog function in Matlab software, and the calculated acetic acid flux (r19) was obtained. Also, the acetic acid flux can be measured according to its production rate, details shown in Table 3. The correlation coefficient between the calculated acetic acid flux by the model and the measured acetic acid flux was up to 0.99 (Figure 4), which indicated that the established metabolic flux balance model of propionic acid in P. acidipropionici was reliable.
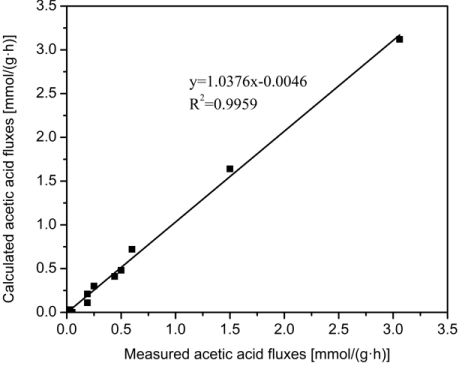
Figure 4: The correlation between the calculated acetic acid flux by the model and the measured acetic acid flux in Propionibacterium acidipropionici fermentation
process.
Time (h)
Concentrations (g/L)
Propionic acid flux [mmol/(g·h)]
Succinic acid flux [mmol/(g·h)]
Acetic acid flux [mmol/(g·h)]
Propionic acid
Succinic acid
Acetic acid
Measured
Calculated
0
5.18±0.47
0
1.61±0.11
-
-
12
7.17±0.58
0
3.80±0.70
2.29
0
3.06
3.12
24
12.32±0.63
1.30±0.10
4.67±0.80
2.96
0.48
0.6
0.72
36
13.81±0.28
4.70±0.40
7.91±0.87
0.63
0.79
1.5
1.64
48
16.79±0.74
7.41±0.56
8.45±0.48
0.87
0.45
0.19
0.11
60
19.03±0.29
8.67±0.10
10.28±0.47
0.61
0.18
0.5
0.48
72
22.35±0.98
9.20±1.03
12.17±0.86
0.66
0.07
0.44
0.41
84
23.66±0.20
9.85±0.41
13.12±0.18
0.18
0.07
0.19
0.21
96
24.33±0.44
10.82±0.41
13.27±0.40
0.08
0.08
0.03
0.03
108
25.57±0.42
11.12±1.18
14.89±0.50
0.14
0.03
0.25
0.3
120
26.78±0.58
10.05±1.19
14.29±0.41
0.11
-
-
-1.95
132
28.83±0.59
11.88±0.92
14.67±1.05
0.17
0.06
0.05
0
Table 3: Metabolic flux of propionic acid and succinic acid in the fermentation process of Propionibacterium acidipropionici and validation of metabolic flux balance model.
Metabolic flux analysis of key nodes
To investigate the intracellular metabolic process of propionic acid [28-31], the metabolic flux of key nodes was analyzed and used to guide the regulation of fermentation process.
Metabolic flux analysis of glucose-6-phosphate node: Glucose- 6-phosphate was the first key metabolic node, participating in the pentose phosphate pathway and glycolysis pathway. It was found that glycerol could affect the metabolic flux distribution of glucose- 6-phosphate node. When the ratio of glucose to glycerol was 4:1, the flux to ribulose-5-phosphate and fructose-6-phosphate was 64.36: 35.66, which implied that the pentose phosphate pathway was one of the main ways to provide the precursor (ribose-5-phosphate, erythrose-4-phosphate, etc.) and reducing power [H] required for cell growth. When the glucose/glycerol ratio was 2:3, the flux to ribulose- 5-phosphate was only 7.48 due to the high reducing power of glycerol (Figure 5), which weakened the pentose phosphate pathway, thereby reducing the synthesis of precursors such as erythrose-4-phosphate.
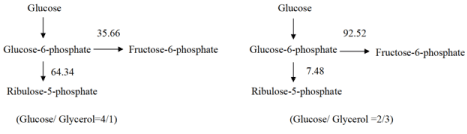
Figure 5: Metabolic flux distribution at glucose-6-phosphate node with different ratios of glucose/glycerol as the carbon source.
Metabolic flux analysis of pyruvate node: Pyruvate was another key metabolic node in the simplified metabolic network of propionic acid. Pyruvate participated in the metabolism process, including: (1) Direct generation of lactate by catalysis with lactate dehydrogenase; (2) Generation of acetyl-CoA by catalysis with pyruvate dehydrogenase, which further produced acetate; and (3) Generation of oxaloacetate via the pyruvate carboxylation pathway, and then further production of succinate. It was found that the flux of the pyruvate node to the oxaloacetate branch decreased in the later stage of fermentation, which explained the mechanism of feedback inhibition of propionic acid on its productivity. According to the results, the maximum metabolic flux to the oxaloacetate branch with a value of 33.64 was obtained with a glucose/glycerol ratio of 4:1, which ultimately led to an increase in the production of propionic acid, details shown in Figure 6.

Figure 6: Metabolic flux distribution at pyruvate node with different ratios of glucose/glycerol as the carbon source.
Therefore, the optimized carbon source combination can improve the fermentation efficiency by strengthening the metabolic distribution of key nodes. In addition, metabolic flux analysis indicated that the pyruvate carboxylation pathway was the key step affecting the synthesis of propionic acid. According to the conclusion, the introduction of carbonate during the fermentation process can promote the pyruvate carboxylation reaction. The preliminary results showed that substituting carbonate for part of NH3·H2O (approximately 15-20%) to adjust pH can increase the concentration of propionic acid by 10-12% (unpublished).
Fed-batch fermentation of P. acidipropionici and scaledup
After optimization, the fed-batch fermentation process of P. acidipropionici was established and scaled-up step by step. Finally, the 150-L fed-batch fermentation of P. acidipropionici was carried out based on the optimized conditions. The results indicated that the combination of glucose and glycerol could effectively promote the metabolic synthesis of propionic acid (Figure 7). When the glucose/glycerol ratio was 4:1, the concentration of propionic acid reached 51.75 ± 3.62 g/L with a fermentation period of 132h, an increase of 79.25% relative to the use of glucose alone, corresponding the productivity and yield of 0.39g/(L·h) and 0.52g/g, respectively. The concentration of succinic acid was only 8.26g/L, which was a decrease of 48.29% relative to the use of glucose alone. Therefore, the productivity of propionic acid was generally higher than that in the small-scale fermentation process, which was consistent with the experimental results. In the large-scale anaerobic fermentation process, due to the low substance diffusion coefficient, the concentration of carbon dioxide in the fermentation broth is higher. This may be due to the strengthening of the carboxylation pathway of pyruvate to increase the production of oxaloacetate, thereby increasing the flux of pyruvate to oxaloacetic acid branches. Therefore, the productivity of propionic acid is generally higher than that of the small-scale fermentation process, which is consistent with the experimental results. In addition, methylmalonyl mutase can catalyze the conversion of succinyl-CoA to methylmalonyl-CoA, and then into propionyl-CoA, so it is also a key enzyme that affects the production of propionic acid. The research technology is limited, so the enzyme activity cannot be verified.
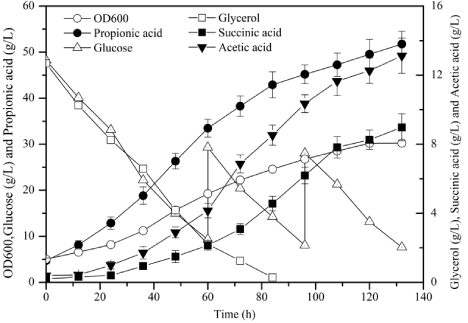
Figure 7: Time courses of OD600, glucose, glycerol, propionic acid, succinic acid, and acetic acid in the 150-L fed-batch fermentation process of Propionibacterium acidipropionici.
Currently, propionic acid is produced almost exclusively through petrochemical processes by the oxidation of propane or propionaldehyde as raw materials [2]. With the increasing interest in sustainable production of chemicals, the production of propionic acid by microbial fermentation (mainly Propionibacterium) has been extensively investigated [32]. To improve the fermentation efficiency, many strategies have been applied to propionic acid biosynthesis, such as genetic engineering, cell immobilization, ISPR processes. However, all these routes have not gone beyond research scale. From an industrial point of view, the application of conventional expensive systems of fermentation is limited. Therefore, the fermentation processes of batch and fed-batch are frequently utilized in the production of propionic acid. The application of cheap commercially available substrate (e.g., glycerol) and renewable feedstock (e.g., molasses, bagasse) can be economically justified. The current state of propionic acid production by Propionibacterium is shown in Table 4. At present, the fermentation of propionic acid is regarded as an economically disadvantage fermentation process in terms of fastidiousness of task, time-consumption, end product inhibition, expensive downstream processing of recovery and concentration [21]. However, it should be believed that a robust cell factory will eventually be constructed successfully in the near future with the development of synthetic biology and systems biology, and its economics can finally be comparable to chemical processes.
Microorganism
Carbon source
Culture system
Propionic acid
References
Production (g/L)
Productivity [g/(L·h)]
Yield (g/g)
P.freudenreichii
glucose
Fed-batch
52.5
0.33
0.66
[34]
P.acidipropionici
glycerol
Batch
20
0.24
0.67
[18]
P.acidipropionici
flour hydrolysate
Fed-batch
30
0.33
--
[35]
P.acidipropionici
glucose/lactate
Fed-batch
~30
--
--
[36]
P.acidipropionici
glycerol
Fed-batch
44.62
0.2
0.56
[37]
P.acidipropionici
glycerol
PEI-Poraver bioreactor
35.23
0.35
0.48
[17]
P.acidipropionici
glucose
Semi-continuous
47
0.37
0.45
[38]
P.acidipropionici (engineered)
glycerol
FBR-bioreactor
106
0.04
0.56
[28]
P. acidipropionici
glucose/glycerol
Fed-batch
51.75
0.39
0.52
This study
Table 4: The current state-of propionic acid production by Propionibacteria.
Conclusion
The production of propionic acid by P. acidipropionici has attracted wide attention. To improve the fermentation efficiency of propionic acid, the effect of glucose and glycerol on product synthesis was analyzed and the combination of glucose and glycerol was optimized. The highest propionic acid concentration of 36.62 ± 1.89 g/L was obtained with a glucose/glycerol ratio of 4:1, which was an improvement of 26.84% relative to the use of glucose alone. The simplified metabolic network [28,33] of propionic acid production in P. acidipropionici was established and the metabolic flux distribution was analyzed to better understand the distribution of intracellular materials and energy. It was found that the fermentation efficiency could be improved by enhancing the synthesis of pyruvate and its flux distribution to the oxaloacetate branch. After adding carbonate ions to the fermentation broth, the concentration of propionic acid of P. acidipropionici increased by 12% compared to the control experiment. Upon performing the scaled-up fed-batch fermentation of P. acidipropionici, the concentration of propionic acid reached 51.75 ± 3.62 g/L with a glucose/glycerol ratio of 4:1, and the corresponding productivity and yield were 0.39g/(L·h) and 0.52g/g, respectively. Therefore, the combination of glucose and glycerol significantly improved the fermentation efficiency and has good prospects for industrial application.
Highlights
• A simplified metabolic network of propionic acid production in Propionibacterium acidipropionici was established.
• The metabolic flux distribution at key nodes was analyzed and used to guide the optimization of P. acidipropionici fermentation process.
• The combination of glucose and glycerol was optimized and the scaled-up fed-batch fermentation of P. acidipropionici was conducted.
Declaration
Credit authorship contribution statement: Yuhan Zhang: Methodology, Validation, Writing-Original Draft; Xiaolian Li: Methodology, Writing-Original Draft; Ziqiang Wang: Conceptualization, Writing-Review & Editing, Funding acquisition, Project administration; Yunshan Wang: Resources, Supervision, Project administration; Zhiguo Su: Resources, Supervision.
Acknowledgment: This work was financially supported by the National Natural Science Foundation of China (Grant No.21506227).
References
- Vidra A, Németh Á. Bio-produced propionic acid: a review. Period. Polytech. Chem. Eng. 2018; 62: 57-67.
- Ahmadi N, Khosravi-Darani K, Mortazavian AM. An overview of biotechnological production of propionic acid: From upstream to downstream processes. Electron. J. Biotechn. 2017; 28: 67-75.
- Bozell JJ. Feedstocks for the future-biorefinery production of chemicals from renewable carbon. CLEAN-Soil Air Water. 2018; 36: 641-647.
- Feng XH, Fei C, Hong X, Wu B, Yao J, Ying HJ, et al. Propionic acid fermentation by Propionibacterium freudenreichii CCTCC M207015 in a multi-point fibrous-bed bioreactor. Biopro. Biosyst. Eng. 2010; 33: 1077-1085.
- Hettinga DH, Reinbold GW. The propionic-acid bacteria-a review: I. Growth. J. Milk. Food Technol. 1972; 35: 295-301.
- Jin Z, Yang ST. Extractive fermentation for enhanced propionic acid production from lactose by Propionibacterium acidipropionici. Biotechnol. Prog. 1998; 14: 457-465.
- Suwannakham S, Yang ST. Enhanced propionic acid fermentation by Propionibacterium acidipropionici mutant obtained by adaptation in a fibrousbed bioreactor. Biotechnol. Bioeng. 2005; 91: 325-337.
- Ammar EM, Jin Y, Wang Z, Yang ST. Metabolic engineering of Propionibacterium freudenreichii: effect of expressing phosphoenolpyruvate carboxylase on propionic acid production. Appl. Microbiol. Biotechnol. 2014; 98: 7761-7772.
- Wang Z, Lin M, Wang L, Ammar EM, Yang ST. Metabolic engineering of Propionibacterium freudenreichii subsp. shermanii for enhanced propionic acid fermentation: effects of overexpressing three biotin-dependent carboxylases. Process Biochem. 2015; 50: 194-204.
- Wei P, Lin M, Wang Z, Fu H, Yang H, Jiang W, et al. Metabolic engineering of Propionibacterium freudenreichii subsp. shermanii for xylose fermentation. Bioresour. Technol. 2016; 219: 91-97.
- Kosmider A, Bialas W, Kubiak P, Drozdzynska A, Czaczyk K. Vitamin B12 production from crude glycerol by Propionibacterium freudenreichii ssp. shermanii: optimization of medium composition through statistical experimental designs. Bioresour. Technol. 2012; 105: 128-133.
- Dishisha T, Ståhl Å, Lundmark S, Hatti-Kaul R. An economical biorefinery process for propionic acid production from glycerol and potato juice using high cell density fermentation. Bioresour. Technol. 2013; 135: 504-512.
- Goswami V, Srivastava A. Propionic acid production in an in situ cell retention bioreactor. Appl. Microbiol. Biotechnol. 2001; 56: 676-680.
- Feng X, Chen F, Xu H, Wu B, Li H, Li S, et al. Green and economical production of propionic acid by Propionibacterium freudenreichii CCTCC M207015 in plant fibrous-bed bioreactor. Bioresour. Technol. 2011; 102: 6141-6146.
- Chen F, Feng X, Xu H, Xu D, Ouyang PK. Propionic acid production in a plant fibrous-bed bioreactor with immobilized Propionibacterium freudenreichii CCTCC M207015. J. Biotechnol. 2013; 164: 202-210.
- Zhu L, Wei P, Cai J, Zhu XC, Wang ZM, Huang L, et al. Improving the productivity of propionic acid with FBB-immobilized cells of an adapted acidtolerant Propionibacterium acidipropionici. Bioresour. Technol. 2012; 112: 248-253.
- Dishisha T, Alvarez MT, Hatti-Kaul R. Batch-and continuous propionic acid production from glycerol using free and immobilized cells of Propionibacterium acidipropionici. Bioresour. Technol. 2012; 118: 553-562.
- Barbirato F, Chedaille D, Bories A. Propionic acid fermentation from glycerol: comparison with conventional substrates. Appl. Microbiol. Biotechnol. 1997; 47: 441-446.
- Himmi EH, Bories A, Boussaid A, Hassani A, Boussaid L. Propionic acid fermentation of glycerol and glucose by Propionibacterium acidipropionici and Propionibacterium freudenreichii ssp. shermanii. Appl. Microbiol. Biotechnol. 2000; 53: 435-440.
- Ammar EM, Martin J, Brabo-Catala L, Philippidis GP. Propionic acid production by Propionibacterium freudenreichii using sweet sorghum bagasse hydrolysate. Appl. Microbiol. Biotechnol. 2020; 104: 9619-9629.
- Wang Z, Yang ST. Propionic acid production in glycerol/glucose cofermentation by Propionibacterium freudenreichii subsp. shermanii. Bioresour. Technol. 2013; 137: 116-123.
- Kosmider A, Drozdzynska A, Blaszka K, Leja K, Czaczyk K. Propionic acid production by Propionibacterium freudenreichii ssp. shermanii using industrial wastes: crude glycerol and whey lactose. Pol. J. Environ. Stud. 2010; 19: 1249-1253.
- Hendry JI, Dinh HV, Foster C, Gopalakrishnan S, Wang L, Maranas CD. Metabolic flux analysis reaching genome wide coverage: lessons learned and future perspectives. Curr. Opin. Chem. Eng. 2020; 30: 17-25.
- Kim HU, Kim TY, Lee SY. Metabolic flux analysis and metabolic engineering of microorganisms. Mol. Biosyst. 2008; 4: 113-120.
- Veras HC, Campos CG, Nascimento IF, Abdelnur PV, Almeida GR, Parachin NS. Metabolic flux analysis for metabolome data validation of naturally xylose-fermenting yeasts. BMC Biotechnol. 2019; 58: 19.
- Huang C, Li X, Xu CQ, Zhang XL, Ye H, Cheng B, et al. Metabolic flux analysis of the β-mannanase production by genetically engineered Escherichia coli BL21. China Brewing. 2009; 0254-5071.
- Lynch HC, Yang Y. Degradation products of clavulanic acid promote clavulanic acid production in cultures of Streptomyces clavuligerus. Enzyme Microb. Tech. 2004; 34: 48-54.
- Zhang A, Yang ST. Propionic acid production from glycerol by metabolically engineered Propionibacterium acidipropionici. Process Biochem. 2009; 44: 1346-1351.
- Antoniewicz MR. A guide to metabolic flux analysis in metabolic engineering: Methods, tools and applications. Metab. Eng. 2020; 63: 2-12.
- Wang Y, Wondisford FE, Song C, Zhang T, Su XY. Metabolic flux analysislinking isotope labeling and metabolic fluxes. Metabolites. 2020; 10: 447.
- Carthew RW. Gene regulation and cellular metabolism: An Essential Partnership. Trends Genet. 2021; 37: 389-400.
- Liu L, Zhu Y, Li J, Wang M, Lee P, Du GC, et al. Microbial production of propionic acid from Propionibacteria: current state, challenges and perspectives. Crit. Rev. Biotechnol. 2012; 32: 374-381.
- Su H, Lin J, Wang GW. Metabolic engineering of Corynebacterium crenatium for enhancing production of higher alcohols. Sci. Rep. 2016; 6: 1-20.
- Wang P, Wang Y, Liu Y, Shi H, Su ZG. Novel in situ product removal technique for simultaneous production of propionic acid and vitamin B12 by expanded bed adsorption bioreactor. Bioresour. Technol. 2012; 104: 652-659.
- Sabra W, Dietz D, Zeng AP. Substrate-limited co-culture for efficient production of propionic acid from flour hydrolysate. Appl. Microbiol. Biotechnol. 2013; 97: 5771-5777.
- Martínez-Campos R, de la Torre M. Production of propionate by fed-batch fermentation of Propionibacterium acidipropionici using mixed feed of lactate and glucose. Biotechnol. Lett. 2002; 24: 427-431.
- Zhu Y, Li J, Tan M, Liu L, Jiang LL, Sun J, et al. Optimization and scaleup of propionic acid production by propionic acid-tolerant Propionibacterium acidipropionici with glycerol as the carbon source. Bioresour. Technol. 2010; 101: 8902-8906.
- Woskow SA, Glatz BA. Propionic acid production by a propionic acid-tolerant strain of Propionibacterium acidipropionici in batch and semi-continuous fermentation. Appl. Environ. Microbiol. 1991; 57: 2821-2828.