
Review Article
Int J Nutr Sci. 2020; 5(1): 1040.
A Review on Remdesivir: An Alternative Antiviral Drug to Fight against COVID-19
Singh N*, Suthar B, Mehta A and Pandey A
Department of Chemistry, Dr. H.S. Gour University, INDIA
*Corresponding author: Nimesh Singh, Department of Chemistry, Dr. H.S. Gour University, Sagar, Flax Laboratories, B-29/1, Mahad MIDC, Taluka- Mahad, Distt. – Raigad, M.H., India
Received: June 01, 2020; Accepted: June 27, 2020; Published: July 04, 2020
Abstract
A coronavirus infection that was discovered in early 2019 has already hampered the world’s purpose so far. The number of infectious cases has grown worldwide to 35 lakhs and so the outbreak has been described as a pandemic by the planet’s health organization, but there have not been any “specific drugs” or vaccines available to date. Relevant reports have identified a novel coronavirus with 80% homology with SARS. So there are few indications available when other countries are using the antiviral drug Remdesivir. The specific antiviral drug regimen for the treatment of patients with acute coronavirus disease 2019 (COVID-19) has not been proven. Remdecivir (GS-5734), a nucleoside analog product, has inhibitory effects on pathogenic animal and human coronaviruses including acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in vitro, and Middle East respiratory syndrome, NiR. -1, and SARS-CoV-2 replication in animal models.
Keywords: Anti-viral drug; Remdesivir; COVID-19; SARS
Introduction
The epidemic of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has led to more than 4 692 797 cases and 195 920 deaths worldwide as of April 9, 2020. Half of Coronavirus Disease 2019 (COVID-19) patients requiring minimally invasive mechanical ventilation often require hospitalization, and overload in health care systems, especially intensive care units, are prevalent in many affected countries.
Remdesivir is an antibiotic developed by an American company known as biopharmaceutical Gilead. Is a nucleotide analog, a direct analogue of adenosine, which is inserted into the viral RNA chains, making its termination premature. Is being studied in 2020 as a treatment option after COVID-19 infection [1]. In January 2020, Gilead launched a labdreivir trial against SARS-CoV-2, stating that transfers were shown to be inconsistent with SARS and MERS in animal models [2-4]. In March 2020, the a little emergence of remdesivir in rhesus macaque monkeys with COVID-19 disease was found to inhibit disease progression [5,6]. On January 21, 2020, the Wuhan Institute of Virology applied for a Chinese patent for “COVID-19” [7]. On 18 March 2020 the planet Health Organization (WHO) announced the launch of a four-arm health trial that could include one group of patients treated with remdesivir [8,9]. While a cohort study published in April 2020 identified potential improvements, finding out whether or not this drug is effective requires randomized controlled trials [10].
As of April 2020, remdesivir was viewed because the most promising treatment for CCIDID-19 is Johns Hopkins University [11] and there have been several ongoing clinical trials or schedules [12-22].
Gilead has initiated two Phase 3 clinical studies to ensure the safety and efficacy of COVID-19-diagnosed adults following the review and immediate approval of the new Gilead Investigation (IND) drug. These randomized, open-ended, multicenter coding studies began enrolling patients in March 2020 and could enroll approximately 1,000 patients in the first phase of the study, in countries with the highest COVID-19.
Mechanism of action
Antiviral drugs are a class of drugs used to treat viral infections [23]. Most antivirals are focused on specific viruses, while a broadspectrum antiviral is effective against a good list of viruses [24]. Unlike many antibiotics, antibiotics do not destroy their pathogen; instead they hinder their growth.
Antiviral drugs are a single class of antimicrobials, a large and compound group of antibiotics (also called antibacterial), antifungal and antiparasite drugs [25], or antiviral drugs supported by monoclonal antibodies [26]. Most antiviral agents are considered to be harmless to the housekeeper, and as a result are often therapeutic. They should be separated from the viricides, which are nonpharmacological but activate or destroy viral particles, either inside or outside the body. Natural viricides are produced by other plants such as eucalyptus and Australian tea trees [27].
Virus life cycle
Viruses include the gene and sometimes enzymes that are stored in a protein-made capsule (called a capsid), and are sometimes covered with a lipid layer (sometimes called an ‘envelope’). Viruses cannot reproduce on their own and instead propagate by suppressing a number cell to supply their copies, thereby producing the next generation.
Researchers working on such “rational design” strategies for the development of antivirals have tried to attack viruses at every stage of their life cycles. Mushrooms of some species have been found to contain multiple antiviral chemicals with synergistic effects. Compounds isolated from fruiting and varied mushroom filtrates have broad-spectrum antiviral activity, but the successful production and availability of compounds such as frontline antiviral may be far from over [29]. Viral life cycles vary in their exact details of the type of virus, but they all share a common pattern:
1. Attachment to a number cell.
2. Release of viral genes and enzymes into the host cell.
3. Replication of viral components using host-cell machinery.
4. Assembly of viral components into complete viral cells.
5. Release of viral cells to infect new host cells.
Remdesivir as key medicine to treat COVID-19
Since the primary instances had been said in December 2019, contamination with the Severe Acute breathing Coronavirus 2 (SARS-CoV-2) has grow to be a global pandemic [30,31]. Covid-19- the illness as a result of SARS-CoV-2 — is overwhelming health care systems globally [32,33]. The signs of SARS-CoV-2 contamination vary widely, from asymptomatic ailment to pneumonia and lifethreatening complications, along with acute respiration misery syndrome, multisystem organ failure, and ultimately, death [34- 37]. Older sufferers and those with preexisting respiratory or cardiovascular situations appear to be at the great danger for intense complications [35,36]. Inside the absence of a proven effective therapy, current management consists of supportive care, consisting of invasive and noninvasive oxygen guide and remedy with antibiotics [37,38]. Additionally, many sufferers have obtained off-label or compassionate-use therapies, along with antiretrovirals, antiparasitic agents, antiinflammatory compounds, and convalescent plasma [39-42].
Remdesivir can be a prodrug of a nucleotide analogue that’s intracellularly metabolized to an analogue of ATP that inhibits viral RNA polymerases. Remdesivir has broad-spectrum interest in opposition to participants of numerous virus families, together with filoviruses (e.G., Ebola) and coronaviruses (e.G., SARS-CoV and Middle East Respiratory Syndrome Coronavirus [MERS-CoV]) and has shown prophylactic and healing efficacy in nonclinical models of those coronaviruses [43-46]. In vitro trying out has additionally proven that remdesivir has pastime in opposition to SARS-CoV-2.
Remdesivir appears to own a high-quality clinical protection profile,as stated on the idea of experience in approximately 500 persons, along with wholesome volunteers and sufferers handled for acute Ebola virus contamination [47,48], and supported by means of our data (on document and shared with the planet Health Organization [WHO]). At some stage in this report, we describe outcomes at some stage in a cohort of patients hospitalized for extreme Covid-19 who have been dealt with with remdesivir on a compassionate-use basis.
Covid-19 cause and symptoms
Covid-19 may be a disease caused by a completely unique humaninfecting beta coronavirus. The coronavirus was identified in Wuhan city of Hubei Province in China, using next-generation sequencing and real-time RT-PCR, in December 2019. The disease commonly spreads through respiratory droplets of the infected people. The first symptoms of the disease are fever, cough and shortness of breath. The people infected with novel coronavirus mostly suffer from pneumonia
Remdesivir mechanism of action
Remdesivir is a 1'-cyano-substituted adenosine nucleotide analogue with broad-spectrum antiviral activity against various RNA viruses. The compound undergoes a metabolic mechanism, activating nucleoside triphosphate metabolite for inhibiting viral RNA polymerases (Figure 1).
1 SARS-CoV-2 enters target cells by binding the S protein to the ACE2 receptor on the cell surface; 2 Remdeivir, the nucleotide analogues, act as RdRp inhibitors, can provide a scheme for blocking RNA replication; 3Once remdesivir added into the growing chain (i position), is cannot cause an immediate stop. On the contrary, it will continue to extend three more nucleotides down to stop the strand at (i + 3) position; 4 Remdesivir triphosphate cannot be removed by nsp14-ExoN [49].
Conclusion
Remdecivir may be a nucleotide analog product that is currently being evaluated in clinical trials for the treatment of EVD and COVID-19. Preclinical studies have shown that remdecivir exhibits potent antiviral activity against multiple ebolavirus strains. The intracellular levels of NTP pools correlated with increased antiviral activity, the corresponding triphosphate being the active type of inhibitor and hence the viral RdRp target. Here we confirmed this confirmed hanu and demonstrated that remdecivir-TP is a substrate for the purified EBOV RdRp complex. Remdecivir-TP is an adenosine analog and therefore competes with ATP for merging. However, the merged inhibitor does not act as a sequence terminator. Inhibition of RNA synthesis appears mainly in the i + 5 position. Despite subtle sequence-dependent effects, RNA synthesis usually ends in the present. Increasing the concentration of the latter nucleotide does not overcome this effect. Therefore, it is reasonable to conclude that the delayed chain termination is the main cause of the antiviral action of remedicavir.
To date, no treatment efficacy has been demonstrated for patients with Covid-19. This preliminary report describes clinical outcomes during a small set of patients who were critically ill and treated with Remedicivir with Covid-19. Data from several ongoing randomized, controlled trials will soon provide further information about the safety and efficacy of Remedicivir for Covid-19, however, the results observed in this compassionate-use program are currently the simplest data available. In particular, an improvement in oxygensupport status was observed in 68% of patients, and overall mortality was 13% over the 18-day median follow-up. In a recent randomized, controlled trial of lopinavir-ritonavir in patients hospitalized for covid-19, 28-day mortality was 22%. [50].
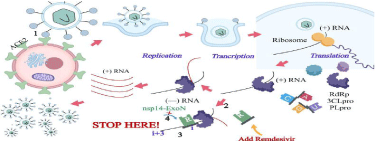
Figure 1: SARS-CoV-2 invasion process and how remdesivir works.
No new safety signals were found during the short-term remedicavir treatment during this compassionate-use cohort. Nonclinical toxicology studies have shown renal impairment, but nephrotoxicity thanks to remedicavir therapy remains unclear. Mild to moderate elevations were observed in ALT, AST, or both during the cohort of patients with severe Covid-19, as reported in studies in healthy volunteers and Ebola-infected patients [51].
References
- Brunk D. Remdesivir Under Study as Treatment for Novel Coronavirus. Medscape. 2020.
- de Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci. USA. 2020; 117: 6771–6776.
- Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Science Translational Medicine. 2017; 9.
- Joseph SS, Samuel M. Gilead working with China to test Ebola drug as new coronavirus treatment. Thomson Reuters. 2020.
- Munster V, Feldmann F, Williamson B, Van Doremalen N, Perez-Perez L, Schultz J, et al. Respiratory disease and virus shedding in rhesus macaques inoculated with SARS-CoV-2. bioRxiv. 2020.
- Williamson B, Feldmann F, Schwarz B, Meade-White K, Porter D, Schulz J, et al. Clinical benefit of remdesivir in rhesus macaques infected with SARSCoV- 2. bioRxiv. 2020.
- “Solidarity” clinical trial for COVID-19 treatments. WHO. 2020.
- UN health chief announces global ‘solidarity trial’ to jumpstart search for COVID-19 treatment. UN News. 2020.
- Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. The New England Journal of Medicine. 2020.
- Coronavirus COVID-19 (SARS-CoV-2). Johns Hopkins ABX Guide. 2020.
- Remdesivir Clinical Trials. Gilead Sciences. 2020.
- “7 Studies found for: Remdesivir & Recruiting, Not yet recruiting, Active, not recruiting, Completed, Enrolling by invitation Studies & COVID-19”. Clinical Trials. gov. U.S. National Library of Medicine. 2020.
- A Trial of Remdesivir in Adults With Severe COVID-19. ClinicalTrials.gov. 2020.
- A Trial of Remdesivir in Adults With Mild and Moderate COVID-19. ClinicalTrials.gov. 2020.
- Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734) in Participants With Moderate Coronavirus Disease (COVID-19) Compared to Standard of Care Treatment. ClinicalTrials.gov. 2020.
- Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734) in Participants With Severe Coronavirus Disease (COVID-19). ClinicalTrials. gov. 2020.
- Adaptive COVID-19 Treatment Trial (ACTT). ClinicalTrials.gov. 2020.
- Trial of Treatments for COVID-19 in Hospitalized Adults (DisCoVeRy). ClinicalTrials.gov. 2020.
- Expanded Access Remdesivir (RDV; GS-5734). ClinicalTrials.gov. 2020.
- Expanded Access Treatment Protocol: Remdesivir (RDV; GS-5734) for the Treatment of SARS-CoV2 (CoV) Infection (COVID-19). ClinicalTrials.gov. 2020.
- Multi-centre, adaptive, randomized trial of the safety and efficacy of treatments of COVID-19 in hospitalized adults (DisCoVeRy). European Union Clinical Trials Register. 2020.
- Barmann J. Bay Area-Based Gilead Sees Potential Legal Conflict With China Over Its Coronavirus Drug. SFist. Impress Media. 2020.
- Medmicro Chapter 52. Archived from the original on 18 August 2000. Retrieved 21 February 2009.
- Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antiviral Res. 2014; 110: 94–103.
- Rick Daniels, Leslie H. Nicoll. Pharmacology – Nursing Management. Contemporary Medical-Surgical Nursing. Cengage Learning. 2011.
- Kisung Ko, Yoram Tekoah, Pauline M. Rudd, David J. Harvey, Raymond A. Dwek, Sergei Spitsin, et al. Function and glycosylation of plant-derived antiviral monoclonal antibody. PNAS. 2003; 100: 8013–8018.
- Schnitzler P, Schön K, Reichling J. Antiviral activity of Australian tea tree oil and eucalyptus oil against herpes simplex virus in cell culture. Die Pharmazie. 2001; 56: 343–347.
- Lindequist U, Niedermeyer THJ, Wolf-Dieter WD. The Pharmacological Potential of Mushrooms. Evidence-Based Complementary and Alternative Medicine. 2005; 2: 285–299.
- Prabin P, Vidya M, Feraz AM. Chandra AD, Muralikrishnan D, (editors). Antiviral Potency of Mushroom Constituents. Medicinal Mushrooms: Recent Progress in Research and Development. Springer Singapore. 2019; 275–297.
- Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020; 91: 157-160.
- Spinelli A, Pellino G. COVID-19 pandemic: perspectives on an unfolding crisis. Br J Surg. 2020.
- Fauci AS, Lane HC, Redfield RR. Covid-19 — navigating the uncharted. N Engl J Med. 2020; 382: 1268-1269.
- Mahase E, Kmietowicz Z. Covid-19: doctors are told not to perform CPR on patients in cardiac arrest. BMJ 2020; 368.
- Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, RhuviVillamizar-Peña, YeimerHolguin-Rivera, Juan PabloEscalera-Antezana, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020.
- Weiss P, Murdoch DR. Clinical course and mortality risk of severe COVID-19. Lancet. 2020; 395: 1014-1015.
- Wu C, Chen X, Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020.
- Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020.
- Poston JT, Patel BK, Davis AM. Management of critically ill adults with COVID-19. JAMA. 2020.
- Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir– ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020.
- Shen C, Wang Z, Zhao F. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020.
- Touret F, de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020.
- Baden LR, Rubin EJ. Covid-19 — the search for effective therapy. N Engl J Med. 2020.
- de Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A. 2020; 117: 6771-6776.
- Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017; 9.
- Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020; 11: 222-222.
- Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020; 30: 269-271.
- Mulangu S, Dodd LE, Davey RT Jr, Mbaya OT, Proschan M, Mukadi D, et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019; 381: 2293-2303.
- European Medicines Agency. Summary on compassionate use: Remdesivir Gilead. 2020.
- Manli Wang, Ruiyuan Cao, Leike Zhang, Xinglou Yang, Jia Liu, Mingyue Xu, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Research. 2020; 30: 269–271.
- Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir– ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020.
- Cao YC, Deng QX, Dai SX. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: An evaluation of the evidence. Travel Med Infect Dis. 2020.