
Research Article
Int J Nutr Sci. 2019; 4(1): 1028.
Potential Susceptibility to Liver Dysfunction Induced by a Therapeutic Dose of Acetaminophen in Rats Submitted to Gestational and Lactational Protein Malnutrition
Siqueira IR¹*, Vanzin SI², Tramontina AC³, Bianchetti P², Sbaraini S², Almeida LMV4, Stulp S2, Perry MLS4, Gonçalves CAS4 and Feoli AM5
¹Departamento de Farmacologia, Universidade Federal do Rio Grande do Sul, Brazil
²Centro Universitário Univates, Brazil
³Programa de Pós-Graduação em Ambiente e Sustentabilidade, Universidade Estadual do Rio Grande do Sul, Brazil
4Departamento de Bioquímica, Universidade Federal do Rio Grande do Sul, Brazil
5Programa de Pós-Graduação em Psicologia, Escola de Ciências da Saúde, Pontifícia Universidade Católica do Rio Grande do Sul, Brazil
*Corresponding author: Ionara Rodrigues Siqueira, Departamento de Farmacologia, Universidade Federal do Rio Grande do Sul, Rua Sarmento Leite, Porto Alegre, RS, Brazil
Received: October 08, 2018; Accepted: February 13, 2019; Published: February 20, 2019
Abstract
We investigated the potential susceptibility to liver dysfunction induced by a therapeutic dose of acetaminophen in rats submitted to gestational and lactational protein malnutrition using an animal model. Wistar rats were submitted to gestational and lactational protein malnutrition (control, 25% casein, and protein malnutrition, 7% casein). On the 10th (P10) or on the 25th (P25) a therapeutic dose of acetaminophen (15 mg/Kg, i.p.) was administered and rats were decapitated 6 hours after acetaminophen administration. Albumin content, Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT) activities were measured in plasma, as well as the Glutathione (GSH) levels and Glutathione S-Transferase (GST) activity in liver. In both age groups, the treatment with acetaminophen decreased albumin levels and increased ALT and AST activities in plasma. It was also observed decreased hepatic GSH levels and increased GST activity just at P10. An acute therapeutic dose of acetaminophen may induce a mild liver dysfunction in protein malnutrition.
Keywords: Protein malnutrition; Therapeutic dose of acetaminophen; Hepatotoxicity; Rats
Abbreviations
AST: Aspartate Aminotransferase; ALT: Alanine Aminotransferase; GSH: Glutathione; GST: Glutathione S-Transferase; PMN: Protein Malnutrition; PBS: Phosphate Buffered Saline; DTNB: 5,5´-Dithiobis-(2-Nitrobenzoic Acid); CYP: Cytochrome P-450; NAPQI: N-Acetyl-P-Benzoquinone Imine
Introduction
Acetaminophen, also known as paracetamol, is widely used as analgesic and antipyretic medication in the world and is considered to be safe at therapeutic dosages. It is widely accepted that an overdose of acetaminophen can induce severe liver damage in humans and in experimental animals; it is important to note that hepatic injury induced by therapeutic dose has been reported [1,2], however there are few studies in order to identify potential triggers for this condition. There are several evidences suggesting that hepatotoxicity of compounds might be related to protein malnutrition condition, for example, Kwashiorkor seems to be associated with exposure to aflatoxins [3,4].
Protein malnutrition can affect the pathways of acetaminophen metabolism [5-7]. Acetaminophen is metabolized by different pathways such as sulfation, glucuronidation, and some isoforms of hepatic microsomal cytochrome P450s, such as CYP1A2 and CYP3A. The P450 system can generate the reactive metabolite, N-Acetyl-PBenzoquinone Imine (NAPQI), which is subsequently detoxified through conjugation with Glutathione (GSH) by Glutathione S-Transferase (GST) [8]. However, at higher doses, saturation of conjugation pathways and the consequent depletion of GSH can increase NAPQI levels [9]; NAPQI is able to bind to several hepatic proteins, and this binding is associated with development of centrilobular necrosis [10]. Moreover, administration of hepatotoxic doses of acetaminophen can reduce GSH levels in liver and kidney [11]. It is important to note that malnutrition per se induced a reduction in the GSH content [12,13].
Although the hepatotoxicity of several compounds may be associated with protein malnutrition condition, few studies have evaluated the potential toxicity of therapeutic doses of acetaminophen on malnutrition conditions [14]. Therefore, it is reasonable to examine the effect of administration of acetaminophen on hepatic function in animals submitted to protein malnutrition.
Considering that acetaminophen is widely used in children, including those in malnourished conditions, in the present study, the effect of acute administration of a therapeutic dose of acetaminophen on hepatic function in neonatal and young rats, respectively, at 10th and 25thpostnatal days, submitted to pre- and postnatal protein malnutrition. In order to investigate the liver function, different parameters commonly employed as markers for cell damage, namely Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT) and albumin content was quantified in plasma. Furthermore, the involvement of GSH levels and GST activity was studied.
Materials and Methods
Animals
Wistar rats were maintained under standard conditions (12-h light/ 12- h dark, temperature 22 ± 2°C); food and water were given ad libitum. The experimental protocol was developed according to the guidelines of the Committee on Care and Use of Experimental Animal Resources, School of Veterinary Medicine and Animal Science of the Universidade de São Paulo, Brazil, and was approved by local research ethical committee.
Diets
The animals had free access to isocaloric diets (Table 1) containing 25% or 7% protein (casein), salts, and vitamins, as recommended by the Association of Official Analytical Chemists [15] and previously described by our group [16,17].
25% protein diet g/kg
7% protein diet
Casein (87% protein)a
287
80.5
L-Methionine
1.5
1.5
Fat (soybean oil)
150
150
Carbohydrate (corn starch)
501.5
708
Salt mixb
40
40
Vitamin mixc
10
10
Non-nutritive fiber
10
10
*Salt and vitamin compositions are according to Horwitz W., 1980. Energy for both diets was 4.3 kcal/g of diet.
aCasein, purity 87% (from Farmaquímica, Porto Alegre, Brazil), supplemented with 0.15% l-methionine (from Merck, Rio de Janeiro, Brazil).
aCasein, purity 87% (from Farmaquímica, Porto Alegre, Brazil), supplemented with 0.15% l-methionine (from Merck, Rio de Janeiro, Brazil).
bMineral mixture (from Roche, São Paulo, Brazil; mg/100 g of ration): NaCl, 557; KCl, 3.2; KH2PO4, 1556; MgSO4, 229; CaCO3, 1526; FeSO4 · 7H2O, 108; MnSO4 · H2O, 16; ZnSO4 · 7H2O, 2.2; CuSO4 · 5H2O, 1.9; CaCl2 · 6H2O, 0.09.
cVitamin mixture (from Roche, São Paulo, Brazil; mg/100 g of ration): vitamin A, 4; vitamin D, 0.5; vitamin E, 10; menadione, 0.5; choline, 200; P-Aminobenzoic Acid (PABA), 10; inositol, 10; niacin, 4; pantothenic acid, 4; riboflavin, 0.8; thiamin, 0.5; pyridoxine, 0.5; folic acid, 0.2; biotin, 0.04; vitamin B12, 0.003.
Table 1: Nutritional composition of the diets.
Malnutrition model
Prenatal and lactational malnutrition was induced by restricting the protein content of the diet mothers to 7% (control group: 25% protein) during the entire gestation and lactational periods, which results in a decrease in the global amount of nutrients accessible to pups. Malnutrition up to 21 and 25 days was induced by maintaining the same 7% protein diet. Both diets were isocaloric and were given ad libitum. The litter size was adjusted to eight pups per mother on the first postpartum day and animals were maintained at 22°C on a 12h light/12h dark cycle until experimental age. The mean weights were higher in the nourished group at P10 and P25 (respectively, 19.8 ± SD 1.7 and 78.0 ± SD 7.4) compared to protein malnutrition group (12.2 ± SD 1.7 and 22.6 ± SD 1.0).
Acetaminophen treatment
Animals of both gender were weighed on the 10th(P10) or 25th(P25) postnatal days. Acetaminophen (Paracetamol, N-(4- hydroxyphenyl) acetamide, CAS 103-90-2) at therapeutic dose (15mg/kg) was administered intraperitoneally [14]. The control group received physiological solution. The rats were distributed in four experimental groups (seven to eight per group): Normal Protein Diet and Saline (NS); Normal Protein Diet and Acetaminophen (NA); Protein Malnutrition and Saline (PMN S), Protein Malnutrition and Acetaminophen (PMN A).
Preparation of samples
The animals were decapitated 6 hours after acetaminophen administration and trunk blood was collected [14]. The blood was centrifuged at 4000 xg for 10 minutes. Plasma was separated and used for determination of concentration of total protein, albumin and the activity of Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT). Livers were removed immediately and immediately placed in liquid nitrogen.
Determination of plasma enzyme activities
The activities of AST and ALT in the blood plasma were measured by commercial kits (BioDiagnóstica - Laborclin, Paraná, Brazil).
Determination of plasma albumin levels
The plasma albumin content was measured by commercial kits (Labtest Diagnóstica S.A., Minas Gerais, Brazil).
Measurement of liver total GSH
Total glutathione content was determined by a slightly modified assay to that described previously Allen et al, 2001) [18] and first described by Tietze (1969) [19]. Briefly, livers were homogenized in Phosphate Buffered Saline (PBS) (0.01 M, pH 7.6), edetic acid (6.3 mmol, pH 7.5) and Triton-X (0.05%). Aliquots were taken for protein assay (triplicate). Sulfosalicylic acid (1%) was added and the mixture was allowed to precipitate for 2 h at 4°C. After centrifugation at 4000g for 15 min, protein-free supernatant was obtained. For glutathione determination, 25 μl of supernatant was assayed in a total volume of 200 μl phosphate buffer containing 462.6 μM 5,5´-Dithiobis-(2- Nitrobenzoic Acid) (DTNB), 0.5 U/ml glutathione reductase, and 0.3 mM NADPH. The reduction in DTNB was followed at 412 nm and 25°C, in relation to a calibration curve.
Determination of liver GST activity
The activity of GST was measured using 1-chloro-2,4- dinitrobenzene as a substrate [20,21]. Incubation mixtures contained homogenized liver, 1 mM 1-chloro-2,4-dinitrobenzene, 1 mM reduced GSH and 0.1 M potassium phosphate buffer, pH 6.5. GST activity was determined at 340 nm.
Statistical analysis
The data were analyzed using one and two-way ANOVA, considered factors were the nutrition status and acetaminophen treatment. Post hoc comparisons were made using Duncan’s test. A value of P <0.05 was considered to be significant. All data are presented as mean (± SD). The body weight was analyzed by Student’s t-test.
Results
The severity of the protein malnutrition model was confirmed by the decrease body weight on the 10th and 25th postnatal days (Student’s t-test, P <0.0001). Figure 1 presents the plasma transaminase levels, ALT (Figure 1A) and AST (Figure 1B) activities, quantified in order to assess the liver injury. In both ages, the plasma ALT and AST levels did not differ between Nourished (N) and Protein Malnutrition (PMN) groups that received saline (NS and PMNS) and the nourished group that received acetaminophen (NA).
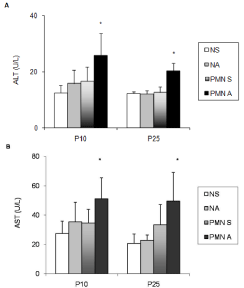
Figure 1: (A,B): Effects of acute acetaminophen treatment on plasma activities
of Alanine Aminotransferase (ALT, panel A) and Aspartate Aminotransferase
(AST, panel B) in rats exposed to pre and post-natal PMN and in nourished
controls at P10, and P25. Normal Protein Diet and Saline-Treated (NS);
Normal Protein Diet and Acetaminophen-Treated (NA); PMN and Saline-
Treated (PMN S), PMN and Acetaminophen-Treated (PMN A).
Columns represent means ±SD (seven to eight rats); Two-way ANOVA
(P<0.05),*, values significantly different from those of NS, NA and PMN S
groups, & value significantly different from PMN A group.
The acute treatment with acetaminophen significantly increased the ALT activity in PMN rats at P10 and P25 (Figure 1A; p=0.0031, p=0.0002, respectively). Two-way ANOVA showed a significant interaction between malnutrition and acetaminophen treatment on plasma ALT activity in both ages (Figure 1A; p = 0.04 and p = 0.003).
AST activity was increased PMN rats treated with acetaminophen at P10 (Figure 1B; p <0.0001); the two-way ANOVA also revealed a significant interaction between malnutrition and acetaminophen treatment factors at P10 (p=0.045). On P25, acetaminophen significantly increased the AST activity in PMN rats comparing to nourished group (p=0.043); although two-way ANOVA did not indicate a significant interaction between malnutrition and acetaminophen treatment.
The treatment with acetaminophen significantly reduced the plasma albumin content in PMN rats at P10 (Figure 2; p=0.0006). Two-way ANOVA showed a significant interaction between malnutrition and acetaminophen treatment on plasma albumin (p=0.0019). On P25, the albumin level was significantly lower in plasma PMN than in the plasma of nourished rats (p <0.0001).
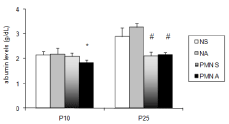
Figure 2: Effects of acute acetaminophen treatment on plasma albumin
content in rats exposed to pre and post-natal PMN and in nourished controls
at P10 and P25. Normal Protein Diet and Saline-Treated (NS); Normal
Protein Diet and Acetaminophen-Treated (NA); PMN and Saline-Treated
(PMN S), PMN and Acetaminophen-Treated (PMN A).
Columns represent means ±SD (seven to eight experiments); Two-way
ANOVA (P<0.05); *, values significantly different to those of NS, NA and PMN
S groups; #, values significantly different from those of NS and NA groups.
The liver GSH concentration and GST activity are presented in Figure 3. The GSH was significantly decreased in liver PMN rats at P10 (Figure 3A; p=0.0001). The acetaminophen administration did not alter the GSH content. No changes in GSH concentration were observed at P25. Saline PMN rats demonstrated a significant increase in hepatic GST activity at P10 (Figure 3B; p=0.0264). On day P25, GST activity did not significantly differ among groups.
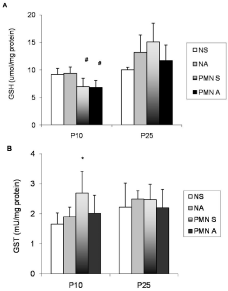
Figure 3: (A,B): Effects of acute acetaminophen treatment on hepatic
glutathione levels (GSH, panel 3A) and glutathione transferase activity (GST,
panel 3B) in rats exposed to pre and post-natal PMN and in controls at P10
and P25. Normal Protein Diet and Saline-Treated (NS); Normal Protein
Diet and Acetaminophen-Treated (NA); PMN and Saline-Treated (PMNS),
PMN and Acetaminophen-Treated (PMN A). Columns represent means
±SD (seven to eight experiments); Two-way ANOVA (P<0.05); *, values
significantly different from those of NS, NA and PMN S groups; #, values
significantly different from those of NS and NA groups.
Discussion
Our results demonstrated that an acute acetaminophen administration associated to gestational and lactational protein malnutrition induced a mild liver dysfunction in neonatal and young rats. Interestingly, it is important to note that acetaminophen treatment at the therapeutic dose did not affect any parameters studied in the nourished group.
Circulating transaminases have been considered as sensitive indicators of liver damage [22]. Injury to the hepatocytes alters their transport function and membrane permeability, leading to leakage of enzymes from the cells; this leakage causes increases in serum ALT and AST levels [23]. Undoubtedly, treatment with acetaminophen changed plasma ALT and AST in the PMN group. There was a significant interaction between PMN condition and acetaminophen administration on liver function. These results are in agreement with the hypothesis that protein malnutrition is an important risk factor and contributes to hepatotoxicity of therapeutic acetaminophen doses. These data have clinical relevance, since acetaminophen is used in children and pregnant women, including those in malnourished conditions. A similar effect of susceptibility to hepatotoxicity was observed using undernourished male rats at 18 weeks of age [14].
At P10, in saline-treated PMN rats, plasma albumin levels were similar to those of nourished groups; however, hypoalbuminemia (about 15%) was induced in the acetaminophen PMN group. Our results are in accordance with those postulated by Fuhrman et al (2004) [24], that the impact of protein malnutrition on serum albumin is neither immediate nor dramatic. In contrast to the reports of other authors suggesting that total plasma protein levels and serum albumin are inadequate indicators of malnutrition effects in drug metabolism studies [25], we observed a significant interaction of the PMN condition and the therapeutic dose of acetaminophen on the plasma albumin levels at P10 in rats. The reduction in plasma albumin content can be related to a decline in the synthesis of aminoacid precursors of albumin [26]. It is important to comment that previously acetaminophen reduced the serum albumin, globulin and total protein levels, which were attributed to histological damage, such as decreased hepatocytes for protein synthesis due to apoptosis and necrosis [27,28].
Reductions in the GSH content at P10 induced by malnutrition are in agreement with those findings obtained by Cho and colleagues [29] and Li and colleagues [13]. Furthermore, recently, we found that the brain glutathione content was significantly lower in PMN rats than in controls at P2 [17]. GSH is involved in a wide range of metabolic functions, including maintenance of protein sulfydryl status and detoxification of electrophilic xenobiotics, as well as an antioxidant function. Lower hepatic GSH levels in PMN group could indicate increased NAPQI levels that bind to hepatic protein targets, associated to centrilobular necrosis [10]. Conversely, the observed increased in plasma transaminase activities in the acetaminophentreated PMN group might not be associated with GSH levels, since we did not observe any changes on GSH levels at P25. At this age, we did not found any changes on GSH levels in acetaminophen-treated groups. This result suggests a lack of involvement of alterations on GSH content and the hepatotoxicity indexes, assessed by plasma transaminase activities. Previous studies may support this finding, since acute fasting increases acetaminophen-induced hepatic necrosis in male hamsters, even in the presence of increased hepatic levels of GSH [30]; besides Redegeld and colleagues demonstrated that the depletion of glutathione per se did not redue the cell viability in hepatocytes, however its depletion associated with reduced ATP levels can induce cytotoxic [31].
Interestingly, the generation of the glucuronide and sulfate conjugates may be altered by protein malnutrition. Protein malnutrition can reduce the sulfate levels [5]; in accordance Jung [32] demonstrated that protein malnutrition decreases the acetaminophen sulfate levels. The depletion of sulfate during PMN might be involved with maintenance of cysteine metabolism for GSH synthesis, as well as to the fact that the transamination pathway is altered, since the catalysis of cysteine sulfinate is performed by AST, yielding pyruvate and sulfate [33]. Subsequently, we can suggest that the level of sulfate might be lowered in treated PMN rats, given that there were an increase on plasma AST levels and probably a decrease on AST levels in hepatocyte, reducing the transamination of cysteine sulfinate to pyruvate and sulfate. PMN affects levels of two isoforms of hepatic microsomal cytochrome P450s, the CYP1A2 and CYP3A proteins, responsible for the metabolism of acetaminophen [7]. Besides, the acetaminophen glucuronide concentrations are altered in proteindeficient rats [33].
Interestingly, PMN rats demonstrated a significant increase in hepatic GST; this altered enzyme activity may be a compensatory mechanism that responds to altered GSH content. These findings are in agreement with previous studies where PMN increased rat liver GST activity [34,35]. Ramdath and Golden [36] observed that severe malnutrition results in the induction of GSTs in erythrocytes in malnourished children (malnourished; marasmic; kwashiorkor; marasmic-kwashiorkor) and suggested that the induction of erythrocyte GST may result in oxidative stress.
We concluded that protein malnutrition may produce biochemical changes, leading to a susceptibility to liver dysfunction induced by acetaminophen in neonatal and young rats. This vulnerability was demonstrated by alterations on plasma transaminase activities and albumin content in malnourished rats that received an acute therapeutic dose of acetaminophen. Further studies are necessary to determine the exact role of malnutrition on acetaminophen toxicity, however this study reinforce the idea that additional caution is needed in children submitted to gestational and postnatal protein malnutrition.
Acknowledgment
We gratefully acknowledge financial support by CNPq, UNIVATES and FUNADESP.
References
- Moling O, Cairon E, Rimenti G, Rizza F, Pristera R, Mian P. Severe hepatotoxicity after therapeutic doses of acetaminophen. Clin Ther. 2006; 28: 755-760.
- Black M. Acetaminophen hepatotoxicity. Annu Rev Med. 1984; 35: 577-593.
- Hendrickse RG. Kwashiorkor and aflatoxins. J Pediatr Gastroenterol Nutr. 1988; 7: 633-636.
- Oyelami OA, Maxwell SM, Adelusola KA, Aladekoma TA, Oyelese AO. Aflatoxins in autopsy kidney specimens from children in Nigeria. J Toxicol Environ Health. 1998; A55: 317-323.
- Galinsky RE, Levy G. Dose-and time-dependent elimination of acetaminophen in rats: pharmacokinetic implications of cosubstrate depletion. J Pharmacol Exp Ther. 1981; 219: 14-20.
- Jung D. Disposition of acetaminophen in protein-calorie malnutrition. J Pharmacol Exp Ther. 1985; 232: 178-182.
- Lee PC, Struve MF, Bezerra JA, Duncan B. Effects of protein malnutrition on liver cytochrome p450s. Nutr Res. 1997; 17: 1577-1587.
- Dahlin DC, Miwa GT, Lu AY, Nelson SD. N-acetyl-pbenzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci. 1984; 81: 1327-1331.
- Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie B. Acetaminophen-induced hepatic necrosis: I. Role of drug metabolism. J Pharmacol Exp. 1973; 187: 185-194.
- Qiu Y, Benet LZ, Burlingame AL. Identification of the hepatic protein targets of reactive metabolites of acetaminophen in vivo in mice using two-dimensional gel electrophoresis and mass spectrometry. J Biol Chem. 1998; 273: 17940- 17953.
- Tolson JK, Dix DJ, Voellmy RW, Roberts SM. Increased hepatotoxicity of acetaminophen in Hsp70i knockout mice. Toxicol Appl Pharmacol. 2006; 210: 157-162.
- Cho MK, Kim YG, Lee MG, Kim SG. The effect of cysteine on the altered expression of class alpha and mu glutathione S-transferase genes in the rat liver during protein-calorie malnutrition. Biochim Biophys Acta. 2000; 1502: 235-246.
- Li J, Wang H, Stoner GD, Bray TM. Dietary supplementation with cysteine prodrugs selectively restores tissue glutathione levels and redox status in protein-malnourished mice. J Nutr Biochem. 2002; 13: 625-633.
- González-Mendoza M, Vicuña-Fernandez N. Modification of liver enzymes in undernourished rats treated with acetaminophen. Gac Med Mex. 2003; 139: 429-433.
- Horwitz W. Official methods of analysis of the Association of Official Analytical Chemists AOAC. Washington, DC, USA. 1980.
- Schweigert I, de Oliveira DL, Scheibel F, da Costa F, Wofchuk ST, Souza D, et al. Gestational and postnatal malnutrition affects sensitivity of young rats to picrotoxin and quinolinic acid and uptake of GABA by cortical and hippocampal slices. Dev Brain Res. 2005; 154:177-185.
- Feoli AM, Siqueira I, Almeida LM, Tramontina AC, Battu C, Wofchuk ST, et al. Brain glutathione content and glutamate uptake are reduced in rats exposed to pre- and postnatal protein malnutrition. J Nutr. 2006; 136: 2357-2361.
- Allen S, Shea JM, Felmet T, Gadra J, Dehn P. A kinetic microassay for glutathione in cells plated on 96-well microtiter plates. Methods in Cell Sci. 2001; 22: 305-312.
- Tietze F. Enzimatic method for quantitative determination of nanogram amount of total oxidized glutathione: Applications to mammalian blood and other tissues. Anal Biochem. 1969; 27: 502-522.
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974; 249: 7130- 7139.
- Habig WH, Jakoby WB. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981; 77: 398-405.
- Molander DW, Wroblewski F, Ladue JS. Serum glutamic oxalacetic transaminase as an index of hepatocellular integrity. J Lab Clin Med. 1955; 46: 831-839.
- Yadav NP, Dixit VK. Hepatoprotective activity of leaves of Kalanchoe pinnata. Pers J Ethnopharmacol. 2003; 86: 197-202.
- Fuhrman MP, Charney P, Mueller CM. Hepatic proteins and nutrition assessment. J Am Diet Assoc. 2004; 104: 1258-1264.
- Mao ZL, Tam YK, Coutts RT. Effect of protein and calorie malnutrition on drug metabolism in rat - in vitro. J Pharm Pharm Sci. 2006; 9: 60-70.
- Davenport DJ, Mostardi RA, Richardson DC, Gross KL, Greene KA, Blair K. Protein-deficient diet alters serum alkaline phosphatase, bile acids, proteins and urea nitrogen in dogs. J Nutr. 1994; 124: 2677S-2679S.
- Yousef MI, Omar SAM, El-Guendi MI, Abdelmegid LA. Potential protective effects of quercetin and curcumin on paracetamol-induced histological changes, oxidative stress, impaired liver and kidney functions and haematotoxicity in rat. Food Chem Toxicol. 2010; 48: 3246-3261.
- El-Maddawy ZK, El-Sayed YS. Comparative analysis of the protective effects of curcumin and N-acetyl cysteine against paracetamol-induced hepatic, renal, and testicular toxicity in Wistar rats. Environ Sci Pollut Res Int. 2018; 25: 3468-3479.
- Cho MK, Kim YG, Lee MG, Kim SG. The effect of cysteine on the altered expression of class alpha and mu glutathione S-transferase genes in the rat liver during protein-calorie malnutrition. Biochim Biophys Acta. 2000; 1502: 235-246.
- Miller MG, Price VF, Jollow DJ. Anomalous susceptibility of the fasted hamster to acetaminophen hepatotoxicity. Biochem Pharmacol. 1986; 35: 817-825.
- Redegeld FA, Moison RM, Koster AS, Noordhoek J. Depletion of ATP but not of GSH affects viability of rat hepatocytes. J Eur Pharmacol. 1992; 228: 229-326.
- Jung D. Disposition of acetaminophen in protein-calorie malnutrition. J Pharmacol Exp Ther. 1985; 232:178-182.
- Stipanuk MH, Londono M, Lee JI, Hu M, Yu AF. Enzymes and metabolites of cysteine metabolism in nonhepatic tissues of rats show little response to changes in dietary protein or sulfur amino acid levels. J Nutr. 2002; 132: 3369-3378.
- Sidhu P, Garg ML, Dhawan DK. Protective effects of zinc on oxidative stress enzymes in liver of protein deficient rats. Nutr Hosp. 2004; 19: 341-347.
- Zhang W, Parentau H, Greenly RL, Metz CA, Aggarwal S, Wainer IW, et al. Effect of protein-calorie malnutrition on cytochromes P450 and glutathione S-transferase. Eur J Drug Metab Pharmacokinet. 1999; 24: 141-147.
- Ramdath DD, Golden MH. Elevated glutathione S-transferase activity in erythrocytes from malnourished children. Eur J Clin Nutr. 1993; 47: 658-665.