
Review Article
Austin J Nutr Metab. 2022; 9(1): 1122.
Review on Development System of Traceability in Dairy Processing
Shahin AA¹ and Shaaban HA²*
1Department Food Technology Research Institute, Agriculture Research Centre, Egypt
2Department of Chemistry of Flavour and Aroma, National Research Center, Dokki, Giza, Egypt
*Corresponding author: Hamdy A Shaaban, Department of Chemistry of Flavour and Aroma, National Research Center, Dokki, Giza, Egypt
Received: December 20, 2021; Accepted: January 31, 2022; Published: February 07, 2022
Abstract
The application of molecular methods is widely implemented in the traceability systems in the food sector, covering many types of food, as it is shown in this review. The rapid implementation of traceability systems in the food system is due to these techniques with high specificity, sensitivity, efficiency, and speed. In addition, compared with protein-based techniques, DNA-based techniques can be applied to any type of product, regardless of the treatment of processing to which it has been subjected. Future trends in the development of molecular or genetic tools for food traceability are focused on the search of techniques to obtain more information in the shortest time possible. Techniques that allow the identification, both at the species and population level, also facilitate the determination of the geographic origin. At present, one of the most promising techniques is the Digital PCR (dPCR), which allows the analysis of samples containing mixtures of species with high sensitivity and in a single assay, making multiple reactions in parallel through a nanofluidic chip. The application of emerging technologies, microfluidics, and nanotechnology in the development of molecular methods will obtain greater sensitivity, discriminatory power, reproducibility, and speed, thus increasing its potential in the traceability of the food sector.
Keywords: Food safety; Traceability system; Dairy processing
Introduction
The utilization of Radio-Frequency Identification (RFID) has a great potential for traceability, logistics, supply chain management, and quick response systems. RFID is a mean of product automatic identification (Auto-ID) where the product can even identify itself without human intervention, independently of line-of-sight, in motion, and simultaneously with other items.
The automated monitoring of processing, displacement, and storage of food items or lots of bulk materials, defined in traceable units, has a precise state-of-the art manufacturing process which is not only considered merely an internal traceability system, but an extraordinary tool to control, manage, and model the process, as well as to share traceability data with the other stakeholders, improving the performances of the supply chain [32].
Besides, certain RFID applications play a role in continuously sensing and monitoring parameters which are highly involved in value creation in perishable product food chains.
Genetic methods are the most commonly used because of their advantages over the morphological characters or proteinbased methods. Although DNA may be altered with various food processes, this molecule is far more resistant and heat-stable than proteins, allowing the amplification by PCR of small DNA regions which are sufficient to enable identification even in the case of DNA fragmentation.
Besides, given the degeneration of the genetic code and the presence of non-coding regions, this molecule provides more information than proteins [15]. Another advantage of DNA is that this marker can potentially be extracted from any species in virtually any kind of organic substrate, such as muscle, fin, or blood, because it is present in all cells of an organism [17,35].
The verification of traceability in food products by genetic techniques consists in checking the identity of the species that make those products. This gives a response to one or more of the above questions [11].
The main types of molecular techniques for species identification, detection, and authentication are Polymerase Chain Reaction (PCR), PCR-Restriction Fragment Length Polymorphism (PCR-RFLP), Forensically Informative Nucleotide Sequencing (FINS), Real- Time PCR (RT-PCR), digital PCR (dPCR), and Next-Generation Sequencing (NGS). Other techniques applied to traceability are the PCR-Length Polymorphism (PCR-LP), used, for instance, in the case of the microsatellites analysis and the Single Nucleotide Polymorphism (SNP) used for large-scale genotyping using highthroughput technologies. These last two techniques are used to determine at the population, stock, variety, or cultivar level and are particularly relevant when it comes to authenticate the origin or identity of products included in the European marks of Protected Designation of Origin (PDO) or Protected Geographical Indication (PGI).
The coupling of mass spectrometry to plasma methods of atomization/ionization of samples provides one of the most sensitive techniques for the determination of trace and ultra-trace elements: Inductively Coupled Plasma-Mass Spectrometry or ICP-MS. This technique has been used since the 1980s in several fields of application, and it has been at least since the 1990s that it has shown its potentiality in the authentication of foodstuffs [22].
The ICP-MS Technique Exploits Particular Features
High rate of conversion of analytes from solution into elemental ions, yielded by the plasma temperature (6.000–10.000K); high selectivity of the mass spectrometer filter; and high sensitivity of the detection system.
RFID System Consists
An RFID system consists basically in the communication between an interrogator (and reader) and a tag (or transponder) (Figure 5.1). Both interrogator and tag contain an antenna, which transmits and receives in a communication channel where the reader sends information to the tag (forward link) and the tag answers to the reader (reverse link).
Tags can be categorized as passive, semi passive, or active: passive tags do not contain a battery and are powered by the energy harvested from the electromagnetic field emitted by the interrogator; semi passive tags or Battery Assisted Passive (BAP) contain a battery which is used only to power the tag IC and is not for communication; and active tags, which have a battery that supplies power to all functions.
In farm dairies, radio-frequency systems for livestock traceability are used coupled to automatic milking systems to trace milk lots delivered to cheese manufacturing plants, Milking yields, oestrus detection, rumination, and individual grain feeding can be monitored individually using RFID coupled to other sensing devices and actuators.
The traceability of milk presents the same criticalities as other liquid or bulk products, which are usually stored in tanks and progressively merged during the production process [6]. Farm milk records (farm number, size of the lot, date, and hour of delivery) as well as analytical determined quality parameters (eg, pH, presence of antibiotic residues, protein and fat content, somatic cell number, and total microbial count) have to be recorded.
De Las Morenas [9], proposed an RFID solution to trace and monitor the temperature of milk sample vials collected in farms during the transport, which improves reliability in milk analysis for high-quality milk bonus payment.
During the steps of merging milk stored in silos to the first operations in cheese production, traceability can be maintained at the processed milk lot level since the cheese reaches its solid state. Then, Auto-ID technologies can be put in place to identify smaller cheese lots or single cheese wheels.
Cheese identification is very critical due to the environmental conditions during the manufacturing phases (curd molding, pressing, dry or brine salting, ripening) and frequent product handling (eg, daily turning, brushing, and scraping during ripening). For these reasons, traditional identification methods cannot be used for itemlevel identification.
Research studies have demonstrated [3,29,28,30] that, if properly coated by special resins or plastic materials approved for food contact, RFID passive (LF or HF) transponders could be directly inserted in the cheese rind (Figure 1). In this case the tag can be reutilized after a proper sanitization, reducing costs.
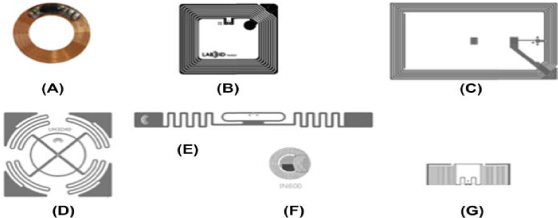
Figure 1: Passive tags at different frequencies. Type (A) is a Low-Frequency tag copper coil of an half duplex (HDX) transponder used in RF animal ear tag
identifiers (134 kHz). Tag models (B) and (C) have HF square coils (13.56 MHz), while those identified by the letters (D), (E), (F), and (G) are UHF tag antennas of
different shapes. Courtesy of Lab-ID, Castel Maggiore, Bologna, Italy.

Figure 2: Cheese wheels identified by means of circular LF or HF tags. Transponders are applied under a casein disk during curd molding on the side (up, left),
or on the cheese wheel face (up, right). During ripening the remains included in the cheese rind and can be detected by different handled models when placed on
shelves in the storage room (down, left and right figures).
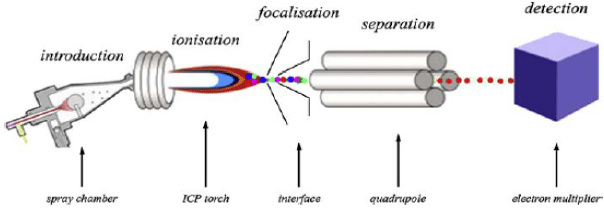
Figure 3: Shows a generic scheme of an ICP-MS system.
In the case of fresh, small, portioned, and packed cheese the RFID tracking can be applied identifying assets and packaging by RFID labels. In seasoned cheese, by monitoring the stock level in storage rooms, the scheduled processing can be driven by the demand and on the basis of cheese shelf life avoiding overproduction, which causes a decrease in cheese quality, the failure in respecting established rules for certified cheese such as PGI and PDO, the loss of value, and avoiding costs due to overtime labor.
The transparency in dairy products is an important issue regarding interests of the consumer due not only to the economic point of view, but also sanitary requirements, food allergies, or religious practices [14,20,25]. Also the absence of proper labeling, indicating the possibility of even traces of determinate milk in any dairy products, can be a risk for allergic persons, becoming a safety issue [2]. The detection of milk species is important in cheese making too, especially those made from one pure species and with PDO, such as pure sheep or pure goat cheeses. In addition, some cheeses are manufactured with defined proportions of each type of milk, making the quantification in traceability system important [5,37]. Several analytical approaches have been developed to apply in the traceability of milk products, the principal ones being electrophoretic, immunological, chromatographic, and molecular [20]. Among these analytical strategies the molecular techniques stand out for their robustness, reliability, sensitivity, and specificity, and their applicability, regardless of the treatment to which the product has been submitted (thermally-treated milks such as pasteurized milk, ultra-pasteurized, and powder milks). Among the molecular methods applied to the traceability of dairy products are mainly PCR, PCRRFLP, and RT-PCR [1,7,10,16,18,19,21].
Many works focused on differentiation of the species in dairy products are based on primers developed for the differentiation of species of meat. These ones are combined with new primers, and the conditions for validating the methodology and its application are optimized. The most recent works combine the simultaneous identification of various species and quantification by RT-PCR [7,10,19].
Among the methodologies developed for the simultaneous identification of species in dairy products are the work of [13], who developed a method for the identification of cows, sheep, goats, and water buffalo in dairy products by multiplex PCR followed by fragment size analysis by capillary electrophoresis. Other works comprise the identification and quantification of species in dairy products. [10] Describe a Real-Time PCR methodology for calculating the bovine and buffalo content in milk and meat-derived food products. It also highlights the work of [2], who developed a quadruplex quantitative Real-Time PCR (qxPCR) methodology for the rapid identification of DNA of cows, goats, sheep and buffalo in dairy products, as well as quantification of cow DNA.
The evolution of technology and the introduction of new techniques, such as digital PCR, will allow the development of increasingly sensitive and specific methodologies, promoting its implementation in the dairy sector and routine control tools as an indispensable part of the traceability system.
The Fundamental Parts of an ICP-MS Instrument are the Following
Introduction system: a device carrying samples to the nebulizer, usually in liquid form. A peristaltic pump is the most common device, providing a constant uptake flow rate. An automatized system, such as an auto sampler, can be present in order to optimize analysis time and consumption of reagents.
Nebulization system: its task is transforming the sample from a liquid solution to a spray composed of light drops by means of an incident argon flow. The lightest are the drops, where the most analytes are carried to the plasma.
ICP compartment: the argon plasma is generated on top of a torch, and it is fed by interaction with the electromagnetic field generated by a radiofrequency source. When the nebulized sample enters in the plasma, it is instantaneously desolvated and atomized; then atoms are turned into ions depending on the ionization potential of the respective elements.
Interface: the vapor containing ions and atoms is driven by argon through an interface made of two subsequent cones, the skimmer and the sampler cone, which allow the focalization of ions into a small volume.
Mass spectrometer: a quadrupole or a hexapole allowing the separation of ions according to their m/z ratios.
Detection system: an electron multiplier that transform ion signals into electric pulses.
ICP-MS instruments with low-resolution quadrupole mass spectrometers allow determination of trace and ultra-trace elements with good accuracy and sensitivity. Using high-resolution MS systems, higher accuracy, precision, and sensitivity are guaranteed and, in addition, it becomes possible to determine isotope ratios of heavy elements (strontium, lead, etc.) which are considered among the best chemical markers for food authentication and traceability; with these features, ICP-MS becomes a member of the group of techniques allowing the determination of isotope ratios which is collectively termed Isotope Ratio Mass Spectrometry, or IRMS, which in turn is a major part of Stable Isotope Analysis, or SIA.
The scientific literature on ICP-MS is at present huge, both in terms of books and journal articles. In-depth analysis on the theoretical basis of the technique and on its applications can be found, among others, in [23,36].
There are good perspectives for the application of ICP-MS in the field of food classification. Its use for the determination of inorganic parameters in foodstuffs will surely increase, in particular to complement other techniques such as SIA of light elements. Instrumentations of higher sensitivity and resolution are being developed that yield better analytical results in terms of reliability of data. The great potential of strontium and trace elements as geochemical markers will be surely improved by these performances. A key role will also be played by a greater comprehension of the mechanisms involved in the relationship among soil and plants and of the processes involved in food production chains.
The production chain of milk is relatively easy to manage. From grass to milk passing through the stomach of the producing animal, inorganic compounds (be them nutrients or contaminants) undergo a fast transfer. Therefore, milk composition should reflect well the environmental conditions to which the animal is exposed, including soil, water, and diet. It must be considered, though, that the commercial value of milk hardly justifies a classification study. A particular case is the one of buffalo milk, which is used to produce Mozzarella di Bufala Campana DOP cheese, a well-known product of the Italian dairy industry.
Benincasa [4] analyzed cow and buffalo milk samples produced in the same farm under identical environmental, feeding, and animal husbandry conditions; multielement profiling from major to trace elements and subsequent chemometric treatment of data with LDA discriminated the two animal species, providing a good way of identifying the fraudulent labeling of milk and associated by-products such as Mozzarella cheese.
Definitely more complicated is the production chain of dairy products. The composition of such products starts from milk but further on is strongly influenced by factors involving chemical transformations: processing, aging, activity and quality of microbial flora, addition of substances with various roles, etc. In addition, most dairy products are indeed alive products in the sense that they host microbial species breeding on a rich substrate; their metabolism generates continuously evolving chemical compounds. According to these features, it is hard to find the link with soil; a rare case is the one presented by [34], who analyzed Bryndza sheep cheese produced in nine regions of Slovakia, finding good correlations among trace element concentrations in pasture soils, grasses, milk and cheese products, and by-products; apparently, that particular cheese-making process did not significantly affect the link between the soil and the final product. Similar results were obtained by [33], who found an excellent agreement in the values of d87Sr in soils, milk, and cheese samples from the same site, confirming the role of strontium isotopes as soil tracers.
Authentication schemes can be developed more easily on the basis of inorganic parameters. [31] Suggested the use of isotope ratio analysis, including d87Sr, in order to determine the geographic provenance of butter [29]. were able to distinguish Emmental cheeses produced in six European regions (Allgau in Germany; Bretagne and Savoie in France, Finland, and Switzerland; and Vorarlberg in Austria) with multielement profiling coupled with stable isotope ratios (d87Sr) and determination of radioactive elements (90Sr, 234U, 238U). Classification succeeded using all the variables determined, including isotope ratios of light elements, but it was only partially efficient when a single class of parameters was used alone. [24] Correctly classified a large number of PDO cheeses produced in Northern Spain using Ca, P, and Na as variables.
Conclusion
There are good perspectives for the application of ICP-MS in the field of food classification. Its use for the determination of inorganic parameters in foodstuffs will surely increase, in particular to complement other techniques such as SIA of light elements. Instrumentations of higher sensitivity and resolution are being developed that yield better analytical results in terms of reliability of data. The great potential of strontium and trace elements as geochemical markers will be surely improved by these performances. A key role will also be played by a greater comprehension of the mechanisms involved in the relationship among soil and plants and of the processes involved in food production chains.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abdel-Rahman SM, Ahmed MMM. Rapid and sensitive identification of buffalo’s, cattle’s and sheep’s milk using species-specific PCR and PCRRFLP techniques. Food Control. 2007; 18: 1246–1249.
- Agrimonti C, Pirondini A, Marmiroli M, Marmiroli N. A quadruplex PCR (qxPCR) assay for adulteration in dairy products. Food chemistry. 2015; 187: 58-64.
- Barge P, Gay P, Merlino V, Tortia C. Item-level radio-frequency identification for the traceability of food products: application on a dairy product. Journal of Food Engineering. 2014; 125: 119–130.
- Benincasa C, Lewis J, Sindona G, Tagarelli A. The use of multi element profiling to differentiate between cowand buffalo milk. Food chemistry. 2008; 110: 257-262.
- Bottero MT, Civera T. A multiplex polymerase chain reaction for the identification of cow’s, goat’s and sheep’s milk in dairy products. International Dairy Journal. 2003; 13: 277–282.
- Comba L, Belforte G, Dabbene F, Gay P. Methods for traceability in food production processes involving bulk products. Biosystems Engineering. 2013; 116: 51–63.
- Dalmasso A, Civera T. Simultaneous detection of cow and buffalo milk in mozzarella cheese by real-time PCR assay. Food Chemistry. 2011; 124: 362–366.
- Dalmasso A, Fontanella E, Piatti P, Civera T, Secchi C, Bottero MT. Identification of Four Tuna Species by Means of Real-Time PCR and Melting Curve Analysis. Veterinary Research Communications. 2007; 31: 355-357.
- De Las Morenas J, Garcia A, Blanco J. Prototype traceability system for the dairy industry. Computers and Electronics in Agriculture. 2014; 101: 34–41.
- Drummond MG, Brasil B. A versatile real-time PCR method to quantify bovine contamination in buffalo products. Food Control. 2013; 29: 131–137.
- Espineira M, Alonso M. Advances in authenticity testing for fish speciation. In: Downey, G., Sykes, R. (Eds.), Advances in Food Authenticity Testing. Woodhead Publishing (in press). 2016.
- Espiñeira M. Santaclara F J. Advances in Food Traceability Techniques and Technologies, Improving Quality Throughout the Food Chain. Woodhead Publishing Series in Food Science, Technology and Nutrition: 2016; Number 301.
- Gonçalves J, Pereira F, Amorim A, Asch BV. New method for the simultaneous identification of cow, sheep, goat, and water buffalo in dairy products by analysis of short species-specific mitochondrial DNA targets. Journal of agricultural and food chemistry. 2012; 60: 10480-10485.
- Guerreiro JS, Fernandes P. Identification of the species of origin of milk in cheeses by multivariate statistical analysis of polymerase chain reaction electrophoretic patterns. International Dairy Journal. 2012; 25: 42–45.
- Lago FC, Alonso M. Fish and seafood authenticity – species identification. In:Boziaris, I.S. (Ed.), Seafood Processing. John Wiley & Sons, Ltd. 2014; 419–452.
- Lanzilao I, Burgalassi F, Fancelli S, Settimelli M, Fani R. Polymerase chain reaction-restriction fragment length polymorphism analysis of mitochondrial cytb gene from species of dairy interest. Journal of AOAC International. 2005; 88: 128-135.
- Lockley AK, Bardsley RG. DNA-based methods for food authentication. Trends in Food Science & Technology. 2000; 11: 67–77.
- Lpez-Calleja I, Gonzalez I. Application of polymerase chain reaction to detect adulteration of sheep’s milk with goat’s milk. Journal of Dairy Science. 2005; 88: 3115–3120.
- Lpez-Calleja I, Gonzalez I. Application of a polymerase chain reaction to detect adulteration of ovine cheeses with caprine milk. European Food Research and Technology. 2007; 225: 345–349.
- Mafra I, Ferreira IM. Food authentication by PCR-based methods. European Food Research and Technology. 2008; 227: 649–665.
- Mafra I, Roxo A. A duplex polymerase chain reaction for the quantitative detection of cow’s milk in goat’s milk cheese. International Dairy Journal. 2007; 17: 1132–1138.
- Mccurdy E, Potter D, Medina M. Trace elements in wine. Laboratory News. 1992; 9: 10–11.
- Montaser A. Inductively Coupled Plasma – Mass Spectrometry, third ed. Wiley-VCH, New York. 1998.
- Moreno-Rojas R, Camara-Martos F, Sanchez-Segarra PJ, Amaro-Lpez MA. Influence of manufacturing conditions and discrimination of Northern Spanish cheeses using multi-element analysis. International Journal of Dairy Technology. 2012; 65: 594–602.
- Nollet LML, Toldra F. Handbook of Dairy Foods Analysis. CRC Press. 2009.
- Jae-Don Oh, Ki-Duk Song, Joo-Hee Seo, Duk-Kyung Kim, Sung-Hoon Kim, Kang-Seok Seo, et al. Genetic traceability of black pig meats using microsatellite markers. Asian-Australasian Journal of Animal Sciences. 2014; 27: 926-931.
- Papetti P, Costa C, Antonucci F, Frigorilli S, Solaini S, Menesatti P. RFID webbased info tracing system for the artisanal Italian cheese quality traceability. Food Control. 2012; 27: 234–241.
- Pérez-Aloe R, Valverde JM, Lara A, Castao? F, Carrillo JM, Gonzalez J, et al. Use of RFID tags for data storage on quality control in cheese industries. In: Balasa, F.(Ed.), Data Storage. InTech. ISBN: 978-953-307-063-6. 2010.
- Pillonel L, Badertscher R, Froidevaux P, Haberhauer G, Hl?zl S, Horn P, et al. Stable isotope ratios, major, trace and radioactive elements in emmental cheeses of different origins. Lebensmittel-Wissenschaft und –Technologie. 2003; 36: 615–623.
- Regattieri A, Gamberi M, Manzini R. Traceability of food products: general framework and experimental evidence. Journal of Food Engineering. 2007; 81: 347–356.
- Rossmann A, Haberhauer G, Hl?zl S, Horn P, Pichlmayer F, Voerkelius S. The potential of multielement stable isotope analysis for regional assignment of butter. European Food Research and Technology. 2000; 211: 32–40.
- Ruiz-Garcia L, Lunadei L. The role of RFID in agriculture: applications, limitations and challenges. Computers and Electronics in Agriculture. 2011; 79: 42–50.
- Stevenson R, Desrochers S, Helie JF. Stable and radiogenic isotopes as indicators of agri-food provenance: insights from artisanal cheeses from Quebec, Canada. International Dairy Journal. 2015; 49: 37–45.
- Suhaj M, Korenovska M. Correlation and distribution of elemental markers of origin in the production of Bryndza sheep cheese. Food Chemistry. 2008; 107: 551–557.
- Teletchea F. Molecular identification methods of fish species: reassessment and possible applications. Reviews in Fish Biology and Fisheries. 2009; 19: 265–293.
- Thomas R. Practical Guide to ICP-MS: A Tutorial for Beginners, third ed. CRC Press, Boca Raton, FL. 2013.
- Ulberth F, Lees M. Milk and dairy products. Food Authenticity and Traceability. 2003; 357–377.