
Review Article
Int J Nutr Sci. 2016; 1(2): 1007.
Nutrition and Neurodegenerative Diseases: The Role of Carbohydrates and Gluten
Caamao D¹, de la Garza A², Beltrán-Ayala P³ and Chisaguano AM¹*
¹Escuela de Salud Pública. Facultad de Ciencias de la Salud. Universidad San Francisco de Quito
²Department of Nutrition and Food Science, University of Barcelona, Spain
³Colegio de Administración y Economía. Universidad San Francisco de Quito
*Corresponding author: Chisaguano AM, Escuela de Salud Pública. Facultad de Ciencias de la Salud. Universidad San Francisco de Quito
Received: June 01, 2016; Accepted: July 29, 2016; Published: August 01, 2016
Abstract
Neurodegenerative diseases have both genetic and environmental influences. Nutrition is a non-genetic factor that has a transcendental influence on the prevention and treatment of these diseases. This review mainly emphasizes the effects of dietary gluten and carbohydrates on cognitive functions and development in most common neurodegenerative diseases. Thus, early clinical studies prove an association between high glucose levels (A1C), oxidative stress, and glycation caused by high carbohydrate diets, cognitive impairment, and brain tissue loss. Obesity and brain tissue loss linkage has been proven, using BMI and waist-hip ratio. Gluten has shown to cause inflammation and cognitive impairment because of its association with Alzheimer, Parkinson, Multiple Sclerosis, Autism and Depression. Mostly based on epidemiological and clinical data, we suggest considering this knowledge for an integral treatment of patients.
Keywords: Neurodegenerative Diseases; Cognitive Impairment; Gluten Intolerance; Carbohydrates
Neurodegenerative Diseases
Neuro-Degenerative Diseases (NDD) are pathologies affecting body functions such as balance, movement, respiration and diction. The incidence is increasing and becoming a threat of converting into a pandemic disease. NDD affect nearly a billion people all over the world, without sex, education, or income distinction. Approximately 6.8 million people die each year from NDD, plus great amounts of economic resources are invested for their treatment [1]. The economic costs associated with these diseases can be of several types. On one side, direct costs refer to those costs that are directly related to the disease manifestations, characteristics and particular needs. On the other side, indirect costs refer to those costs resulting from the limitations that the disease produces over the patient’s quality of life. Direct costs include the payment for medical care (doctors, medicines, hospitalization) and the payment for non-medical services (caregivers, home care, etc.). Most of non-medical services are provided by unqualified personnel (volunteers, friends, family members) who do not charge for their help, consequently becoming informal costs. However, from an economic perspective, these are resources that carry an opportunity costs (that could be used for an alternative purpose) which is why they also represent an economic cost. Indirect costs, on the other hand, although more complex at the time of being quantified, may achieve significant amounts for society. These costs account for the loss in labor productivity, replacement costs due to retirement and mortality costs. This group should also include the costs associated to the physical, psychological, and emotional impacts that these diseases have on patients and on their nearest environment, as well as the collateral negative effects on their life expectancy, life quality, and on their personal, social and professional development.
Within the neurodegenerative diseases, currently the one with highest incidence is Alzheimer, a chronic ailment with unknown cure, and highly incapacitating. It generally appears within people who are over 65 years old, that is to say, at the end of their productive life, and due to its slow progression, it may extend throughout time, deeply affecting the total costs. In 2015, just in the United States the total cost was estimated at $226 billion [2]. In Spain, according to the last study released in 2014, the annual total cost per patient was estimated in 6325 Euros, it can reach up to 33 and 56 billion in 2030 and 2050, respectively [3].
In many cases, Alzheimer may lead to Dementia. This disease, according to the Tenth International Classification of Mental Diseases (CIE-109) published by the World Health Organization (WHO) in 1992, is characterized by the deterioration of the memory, the intellectual capabilities and the social conduct, and by the lack of control over emotions and motivations. Although age is the major risk factor, this disease is not exclusive of the senescence but it may also be present in young individuals thus increasing the costs related to lost productivity [4]. In 2010 around 35.6 million people worldwide living with dementia and the figure is expected to double every 20 years [5]. In this way, the cost of NDD just in Europe in 2010 has been estimated at 143.000 million Euros, for dementia has been estimated at 16548 Euros and Parkinson´s disease by 11153 Euros for patient [6].
In Latin America, the costs do not vary significantly. However, the difference is that due to the lack of welfare policies, almost the entirety of costs is directly charged onto the patient and his family members. In this sense, poor people are more defenseless and unprotected against the ravages of these alterations [4].
Furthermore, Dementia is directly related to other neurodegenerative diseases. Around 30% of patients with Parkinson may present dementia [7]. Due to its incidence in the total population, this neurodegenerative disease is the most severe after Alzheimer.
Parkinson is a chronic, progressive and irreversible neurodegenerative disease that generally appears in people who are around 60 years old, producing elevated indirect costs [8]. This disease is not fatal by itself; average life expectancy of a patient with Parkinson is generally the same as the people who do not suffer from it. Therefore, direct costs for the family and for society may reach high amounts [9].
Another disease of neurological origin is depression, which, when recurrent, may become degenerative. It generates grave social incapacities and may achieve high mortality figures, making it an expensive and very costly disease for society [10]. Unlike other diseases whose prevalence is given to people over 60 years old, depression may affect people at the peak of their productive age, in the age range between 30 and 40 years old [11]. Furthermore, at the beginning of the millennium, dementia and depression were recognized as a severe public health issue. This recognition obeys not only to the high morbidity and mortality rates associated with theses, diseases, but also to the decrease in the patient’s healthy life years [11].
The high costs, both human and economic, of neurodegenerative diseases, require the urgent development of public policies that include social, economic and scientific elements, so as to prevent, address, approach and restraint the uncontrolled growth of its prevalence. In this way, the dietary components, which can prevent or promote neurologic health, are the key to future interventions for these pathologies. A healthy nutrition in childhood, adolescence and adulthood can prevent the risk of developing cognitive impairment and NDD at elder stages of life. In this way, research has described the power that nutrition has over prevention and treatment of NDD such as Alzheimer, Parkinson, autism, headaches and depression, even in genetic predisposed individuals [12-16]. The omega-3 lipids are nutrients that have shown to have protective effects [17]. Nutrition´s greatest influence is on prevention of physiological states such as inflammation or oxidative stress, which in chronic conditions, promote brain disrepair, neurodegenerative impairment, and in certain cases NDD. On the other hand, certain dietary nutrients have shown to favor states of chronicle inflammation and oxidative stress, previous stages of NDD. Epidemiological, experimental and animal research have established a close relation between high intake of carbohydrates and brain disrepair, caused by brain shrinkage [14,18], cognitive impairment [19,20], or even body weight [21,22].
On the other hand, the incidence of gluten intolerance and celiac disease has grown drastically in recent years, which can be explained by an increased use of genetically engineered foods. These genetic processes have modified food´s composition and the way body metabolizes these new processed foods [17,23]. Gluten intolerance (not synonym of celiac disease) has shown to have an influence on autism development and low brain performance, producing physiological changes in the brain [24,25].
Neurodegenerative Diseases and Diet
Neurodegenerative diseases are illnesses that affect the brain, specifically neurons. The most common symptoms caused by these diseases include malfunctions in balance, respiration, movement, reflexes, motor skills, or even in heart beat activity [26-28]. There are various risk factors that influence the incidence of NDD, these can be genetic, for example, the ApoE e4 protein on Alzheimer, or can be non-genetic factors. Nutrition is the non-genetic factor that has shown the greatest influence on NDD incidence and development [17]. The common factor in most NDD is a state of chronic inflammation, which is greatly influenced by nutrition.
The brain can suffer from inflammation, although it cannot be felt like in other tissues because the brain has no pain receptors, so its inflammation will be impossible to be detected without specific exams. Chronic brain inflammation will trigger accelerated cell death, interfering with proper chemical neuronal functions, causing cognitive impairment [29,30] and, in certain cases, it has been related to an increased risk of suffering from NDD [17,31,32]. Inflammatory processes are localized responses, which will remain activated during oxidative stress, creating a destruction and death cycle impossible to break without antioxidant defenses (Figure 1). This process starts as a body defensive mechanism, but in chronic conditions, it leads to cell impairment and death [17,31-34], the turning point in which changes from a protective mechanism into a chronic inflammation is still unknown.
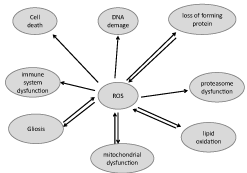
Figure 1: ROS effects over brain functions.
Research has shown that there are excessive amounts of cytosine, beta-amyloid plaque, and pro-inflammatory compounds (microglia and astrocytes) on Alzheimer patient’s brains. Oxidative stress has been shown to be a precursor of neurological impairment, a previous condition to the development of this disease. In addition Reactive Oxygen Species (ROS) reactivity has shown to interrupt nervous terminal activity, causing brain dysfunction, synopsis and memory loss [31,32,34].
Glycation
The excessive carbohydrate intake will lead to increased glucose, blood levels, causing an increase on glycation processes in the body. Glycation refers to the attachment of glucose molecules to amino acids, proteins or lipids [17,35]. These attachments can create post translational modifications in amino groups of proteins or lipoproteins. They can also create chemical modifications, which can change the molecule´s functionality [36]. Glycation´s advanced products will have effects on a protein´s shape and flexibility, leading to increased states of oxidative stress and inflammation processes [17]. Glycation is a normal process in our bodies that is responsible for natural signs of aging such as skin wrinkles or discolored skin.
Dietary factors, such as an excessive intake of carbohydrates, can lead to an increase of this process [17,37] along with harmful effects on organs such as the brain. This process is equally responsible for the vein and artery wall hardening that affect organ functions such as heart and brain, which are related to arteriosclerosis and vascular dementia. A glycated protein has a higher tendency for cross-linking with other proteins provoking a chain reaction that increases 50 times the production of ROS than a non-glycated protein. This process will end in lipid, protein, and DNA impairment and in accelerated cell death, which is a previous state to cognitive impairment and depression [16,17,36].
One of the most relevant glycations with NDD is the cholesterol´s glycation that will form the Low Density Lipoproteins (LDL). This will change its functionality, affecting neuronal health. The main LDL function is to trap cholesterol and transport it to the neurons, where it will participate in vital functions such as the plasmatic membrane synthesis, which is responsible for the neuronal cell’s permeability. An alteration in this lipoprotein will lead to an incapacity to enter to the astrocytes, cells filled with neurons, not only losing their function, but leaving the cells without cholesterol for vital functions [17,38].
Carbohydrates
Carbohydrates are food nutrients, whose main task is to provide energy for body funcitions [39]. The homeostatic balance between energy intake and expenditure has suffered a dramatic change in recent years, causing harmful effects on people’s health. The excessive dietary intake of carbohydrates, in comparison with its use as energy, has caused increased blood glucose levels in people.
This blood glucose level increment seems to be the first step for a series of chain reactions which cause a variety of complications that will increase the risk of suffering of neuronal impairment [40]. An investigation done by Cherbuin et al. has described that high glucose levels have been related with the reduction of certain parts of the brain. They demonstrated that people with “normal” blood glucose levels or people who were on normal superior ranges (above 100mg/ dL) have a higher risk of suffering brain shrinkage (between 6-10% of tissue) in memory and cognitive related areas of the brain. There was also a relation between higher levels of blood glucose and a greater area of brain tissue loss [18].
On the other hand, Kerti et al. analyzed the relation between blood glucose levels, cognitive performance (memory functions), and hippocampal volume, leading to similar results. People who had higher blood glucose levels showed lower scores on cognitive tests. This was explained by micro-structural hippocampal tissue loss [19].
Yaffee et al. in 2012 presented their study results concerning the relation between diabetes and cognitive impairment. When compared to non-diabetic patients, people who suffered from diabetes had substantially lower scores on cognitive tests showing an accelerated cognitive impairment. A direct relation was shown between impairment rate and high levels of glycosylated hemoglobin (A1C), an indicator of long term (90-120 days) glycemic control. The higher the A1C levels were, the faster the cognitive impairment was. This relation was explained by chronic states of inflammation, which were caused by an increased amount of final products of advanced glycation [20].
In addition to these studies, there are studies like those made by Enzinger et al. where they compared different degrees of brain tissue loss with glycosylated hemoglobin levels. They observed that higher levels of A1C duplicated the risk of brain damage in comparison to patients with lower levels of A1C. Therefore, diabetes is a risk factor for Alzheimer doubling the probability to develop this illness [41]. Hamer et al. related A1C with depression, describing the altered glucose metabolism as a risk factor, explained by protein glycation processes that altered the metabolism [42].
Also, obesity has been described as a risk factor for brain impairment. An excessive carbohydrate diet (over 60% of daily caloric intake) increases the risk of cognitive impairment, as described by Geroldi et al, who demonstrated that a higher waist-hip ratio was related with a smaller hippocampal area, which is related with memory functions. The study also observed that the higher the waisthip ratio was the higher chances the person had to suffer from brain strokes in adulthood [43].
Raji et al also determined that patients with a BMI>30kg/m2 (obesity) had similar brains to people 16 years older than people with a “normal” (BMI= 18.5 - 24.99 Kg/m2). They also observed that overweight people (BMI= 25 - 29.99 Kg/m2) had a brain apparently 8 years older than a person with normal weight. Obese patients had 8% less brain tissue than patients with normal weight, and overweight patients had 4% less brain tissue than patients with a normal BMI. The brain areas associated with tissue loss were frontal temporal regions, which are involved in decision making and memory [21].
Also Whitmer et al. evaluated the relation between body fat and dementia. They observed that people with the highest amounts of body fat were twice as likely to develop some sort of mental disorder. Therefore, hey concluded that central obesity (abdominal fat), just like diabetes and cardiovascular diseases, is a risk factor for developing dementia. This increment in body fat is known to be caused by an excessive dietary intake of simple carbohydrates [43,44]. Finally, Bigal and Lipton showed that chronic headaches on a daily basis, were 28% higher in obese participants (BMI > 30 Kg/m2) than in control group patients who had normal weights. Moreover, patients who suffered from morbid obesity had 74% higher probabilities from suffering of chronic headaches than control group [13].
Described evidence is relatively new; nevertheless, different studies lead to similar conclusions: The excessive fat storage, caused by a negative energy balance of high carbohydrate diets, impedes the body to use these fat reserves as an energy source. A high carbohydrate dietary intake continuously produces and releases insulin as a metabolic response for its utilization, but this excessive insulin production will also trigger fat storage, hence in-creasing lipogenesis and inhibiting its use for energetic purposes. As a result, the body will start to accumulate fat that cannot be used by the body as an energy source, because it will be “kidnapped” by constant high levels of insulin, caused by the continuous insulin production as a response to a high dietary intake of carbohydrates, creating a cycle that promotes obesity [17].
Gluten
Gluten is a protein found in most of carbohydrate rich foods, such as cereals like wheat, barley, rye, spelt, oats, and their derivatives [23,45]. They are mainly composed by glutein and gliadin, attached by intramolecular disulfide bonds, which provide elasticity, extensibility, sponginess, stickiness, and volume. The gliadins present in wheat have ideal characteristics for food industries, which is why many foods that are naturally gluten-free, in processed foods they include it [45,46]. Gliadins contain the majority of components that produce adverse effects on genetically predisposed gluten intolerant people. Wheat is the cereal with the greatest amount of gluten, on its majority, composed by gliadin, and it is also the most consumed cereal all over the world. The rest of cereals that contain gluten are composed from toxic peptides which are homologous to gliadins, like hordein in barley, secalin in rye, and avenin in oats [23,46,47].
Nowadays, food industry has modified cereals, causing them to contain 40 times more gluten than cereals that were produced decades ago, these modifications have caused metabolic alterations in people, with a great variety of health effects [17,23].
Seeking methods for optimizing food production, new hybridization and genetic alteration techniques have been developed in wheat and barley seeds. These modifications have altered food´s composition and little chemical and compositional relation is left with unprocessed cereals. Actually, the body is being exposed to ingredients that genetically is not prepared to metabolize [17,48,49].
Celiac disease is defined as “a permanent intolerance to gluten that is presented in genetic predisposed individuals and it is characterized by an inflammatory immune reaction in the intestinal mucus and obstructs the absorption of micro and macro nutrients”. The incidence on celiac disease has suffered a significant increase all over the planet [23,47,50] and estimations predict that 2% of the general population can suffer of celiac disease. On the other hand, gluten intolerance affects 1 out of 17 people and it can provoke different physical alterations, such as inflammation, intestinal damage and cognitive impairment [17,47]. A person can suffer from this gluten intolerance without presenting evident symptoms, reason why it can be confused with symptom like pathologies [17,50].
When the body reacts to a potential harmful exogenous compound, a defensive reaction triggers causing inflammation. The purpose of an inflammatory response is to identify and protect the body from harmful particles, chemical compounds or traumas. Inflammation mechanisms triggered by gluten particles have different causes, which include a lack of digestive enzymes (intestinal glutaminase), an increased production of anti-prolamin antibodies, increased inflammation mediators (histamine, serotonin, kinins, prostaglandins and interleukins) or increased intestinal permeability for macromolecules and antigen proteins [51]. These processes not only affect intestinal tissues which increases the probability of allergy development, but they also produce a high amount of cytokines which cause brain tissue damage [17]. Another problem caused by immune reactions triggered by gluten, are the auto-immune attacks by the anti-gliadins (antibodies in charge of identifying and neutralizing gliadins present in gluten). These anti-gliadins may confuse proteins found in the brain that are similar to gliadins and attack them without differentiation. This autoimmune process will cause an increment on inflammatory cytokines (monocytes, macrophages, and neutrophils) causing reactions that attack brain cells in various fronts [17,52]. Pathologies such as Alzheimer, Parkinson, multiple sclerosis, and autism have reported high cytokine levels, reason why therapists have focused on inflammatory prevention treatments. Hadjivassiliou et al. established that gluten intolerance is common in neurological disease with unknown cause, and it can have etiological relevance. In addition, people with gluten intolerance can have problems in brain function without presenting any gastrointestinal symptoms whatsoever [53,54].
Knowledge of gluten effects on cognitive functions is recent, however, as carbohydrate findings, evidence is concise. One of the first studies analyzing the relation between gluten and brain was performed by Whiteley et al in 1999. They found a conduct improvement on autism patients when they were exposed to a glutenfree diet, and when they consumed gluten accidentally, behavior change was alarming according to parents and professors [24,25].
Murray et al. in 2006, observed an association between progressive cognitive dysfunction and celiac disease. Some of the individuals that underwent a gluten-free diet had a significant cognitive improvement. The authors pointed out that cytokines in charge of inflammatory processes can have an impact on brain function aggravating cognitive problems, which sets a precedent for further and deeper investigations for development of new dementia treatment [55].
The author Ferreti et al. determined that people with celiac disease have an overproduction of ROS, which interact with lipids, proteins and even with DNA. Moreover, apart from the overproduction of ROS, people with this pathology suffer from alterations in antioxidant mechanisms, such as the reduction of glutathione (powerful cellular antioxidant), vitamin E, retinol, and vitamin C, which are compounds in charge of counterbalancing ROS effects. It is like if gluten´s presence would disable the immune system. Immune reactions have been proven to activate ciclooxigenase-2 (COX-2) and alfa tumoral necrosis factor (TNFA a) which are two pro inflammatory compounds characteristically present in Alzheimer disease. Nevertheless, dietary components such as omega-3, flavonoids, and carotenoids have shown to modulate the oxidative stress, caused immune response to gluten [29].
Ludvigsson et al. evaluated both the risk of depression for people that suffered from celiac disease, and the risk of having celiac disease when depressed. It was found that celiac patients had 80% higher risk of suffering depression, while the risk of depressed people to be diagnosed with celiac disease was increased a 230% [56]. James Greenblat, a psychiatric treatment pioneer, mentioned that untreated celiac disease can exacerbate the symptoms of depression, and could even be the underlying cause. He also declares that patients with depression should be evaluated for nutritional deficiencies because it is possible that celiac disease might be the correct diagnosis instead of depression [57].
A rich carbohydrate diet is each time more proven to have a close relation with cognitive impairment processes, increasing oxidative stress and chronic inflammation. Evidence is recent; however, investigations are each time proving with different evidence that is urgent to make changes not only in nutrition, but also on food´s composition and production technologies that can be harming our health. Increased gluten availability in population´s diet is having considerable effects on people´s health that consume it. Food daily choices can define human physiological conditions, which could define health status in time. This is the real importance of underlying the power of food on people´s health. It is highly important and necessary to develop new investigation lines in new areas that could help understand, from an integral point of view, the influence that food choices have on people’s health, for example in prevention and activation of neurodegenerative diseases.
References
- OMS. Los trastornos neurologicos afectan a millones de personas en todo el mundo: Informe de la OMS. Bruselas/Ginebra. 2007.
- Alzheimer Association. 2016 Alzheimer’s disease Facts and Figures. Alzheimer’s Dement 2016. 2016; 12:1-80.
- Madrid UC de. Estudio sobre las enfermedades neurodegenerativas en Espaa y su impacto económico y social. 2016.
- Campillo-Serrano C, Lopez M. Las enfermedades neurologicas. II. Depresión y demencia. Gac Med Mex. 2002; 138: 533-546.
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013; 9: 63-75.
- Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jonsson B, CDBE-2010 study group, European Brain Council. The economic cost of brain disorders in Europe. Eur J Neurol. 2012; 19: 155-162.
- Corona-Vazquez T, Campillo-Serrano C, López M, Mateos-G JH, Soto-Hernández JL. The neurologic diseases. Gac Med Mex. 2002; 138: 533-546.
- Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson's disease: A systematic review and meta-analysis. Mov Disord. 2014; 29: 1583-1590.
- De la Casa Fages B, Federación Espaola de Parkinson (FED). Guia informativa de la Enfermedad de Parkinson. 2015; 5-25.
- Saint Onge JM, Krueger PM, Rogers RG. The relationship between major depression and nonsuicide mortality for U.S. adults: the importance of health behaviors. J Gerontol B Psychol Sci Soc Sci. 2014; 69: 622-632.
- Campillo-Serrano C, López M. Las enferemedades neurologicas. II. Depresion y demencia. Gac Med Mex. 2002;138: 536-540.
- Wolf S, McGoldrick P, Sullvivan M. Pediatric Migraine Management. Pain Medicine News. 2003.
- Bigal ME, Lipton RB. Obesity is a risk factor for transformed migraine but not chronic tension-type headache. Neurology. 2006; 67: 252-257.
- Burns CM, Chen K, Kaszniak AW, Lee W, Alexander GE, Bandy D, et al. Higher serum glucose levels are associated with cerebral hypometabolism in Alzheimer regions. Neurology. 2013; 80: 1557-1564.
- Campdelacreu J. Parkinson disease and Alzheimer disease: environmental risk factors. Neurologia. 2014; 29: 541-549.
- Morgan RE, Palinkas LA, Barrett-Connor EL, Wingard DL. Plasma cholesterol and depressive symptoms in older men. Lancet. 1993; 341: 75-79.
- Perlmutter D, Lober K. Cerebro de Pan. Grijalbo. 2014; 384.
- Cherbuin N, Sachdev P, Anstey KJ. Higher normal fasting plasma glucose is associated with hippocampal atrophy: The PATH Study. Neurol. 2012; 79: 1019-1026.
- Kerti L, Witte AV, Winkler A, Grittner U, Rujescu D, Floel A. Higher glucose levels associated with lower memory and reduced hippocampal microstructure. Neurology. United States; 2013; 81:1746-1752.
- Yaffe K, Falvey C, Hamilton N, Schwartz A V, Simonsick EM, Satterfield S, et al. Diabetes, glucose control, and 9-year cognitive decline among older adults without dementia. Arch Neurol. 2012; 69: 1170-1175.
- Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, et al. Brain structure and obesity. Hum Brain Mapp. 2010; 31: 353-364.
- Roberts RO, Roberts LA, Geda YE, Cha RH, Pankratz VS, O'Connor HM, et al. Relative intake of macronutrients impacts risk of mild cognitive impairment or dementia. J Alzheimers Dis. 2012; 32: 329-339.
- FACE. El Gluten. 2016.
- Whiteley P, Shattock P, Knivsberg AM, Seim A, Reichelt KL, et al. Gluten- and casein-free dietary intervention for autism spectrum conditions. Front Hum Neurosci. 2013; 6: 344.
- Whiteley P. Nutritional management of (some) autism: a case for gluten- and casein-free diets? Proc Nutr Soc. 2015; 74: 202-207.
- NIH. Enfermedades Neurodegenerativas. 2015.
- Abril Carreres, Falguera NT, Garreta R, Rehabilitación S De, Mutua H, et al. Enfermedades neurodegenerativas. Rehabilitación. 2004; 38: 318-324.
- Baquero M, Blasco R, Campos-Garcia A, Garcés M, Fages EM, Andreu-Catala M. Descriptive study of behavioural disorders in mild cognitive impairment. Rev Neurol. 2004; 38: 323-326.
- Ferretti G, Bacchetti T, Masciangelo S, Saturni L. Celiac disease, inflammation and oxidative damage: A nutrigenetic approach. Nutrients. 2012; 4: 243-257.
- Sohal RS, Mockett RJ, Orr WC. Mechanisms of aging: An appraisal of the oxidative stress hypothesis. Free Radic Biol Med. 2002; 33: 575-586.
- Rubio-Perez JM, Morillas-Ruiz JM. A review: Inflammatory process in Alzheimer's disease, role of cytokines. ScientificWorldJournal. 2012; 2012: 756357.
- Agostinho P, Cunha RA, Oliveira C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer's disease. Curr Pharm Des. 2010; 16: 2766-2778.
- Angoa Pérez, Mariana, Arancibia SR. Estrés oxidativo y neurodegeneración: ¿causa o consecuencia? Arch Neurocien. 2007; 12: 45-54.
- Tuppo EE, Arias HR. The role of inflammation in Alzheimer's disease. Int J Biochem Cell Biol. 2005; 37: 289-305.
- Mendez JD. Advanced glycosylation end products and chronic complications of diabetes mellitus. Gac Med Mex. 2003; 139: 49-55.
- Gugliucci A. Glicación de proteínas? rol protagónico de la hiperglicemia en las complicaciones crónicas de la diabetes mellitus. Rev Med Uruguay. 2000; 16: 58-75.
- Rios de Molina MDC. El estrés oxidativo y el destino celular. Departamento de Química Biológica UBA. 2003.
- Elias PK, Elias MF, D’Agostino RB, Sullivan LM, Wolf PA. Serum cholesterol and cognitive performance in the Framingham Heart Study. Psychosom Med. 2005; 67: 24-30.
- Leon A. Hidratos de carbono. Bioquimica. 2008; 14-30.
- Rosado-Pérez J, Mendoza-Núez VM. Inflamación crónica y estrés oxidativo en la diabetes mellitus. Bioquimica. 2007; 32: 58-69.
- Enzinger C, Fazekas F, Matthews PM, Ropele S, Schmidt H, Smith S, et al. Risk factors for progression of brain atrophy in aging: Six-year follow-up of normal subjects. Neurology. 2005; 64: 1704-1711.
- Hamer M, Batty G, Kivimaki M. NIH Public Access. Psycol Med. 2012; 41: 1889-1896.
- Geroldi C, Frisoni GB, Paolisso G, Bandinelli S, Lamponi M, Abbatecola AM, et al. Insulin Resistance in Cognitive Impairment. Arch Neurol. 2005; 62: 1067-1072.
- Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. United States; 2008; 71: 1057-1064.
- American Diabetes Association. Qué Alimentos Contienen Gluten? 2015.
- Flores RV. El gluten del trigo y su rol en la industria de la panificación. Ing Ind. 2014; 32: 231-246.
- Parada A, Araya M. El gluten. Su historia y efectos en la enfermedad celíaca. Rev Med Chil [Internet]. 2010; 138: 1319-1325.
- Arroyo P. La alimentación en la evolución del hombre? su relacion con el riesgo de enfermedades crónico degenerativas. Bol Med Hosp Infant Mex. 2008; 65: 9-10.
- Sanchez Martín T. Plantas Transgénicas. 2008.
- Catassi C, Prof C, Catassi C. El mapa mundial de la enfermedad celíaca. Acta Gastroenterológica Latinoam. 2005; 35: 46-55.
- Bordes González, R, Martinez Beltrán, M, García Olivares, E, Guisado Barrilao R. El Proceso Inflamatorio. Enfermeria. 2010; 11: 9-12.
- Zamora ZB, Borrego A, Lopez OY, Delgado R, Gonzalez R, Menendez S, et al. Effects of ozone oxidative preconditioning on TNF-alpha release and antioxidant-pro-oxidant intracellular balance in mice during endotoxic shock. Mediators Inflamm. United States. 2005; 2005: 16-22.
- Verkasalo Ma, Raitakari OT, Viikari J, Marniemi J, Savilahti E. Undiagnosed silent coeliac disease: A risk for underachievement? 2005; 40:1407-1412.
- Hadjivassiliou M, Grünewald R. The Neurology of Gluten Sensitivity: Science Vs. Conviction. Pract Neurol. 2004; 4: 124-126.
- Murray CJL, Lopez AD. The global burden of disease: a comprehensive assessment of mortality and disability from deceases, injuries and risk factors in 1990 and projected to 2010. Harvard Univ Press. 1996; 1: 1-35.
- Ludvigsson JF, Reutfors J, Osby U, Ekbom A, Montgomery SM. Coeliac disease and risk of mood disorders-a general population-based cohort study. J Affect Disord. Netherlands. 2007; 99: 117-126.
- Greenblatt J. Is Gluten Making You Depressed? The Link between Celiac Disease and Depression. The Breakthrough Depression Solution (blog). Psychology Today. 2011.