
Research Article
Austin J Obes & Metab Synd. 2021; 5(1): 1023.
A Multi-Center Study Exploring the Association of Metabolic Syndrome and Non-Alcoholic Fatty Liver Disease in Cuban Patients
Castellanos-Fernández MI1, Stepanova M2, Infante-Velázquez ME1, Henry L2*, Crespo-Ramírez E3, del Valle-Díaz S4, Elias JD5, Santaló-Rodríguez L5, Corrales-Alonso S6, Morales-Martínez I7, Cedeño-Ramirez E8, Pérez-González T9, González-Suero SM1, Ruenes-Domech C1, Suárez EB10, Racila A11, Guridi ZD1 and Soler EA1
1Institute of Gastroenterology, University of Medical Sciences of Havana, Havana, Cuba
2Center for Outcomes Research in Liver Diseases, Washington, District of Columbia, USA
3HospitalGeneral Docente Abel Santamaría Cuadrado, Pinar del Río, Cuba
4HospitalProvincialSaturnino Lora, Santiago de Cuba, Cuba
5HospitalUniversitario General Calixto García, La Habana, Cuba
6HospitalUniversitario Faustino Pérez, Matanzas, Cuba
7HospitalOncológico Celestino Hernández Robau, Villa Clara, Cuba
8Hospital Clínico Quirúrgico Docente Comandante Manuel Fajardo, La Habana, Cuba
9HospitalGeneral DocenteIvan Portuondo, Artemisa, Cuba
10Hospital Universitario Manuel Ascunce Domenech, Camagüey, Cuba
11Guy and Betty Beatty Center for the Study of Obesity and Outcomes, Inova Health Systems, Falls Church, VA, USA
*Corresponding author: Linda Henry, Center for Outcomes Research in Liver Diseases, Washington, District of Columbia, USA
Received: March 03, 2021; Accepted: April 19, 2021; Published: April 26, 2021
Abstract
Aim: There is a paucity of data on Non-Alcoholic Fatty Liver Disease (NAFLD) and Metabolic Syndrome (MetS) among native Cubans. We aimed to assess the prevalence of MetS in Cubans with NAFLD and the outcomes and predictors for advanced fibrosis.
Methods: A multicenter (outpatient clinics of nine hospitals in seven Cuban provinces) cross-sectional study of adults with NAFLD between September 2018 and May 2019. MetS was defined by the National Cholesterol Education Program Adult Treatment Panel III (NCEP: ATPIII) criteria. Advanced fibrosis was defined using AST to Platelet Ratio Index (APRI) ≥1 and Fibrosis-4 score (FIB-4) ≥2.67.
Results: 819 patients enrolled, 563 (68.7%) had MetS; mean age 54.9 years, 60.3% female, 65.8% white, 95.1% from urban residency, mean BMI 30.7 kg/m². Fibrosis was present in 114 (13.9%); 94 (82.5%) had APRI ≥1; 77 (67.5%) had FIB-4 ≥ 2.67; 57 (50%) both scores were elevated. MetS group had significantly more fibrosis than no MetS, [17% vs. 7% (p=0.0001)]. Patients with fibrosis were older (57.7 vs. 54.5, P=0.0015), of Mestizos ethnicity (36.8% vs. 16.9%, P<0.0001), and from rural residency (17.5% vs. 2.8%, P<0.0001). MetS was independently associated with fibrosis: Odds Ratio (OR) = 2.05 (95% CI 1.10-3.81) (p=0.024), but, rural residency was the strongest fibrosis predictor [OR: 5.30 (95% CI 2.45-11.47, (P<0.0001)]. Other fibrosis predictors were male gender, sedentary life-style, NAFLD family history, and lower estimated glomerular filtration rate (p<0.05). Risk of fibrosis was not associated with age, ethnicity, or smoking (all p>0.05).
Conclusion: Cuban NAFLD patients with MetS have substantial clinical impairment and a higher risk for fibrosis.
Keywords: Fatty liver; Metabolic syndrome; Fibrosis; eGFR; Rural residency
Abbreviations
MetS: Metabolic Syndrome; NAFLD: Non-Alcoholic Fatty Liver Diseases; NCEP: ATPIII: National Cholesterol Education Program Adult Treatment Panel III; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; NIAAA: National Institute on Alcohol Abuse and Alcoholism; BMI: Body Mass Index; ADA: American Diabetic Association; CKD: Chronic Kidney Disease; MDRD: Modification of Diet in Renal Disease; eGFR: Estimated Glomerular Filtration Rate; APRI: AST to Platelet Ratio Index; FIB-4: Fibrosis 4 Score; Scr: Serum Creatinine
Lay Summary
People with metabolic syndrome have high rates of cardiovascular disease and cardiovascular disease related death. Metabolic syndrome is present when one has three or more of the following criteria: waist circumference >102cm in men, >88cm in women, triglycerides 150mg/dl or greater, HDL-cholesterol <40mg/dl in men and <50mg/ dl in women, blood pressure 130/85mmHg or greater, and fasting glucose 100mg/dl or greater. The components of metabolic syndrome are associated with a liver disease called, Non-Alcoholic Fatty Liver Disease (NAFLD). In this study, we found 68.7% of patients from Cuba who had NAFLD also had metabolic syndrome. The majority of those with NAFLD and MetS were women though men were more likely to develop liver fibrosis.
Introduction
Non-Alcoholic Fatty Liver Disease (NAFLD) is defined as the presence of ≥5% of hepatic steatosis, in the absence of competing liver disease etiologies, such as chronic viral hepatitis, use of medications that induce steatosis such as amiodarone or tamoxifen, and other chronic liver diseases, such as autoimmune hepatitis, hemochromatosis, Wilson’s disease, or significant alcohol consumption [1]. NAFLD is also considered to be the Metabolic Syndrome (MetS) of the liver due to its close association with features of metabolic syndrome which include hypertension, hyperlipidemia, type 2 diabetes, and obesity. Many consider the relationship to be bidirectional between Mets, components of MetS, and NAFLD in addition to the presence of MetS being associated with advanced liver fibrosis [2-4].
NAFLD is now one of the most prevalent liver diseases worldwide due to parallel increases in the rates of obesity and type 2 diabetes mellitus. The reported overall global prevalence of NAFLD among adults is 24% but with prevalence rates that range from a high of 32% in the Middle East followed by 31% in South America, Asia at 27%, the United States of America at 24% and Europe at 23% while Africa reports only a prevalence rate of 14% [5]. Mortality related to NAFLD is increasing with a reported age-standardized mortality annual percentage change of 11.3% between 2013-2016 [6]. NAFLD is less common in women than men but the more advanced form of NAFLD, Non-Alcoholic Steatohepatitis (NASH), appears to be more common in women [7]. Older age is also associated with NAFLD, but it is also appearing in the younger population.
The demographic characteristics also differ worldwide [5]. Europe and North America appear to follow similar demographic patterns for the prevalence of NAFLD. However, a recent study which investigated the prevalence of suspected NAFLD among 12,133 Hispanic/Latino persons found that persons of Cuban, Puerto Rican, and Dominican backgrounds had lower rates of suspected NAFLD when compared to persons of Mexican heritage while persons of Central American and South American lineage had a similar prevalence of suspected NAFLD compared to persons of Mexican heritage [7].
However, there is a paucity of data regarding the history of NAFLD patients currently living in their native Caribbean countries, particularly Cuba. In fact, the majority of studies investigating the impact of Latino ethnicity (Dominicans, Cubans and Puerto Ricans) on NAFLD were conducted on persons living in the United States where there are very different environmental and socioeconomic conditions that can impact the course of this liver disease [8-10]. Therefore, despite our current understanding of NAFLD and MetS, [11-13] understanding NAFLD by ethnicity from persons living in their respective Caribbean country is important for further understanding of the interplay between one’s environment and genetic make-up in the development of this metabolically based liver disease.
As such, this study will focus on the impact of metabolic syndrome and NAFLD among native Cubans to assist policy makers in developing targeted NAFLD interventions [14-16] that may be applicable to the surrounding areas as Cuba is the largest of the four islands that make up the Greater Antilles and is comprised of 11,338,138 people. Cuba also has a large ethnic admixture even within the country due to the past conquering forces from Europe/Spain, slave trade from West Africa, and the indigenous population (Overall, 64.1% of the population is European Cuban, 26.6% Mulatto or Mixed, and 9.3% are Afro-Cuban) [17-19]. In addition. Cuba is facing several major health issues, which include the growing prevalence of the metabolic diseases of diabetes, obesity and hypertension [20-22].
Therefore, we aimed to screen for features of MetS in patients with NAFLD; assess whether the NAFLD clinical profile differed by the presence of MetS; and identify predictors of fibrosis among those with NAFLD with the goal of providing a better understanding of the impact the presence of MetS on the outcomes of patients with NAFLD which may also help further the discussion on which terminology is more appropriate for this fatty liver disease.
Methods
Study design and Setting
A multicenter cross-sectional study was performed in adult patients with a well-documented diagnosis of Non-Alcoholic Fatty Liver Diseases (NAFLD). Patients were enrolled from outpatient clinics of nine hospitals in seven provinces of the country, Pinar del Rio, Artemisa, La Habana, Matanzas (Western region), Villa Clara, Camaguey (Central region) and Santiago de Cuba (Eastern region). Patients were continuously enrolled from September 2018 to May 2019. The study was approved by the Institutional Review Board of all the participating hospitals.
Participants
The primary inclusion criteria were age ≥18 years with the presence of hepatic steatosis on ultrasonography in the absence of known secondary causes of liver fat accumulation, according to the criteria of the American Association for the Study of Liver Diseases [1]. Patients with secondary causes of hepatic fat accumulation, current or recent alcohol or drug abuse history, use of potentially hepatotoxic drugs, ischemic liver disease, alpha-1 antitrypsin deficiency, hemochromatosis or Wilson’s disease, viral hepatitis B or C, human immunodeficiency virus infection, were excluded from the study. In addition, those who reported excessive alcohol intake which was defined as more than 4 drinks on any day for men or more than 3 drinks for women, following the National Institute on Alcohol Abuse and Alcoholism (NIAAA) guidelines where one “standard” drink was equivalent to 1 regular beer, or 12 oz of liquor or 5 oz of wine or 1 shot of distilled spirit (https://www.niaaa.nih. gov/what-standard-drink) were also excluded [23]. Social drinker or abstinence was assigned to those who either drank alcohol but did not meet the NIAAA guidelines for being a heavy drink or reported that they did not drink alcohol at all [23]. We also excluded those with hypothyroidism, hypopituitarism and polycystic ovarian syndromes as potentially other known causes of NAFLD.
During an office visit, after giving informed consent, demographic, personal habits, medical history, and clinical data were collected from the patients and their clinical charts. In order to standardize data collection, a pre-approved data collection form was used.
Data sources/ measurement
Metabolic Syndrome (MetS) was defined according to the National Cholesterol Education Program Adult Treatment Panel III (NCEP:ATPIII) criteria [24] and updated by the American Diabetes Association (ADA) for impaired fasting glucose tolerance [25,26] as any three or more of the following criteria: waist circumference >102cm in men, >88cm in women, triglycerides 150mg/dl or greater, HDL-cholesterol <40mg/dl in men and <50mg/dl in women, blood pressure 130/85mmHg or greater, and fasting glucose 100mg/dl or greater.
Collected demographics included age, gender, ethnic groups (based on the color of skin: white, black and mestizo), and current residence (urban or rural area). The rural population refers to people living in rural areas as defined by national statistical office [27]. Demographic characteristics, anthropometric measures, and laboratory tests were recorded within 1 month of recruitment.
Personal habits recorded included: current smoking defined as regular or occasional use of tobacco products, physical activity defined as either sedentary which is the engagement in physical activity for <15 minutes less than three times a week during the last quarter or as the practice of any physical activity for ≤90min/week or regular physical activity which is the practice of regular physical activity or exercise for >90min/week. Activity included any activity that occurred in the occupational, educational, home and community settings as well as mode of transportation [28]. Current alcohol use was defined as the amount of alcohol consumed on a daily basis over the past six months. A family history was determined by querying the patients with regards to first-degree relatives with known NAFLD, cirrhosis, diabetes, obesity or cancer. A medical history was also performed which included an inquiry about a history of arterial hypertension defined as a Systolic Blood Pressure (SBP) ≥130mmHg or Diastolic Blood Pressure (DBP) ≥85mmHg or the self-reported use of anti-hypertensive medications [29].
A medical examination was also performed. The medical history included measurements of a patient’s waist circumference, weight, height, Body Mass Index (BMI) [30]. At each examination, three blood pressure measurements were taken at 10min intervals by centrally trained healthcare staff using an appropriately sized cuff and mercury sphygmomanometer (Kindcare medical system, Zhejiang, China), and the average blood pressure was recorded. Type 2 diabetes was defined as a fasting glucose ≥7mmol/l or if the 2-hour plasma glucose value during a 75g oral glucose tolerance test was ≥11mmol/l or the self-reported use of anti-diabetic medications [31]. Cirrhosis, was established using historic liver biopsy or, if unavailable, imaging and existing clinical medical records. Hyperlipidemia was defined as having a total cholesterol ≥5.2mmol/l and/or triglycerides ≥1.7mmol/l or the self-reported use of statins, fibrates or any other anti-lipid drugs [32].
The laboratory tests included platelet counts, Alanine Aminotransferase (ALT) with standardized values for the Upper Limit Normal (ULN) of <46IU/L, Aspartate Aminotransferase (AST) (ULN <49IU/L), alkaline phosphatase (normal range: 60-290 IU/L), gamma-glutamyltransferase (normal <45IU/L), albumin (normal range: 38-64g/L), glucose (normal range: 4.2-6.1mmol/L), total cholesterol (normal range: 2.9-5.2mmol/L), triglyceride (normal range: 0.46-1.71mmol/L), uric acid (normal ranges: male: 208- 428, female: 155-357mmol/l) and creatinine (normal ranges: 47.6 - 113.4mmol/L). All laboratory tests were performed using routine validated methods. The ULN ranges of AST and ALT used were provided according ALT 4+1 test validated available by HELFA diagnostic: https://es.scribd.com/document/383145547/Catalogode- Tecnicas-HELFA. Hematological tests were performed using the BC-3200 Auto Hematology Analyzer (Mindray, Shenzhen, China) and biochemical parameters were measured using the Cobas C311 Clinical Chemistry Analyzer (Roche, Basel, Switzerland).
Serum Creatinine (Scr) measurements were used to estimate Glomerular Filtration Rate (eGFR) using the following equation: Modification of Diet in Renal Disease (MDRD eGFR). (mL/ min/1.73m²) = 175* (Scr/88.4)^(-1.154)*(Age)^(-0.203)*(0.742 if female)*(1.212 if African American) [33] Kidney function was evaluated through eGFR, Chronic Kidney Disease (CKD) stage 1 if eGFR (CKD-MDRD) ≥90, stage 2, eGFR = 60-89, stage 3, eGFR = 30-59, stage 4, 15-29 and stage 5<15 [34].
The presence of fibrosis was established using the AST to Platelet Ratio Index (APRI) and Fibrosis-4 score (FIB-4) calculated for each patient [35]. The cut-off points for advanced fibrosis were APRI ≥1 and/or FIB-4 ≥2.67 [36]. Fibrosis was presumed in patients meeting one of the two cut-offs.
At the time of enrollment, an upper abdominal ultrasound was obtained using one of the following ultrasound machines: Toshiba Aplio 300 (Toshiba Medical Systems Europe, The Netherlands), Aloka SSD-4000 (Hitachi Aloka Medical Ltd) or EPIQ 5 (Philips Ltd). Each ultrasound was reviewed. Hyperechogenicity of liver (increased hepatorenal echogenicity), blurring of vascular margins, and increased acoustic attenuation defined the presence of NAFLD.
Statistical analysis
Patients’ clinico-demographic characteristics were summarized as N (%) or mean +/- standard deviation. Subgroup analyses were performed on the predefined subgroups of: patients with and without MetS and patients with and without fibrosis based on the definitions described above. Comparison of parameters between groups was done using chi-square test or Mann-Whitney non-parametric test for categorical and continuous parameters, respectively. Independent predictors of fibrosis in patients with NASH were assessed using multiple logistic regression. P-values of less than 0.05 were considered statistically significant.
All analyses were run using SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
Of the 6601 patients who visited a clinic during the study period, 1070 met the eligibility criteria and 819 (76.5%) completed all labs and clinical data; the other 251 patients had insufficient medical records for extraction of required data or did not provide informed consent (Figure 1). Patients included were, on average, 54.9 (±10.9) years of age, 60.3% female, 65.8% white, 95.1% from urban residency, with a mean BMI of 30.7±4.6 kg/m² (Table 1).
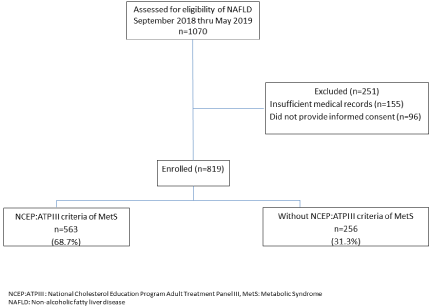
Figure 1: Patient Selection Flowchart.
The mean age of all patients was above 50 years but different means between males and females were seen, woman were older, 53.2 ± 11.1 vs. 56.1 ± 10.6 (p=0.0042) respectively.
By region, the majority of enrolled patients came from the Western provinces (n=638 (77.9%)), 8.1% from the Central provinces (n=66), and 115 (14%) from the Eastern provinces. Sociodemographic profile of the patients by province was a bit different such that 95.5% and 70.1% of patients who came from the Central and Western provinces were white, respectively, while 65.2% of the Eastern provinces were mestizos. Male gender in the Central province was only 24.2% while the Eastern province had the highest percent of males at 47.8%. Of the 40 patients from a rural residency, 18 (45%) lived in the Western province and 20 (55.5%) were from the Eastern (Figure 2 and Graphic 1).
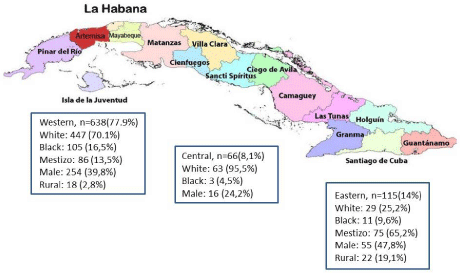
Figure 2: Sociodemographic profile by regions of patients with non-alcoholic fatty liver disease; Cuba 2019.
Metabolic syndrome in NAFLD patients
Of the 819 patients enrolled, 563 (68.7%) met the NCEP:ATPIII criteria for MetS (Figure 1). The demographics and clinical data of NAFLD patients by MetS diagnosis are also summarized in Table 1. The MetS group was of similar age to no MetS. The MetS group had significantly more females (67.5%) compared to the no MetS group (44.5%, P<0.0001). Personal habits like current smoking were most common in the MetS group (21.3% vs. 14.1%, P=0.014) while the practice of regular exercise was significantly more common in no MetS group (81.3% vs. 55.6%, P<0.001), conversely, the MetS group was more sedentary (27.5% vs. 10.9%, P<0.0001). BMI did not differ among groups (p=0.60) though waist circumference for both males and females was significantly higher in the MetS group (P<0.0001) (Table 1).
NAFLD/MetS
NAFLD/No MetS
p
All
Variable
N = 563 (68.7%)
N = 256 (31.3%)
819 (100%)
Age, years
55.7 ± 10.6
53.3 ± 11.2
0.0515
54.9 ± 10.9
Gender (male), n (%)
183 (32.5%)
142 (55.5%)
<0.0001
325 (39.7%)
Ethnic groups
White
372 (66.1%)
167 (65.2%)
0.8142
539 (65.8%)
Black
74 (13.1%)
45 (17.6%)
0.0951
119 (14.5%)
Mestizo
117 (20.8%)
44 (17.2%)
0.2303
161 (19.7%)
Residence
Urban
530 (94.1%)
249 (97.3%)
0.0543
779 (95.1%)
Rural
33 (5.9%)
7 (2.7%)
0.0543
40 (4.9%)
Personal Habits
Current Smokers, n (%)
120 (21.3%)
36 (14.1%)
0.0143
156 (19.0%)
Physical Activity, n (%)
Regular
313 (55.6%)
208 (81.3%)
<0.0001
521 (63.6%)
Sedentary
155 (27.5%)
28 (10.9%)
<0.0001
183 (22.3%)
Body Mass Index, kg/m2
30.8 ± 4.6
30.5 ± 4.7
0.5887
30.7 ± 4.6
Waist Circumference (cm)
Female
99.2 ± 12.4
92.9 ± 11.4
<0.0001
97.8 ± 12.4
Male
103.3 ± 11.6
93.9 ± 10.9
<0.0001
99.2 ± 12.2
Family history, n (%)
NAFLD
89 (17.1%)
13 (5.5%)
<0.0001
102 (13.4%)
Cirrhosis
45 (8.6%)
3 (1.3%)
0.0001
48 (6.3%)
Diabetes
244 (46.8%)
53 (22.3%)
<0.0001
297 (39.1%)
Obesity
187 (35.9%)
42 (17.6%)
<0.0001
229 (30.2%)
Cancer
123 (23.6%)
29 (12.2%)
0.0003
152 (20.0%)
Blood pressure (mmHg)
Systolic
131.3 ± 16.2
123.8 ± 11.3
<0.0001
128.9 ± 15.2
Diastolic
84.1 ± 10.2
81.0 ± 7.8
<0.0001
83.1 ± 9.6
History of n (%)
Fatigue
184 (32.7%)
45 (17.6%)
<0.0001
229 (28.0%)
Abdominal pain
209 (37.1%)
34 (13.3%)
<0.0001
243. 9.7%)
Muscle pain
57 (10.1%)
9 (3.5%)
0.0013
66 (8.1%)
Dyspepsia
134 (23.8%)
39 (15.2%)
0.0054
173 (21.1%)
Hepatomegaly
148 (26.3%)
19 (7.4%)
<0.0001
167 (20.4%)
Xantoms
56 (9.9%)
5 (2.0%)
0.0001
61 (7.4%)
Cirrhosis
36 (6.4%)
1 (0.4%)
0.0001
37 (4.5%)
Type 2 diabetes
203 (36.1%)
17 (6.6%)
<0.0001
220 (26.9%)
Hypertension
358 (63.6%)
56 (21.9%)
<0.0001
414 (50.5%)
Hyperlipidemia
321 (57.0%)
20 (7.8%)
<0.0001
341 (41.6%)
Thyroid diseases
32 (5.7%)
6 (2.3%)
0.0352
38 (4.6%)
Heart attack
142 (25.2%)
5 (2.0%)
<0.0001
147 (17.9%)
Cancer
19 (3.4%)
1 (0.4%)
0.0105
20 (2.4%)
eGFR (mL/min/1.73 m2). CKD/MDRD
77.6 ± 22.3
90.3 ± 27.5
<0.0001
81.5 ± 24.7
eGFR cut-offs (mL/min/1.73 m2), CKD-MDRD, n (%)
stage 1: eGFR=90
129 (25.4%)
110 (48.7%)
0
239 (32.6%)
stage 2: eGFR 60-89
260 (51.2%)
94 (41.6%)
0.0164
354 (48.2%)
stage 3: eGFR 30-59
118 (23.2%)
22 (9.7%)
0
140 (19.1%)
stage 4: eGFR 15-29
1 (0.2%)
0 (0.0%)
0.5045
1 (0.1%)
Fibrosis n (%)
96 (17.1%)
18 (7.0%)
0.0001
114 (13.9%)
APRI (=1.0), n (%)
82 (14.6%)
12 (4.7%)
<0.0001
94 (11.5%)
FIB-4 (=2.67), n (%)
66 (11.7%)
11 (4.3%)
0.0007
77 (9.4%)
APRI score
0.657 ± 0.651
0.467 ± 0.269
0.0003
0.598 ± 0.567
FIB-4 score
1.72 ± 1.16
1.38 ± 0.63
<0.0001
1.62 ± 1.04
Table 1: Demographic and clinical characteristics of patients with NAFLD with and without Metabolic Syndrome (NCEP: ATPIII).
The MetS group also had significantly more first-degree relatives with known NAFLD, cirrhosis, diabetes, obesity, and/or cancer, and a personal history and reports of fatigue, abdominal pain, dyspepsia, thyroid disease, etc (all P <0.03). Of the 20 patients with a previous history of cancer, the most frequent were cervix cancer (n=6) followed by breast and prostate (n=3, each), colon and thyroid (n=2, each), lung, lymphoma, melanoma and hypernephroma (n=1, each) (Table 1). MetS patients also presented with higher average blood pressure readings (systolic 131.3±16.2 vs. 123.8±11.3, P<0.0001 and diastolic 84.1±10.2 vs. 81.0±7.8, P<0.0001) than the no MetS group. The mean eGFR for the MetS group was considerably lower than the no MetS group (77.6±22.3 vs. 90.3±27.5, P<0.0001) (Table 1). Patients with MetS also had higher ALT, AST, GGT, glycaemia, cholesterol, and triglyceride levels (p<0.05) (Table 2).
NAFLD/MetS
NAFLD/No MetS
p
All
Variable
n = 563 (68.7%)
n = 256 (31.3%)
819 (100%)
ALT (U/L)
65.5 ± 46.0
51.7 ± 28.6
0.001
61.2 ± 41.8
AST (U/L)
58.7 ± 46.5
45.5 ± 23.3
0.0057
54.6 ± 41.1
Alkaline phosphatase (U/L)
167.6 ± 91.4
136.0 ± 71.1
<0.0001
157.9 ± 86.8
GGT (U/L)
78.8 ± 84.6
58.3 ± 55.6
0.0036
74.5 ± 79.8
Fasting blood glucose (mmol/L)
6.65 ± 2.41
5.07 ± 1.11
<0.0001
6.16 ± 2.22
Total cholesterol (mmol/L)
5.75 ± 1.49
4.71 ± 1.01
<0.0001
5.53 ± 1.46
Triglycerides (mmol/L)
2.56 ± 1.30
2.13 ± 1.10
<0.0001
2.43 ± 1.25
Albumin (g/L)
44.0 ± 6.3
47.1 ± 6.6
<0.0001
45.0 ± 6.6
Creatinine (mmol/L)
81.0 ± 18.4
76.0 ± 14.7
0.0013
79.5 ± 17.5
Uric acid (mmol/mL)
337.8 ± 94.4
321.8 ± 82.5
0.0775
333.1 ± 91.3
Platelet count/109/L
244.0 ± 64.2
257.7 ± 63.3
0.0057
248.3 ± 64.2
Table 2: Baseline laboratory parameters of patients with NAFLD with and without Metabolic Syndrome (NCEP: ATPIII).
Using the calculated eGFR, the number of patients for each stage of Chronic Kidney Disease (CKD) among the overall group regardless of the metabolic syndrome status (n=819) were as follows: stage 1 ≥90mL/min (n=239) 32.56%; stage 2 eGFR = 60-89 mL/min (n=354) 48.23%; stage 3 eGFR = 30-59 mL/min (n=140) 19.07%, and stage 4 (n=1) 0.14%. We found 85 (10.3%) were missing values to calculate the eGFR.
The majority of NAFLD patients (67.4%) had eGFR below 90mL/ min/1.73m². Patients with MetS had more impairment of renal function where CKD stage 1 was more frequent in NAFLD without MetS (48.7% vs. 25.4%) while stage 2-4 was more prevalent among NAFLD patients with MetS (74.6% vs. 51.3%) (p<0.001).
Advanced fibrosis in NAFLD with Metabolic Syndrome
Fibrosis was present in 114 (13.9%) patients. The MetS group had significantly more fibrosis than the no/MetS group (17% vs. 7%; p=0.0001) (Table 1). Of 114 patients with fibrosis, 94 (82.5%) patients had an APRI ≥1 whereas 77 (67.5%) patients had a FIB-4 ≥2.67.
Comparison of patients with and without fibrosis is shown in Table 3. Patients with fibrosis were older (mean age 57.7 vs. 54.5, P=0.0015), of mestizos ethnicity (36.8% vs. 16.9%, P<0.0001), and more likely from a rural area (17.5% vs. 2.8%, P<0.0001). Personal habits like smoking did not differ among groups but patients with fibrosis were more sedentary (39.5% vs. 19.6%, P<0.0001) with less regular activities (35.1% vs. 68.2%, P<0.0001). BMI mean in fibrosis patient was lower (29.8±4.3 vs. 30.9±4.6, P=0.02) whereas waist circumference did not differ among females; males with fibrosis had more central obesity with a waist circumference of 102.9±11.4 vs. 98.5±12.3 cm, (P=0.002). Patients with fibrosis also had more hyperlipidemia. Except for cancer, family history of NAFLD, cirrhosis, diabetes, and obesity were more frequent in patients with fibrosis. History of hypertension, thyroid diseases, and cancer were similar among patients with or without fibrosis. Patients with fibrosis had a lower mean eGFR (74.2±22.8) when compared to the no fibrosis group (82.8±24.8, P=0.0003) (Table 3).
Fibrosis
No fibrosis
p
All
Variable
n = 114 (13.9%)
n = 705 (86.1%)
819 (100%)
Age. years
57.7 ± 12.7
54.5 ± 10.5
0.0015
54.9 ± 10.9
Gender (male), n (%)
53 (46.5%)
272 (38.6%)
0.1092
325 (39.7%)
Ethnic groups
White
61 (53.5%)
478 (67.8%)
0.0028
539 (65.8%)
Black
11 (9.6%)
108 (15.3%)
0.1109
119 (14.5%)
Mestizo
42 (36.8%)
119 (16.9%)
<0.0001
161 (19.7%)
Residence
Urban
94 (82.5%)
685 (97.2%)
<0.0001
779 (95.1%)
Rural
20 (17.5%)
20 (2.8%)
<0.0001
40 (4.9%)
Personal Habits
Current smokers, n (%)
25 (21.9%)
131 (18.6%)
0.3982
156 (19.0%)
Physical Activity, n (%)
Regular
40 (35.1%)
481 (68.2%)
<0.0001
521 (63.6%)
Sedentary
45 (39.5%)
138 (19.6%)
<0.0001
183 (22.3%)
Body Mass Index, kg/m2
29.8 ± 4.3
30.9 ± 4.6
0.0186
30.7 ± 4.6
Waist Circumference (cm)
Female
99.5 ± 12.5
97.5 ± 12.4
0.0751
97.8 ± 12.4
Male
102.9 ± 11.4
98.5 ± 12.3
0.0023
99.2 ± 12.2
Family history, n (%)
NAFLD
40 (36.7%)
62 (9.5%)
<0.0001
102 (13.4%)
Cirrhosis
18 (16.5%)
30 (4.6%)
<0.0001
48 (6.3%)
Diabetes
59 (54.1%)
238 (36.6%)
<0.0001
297 (39.1%)
Obesity
51 (46.8%)
178 (27.4%)
<0.0001
229 (30.2%)
Cancer
21 (19.3%)
131 (20.2%)
0.8302
152 (20.0%)
Blood pressure (mmHg)
Systolic
130.4 ± 15.1
128.7 ± 15.2
0.2602
128.9 ± 15.2
Diastolic
82.7 ± 10.8
83.2 ± 9.4
0.656
83.1 ± 9.6
History of n (%)
Fatigue
44 (38.6%)
185 (26.2%)
0.0064
229 (28.0%)
Abdominal pain
60 (52.6%)
183 (26.0%)
<0.0001
243 (29.7%)
Muscle pain
30 (26.3%)
36 (5.1%)
<0.0001
66 (8.1%)
Dyspepsia
41 (36.0%)
132 (18.7%)
<0.0001
173 (21.1%)
Hepatomegaly
60 (52.6%)
107 (15.2%)
<0.0001
167 (20.4%)
Xantoms
26 (22.8%)
35 (5.0%)
<0.0001
61 (7.4%)
Cirrhosis
22 (19.3%)
15 (2.1%)
<0.0001
37 (4.5%)
Type 2 diabetes
55 (48.2%)
165 (23.4%)
<0.0001
220 (26.9%)
Hypertension
61 (53.5%)
353 (50.1%)
0.4957
414 (50.5%)
Hyperlipidemia
73 (64.0%)
268 (38.0%)
<0.0001
341 (41.6%)
Thyroid diseases
5 (4.4%)
33 (4.7%)
0.8895
38 (4.6%)
Heart attack
21 (18.4%)
126 (17.9%)
0.8873
147 (17.9%)
Cancer
7 (6.1%)
13 (1.8%)
0.0058
20 (2.4%)
eGFR (mL/min/1.73m2), CKDMDRD
74.2 ± 22.8
82.8 ± 24.8
0.0003
81.5 ± 24.7
Fibrosis non-invasive scores
APRI score
1.57 ± 0.97
0.440 ± 0.214
<0.0001
0.598 ± 0.567
FIB-4 score
3.32 ± 1.71
1.34 ± 0.48
<0.0001
1.62 ± 1.04
Table 3: Analyses of factors related to fibrosis in NAFLD patients. Patients were presumed to have fibrosis if they had APRI >= 1 or FIB-4 >=2.67.
Predictors of fibrosis in patients with NAFLD
Independent predictors of fibrosis in NAFLD patients are summarized in Table 4. As shown, having metabolic syndrome was found to be independently associated with fibrosis in patients with NAFLD: odds ratio = 2.05 (95% CI 1.10-3.81) (p=0.024). Other predictors of fibrosis were male gender, rural residence, sedentary life style, family history of NAFLD, and lower eGFR (p<0.05) (Table 4). In contrast, there was no association of age, ethnicity, and smoking status (all p>0.05) with the risk for fibrosis (Table 4).
Univariate
Predictor
OR
95% CI
p
MetS ATPIII
2.72
1.60-4.60
0.0002
Age per year
1.029
1.010-1.049
0.0035
Male (ref: female)
1.38
0.93-2.06
0.1102
Ethnicity: white
0.55
0.37-0.82
0.0031
Rural residence (ref: urban)
7.29
3.78-14.05
<0.0001
Current smoker
1.23
0.76-1.99
0.3989
Body mass index per kg/m2
0.942
0.898-0.988
0.0137
Waist circumference = 102/88cm for male/female
1.09
0.71-1.66
0.6959
Diabetes (clinical diagnosis or fasting blood glucose >= 5.6mg/dL)
2.22
1.44-3.42
0.0003
Physical activity: Sedentary (<15 min/day <3 days/week)
2.68
1.76-4.08
<0.0001
Family history of cirrhosis
4.09
2.19-7.63
<0.0001
Family history of NAFLD
5.5
3.44-8.79
<0.0001
Family history of obesity
2.33
1.54-3.53
<0.0001
Albumin per g/dL
0.864
0.831-0.897
<0.0001
Total cholesterol per mg/dL
1.388
1.206-1.598
<0.0001
Fasting blood glucose per mg/dL
1.349
1.243-1.463
<0.0001
Triglycerides per mg/dL
1.442
1.254-1.659
<0.0001
eGFR per unit
0.983
0.973-0.993
0.0006
GGT per U/L
1.009
1.006-1.011
<0.0001
Alkaline phosphatase per U/L
1.013
1.011-1.016
<0.0001
Multivariate
Predictor
OR
95% CI
p
MetS ATPIII
2.05
1.10-3.81
0.0239
Age
1.012
0.989-1.036
0.31
Male (ref: female)
3.19
1.87-5.44
<0.0001
Ethnic White
0.67
0.42-1.08
0.1
Rural residence (ref: urban)
5.3
2.45-11.47
<0.0001
Current smoker
0.72
0.40-1.29
0.27
Physical Activity: sedentary
2.55
1.48-4.39
0.0007
Family history of cirrhosis
1.04
0.46-2.37
0.93
Family history of NAFLD
2.81
1.52-5.19
0.001
Family history of obesity
1.2
0.70-2.05
0.51
eGFR per unit
0.986
0.974-0.997
0.0122
Univariate models: Each parameter was tested as a single predictor of fibrosis in a separate regression model; Multivariate: Predictors from the univariate models were not included if collinearity with MetS (by definition diabetes, BMI, waist circumference, laboratory parameters] was found.
Table 4: Predictors of fibrosis in patients with NAFLD.
Discussion
This national study has provided knowledge about the burden of MetS in Cuban patients with NAFLD seen in real-world practices in Cuba. Our study enriches the knowledge regarding the association between NAFLD and MetS especially as the other studies conducted in Cuba were on smaller samples of patients with NAFLD [37-40].
In the current data, we found that there was a high prevalence of MetS in those with NAFLD, the majority of patients with NAFLD were female and those with NAFLD and MetS had a worse clinical profile. Overall, over two out of every three patients with NAFLD had MetS (68.7%) where for every three patients with NAFLD MetS, two were women. The prevalence of smoking and sedentarism, a family history of chronic diseases, and the significant presence of liver cirrhosis were all remarkable in the MetS group.
Our understanding of sex differences in NAFLD is expanding. In a recent review summarizing the current knowledge of sex differences in NAFLD, NAFLD prevalence and incidence were reported higher in men than in premenopausal women (or age ≤ 50-60 years) but was found to be more common in women after menopause (or age ≥ 50-60 years) probably due to the result of the loss of the protective estrogen effect which then caused body fat redistribution to the abdomen, favoring the appearance of MetS [41]. As seen in our study, this phenomenon of fat redistribution was recognized in overweight/obese females in that their waist circumference was, on average, less than four centimeters different than males which is dramatically different than what has been reported to be the male to female difference of approximately 10 centimeters among those not over weight [42]. Additionally, over the past two decades, the obesity prevalence among the female Cuban population became greater than among the males [21]. As such, we suggest that the presence of MetS or features of MetS such as obesity and increased waist circumference in females warrants further assessment for the presence of NAFLD.
Those with MetS were also found to have over two times greater risk for the development of NAFLD related fibrosis than those without MetS. In addition, male gender, rural residence, sedentary life style, family history of NAFLD, and lower eGFR were all predictors of fibrosis. Interesting, despite more females having MetS and fibrosis, males were over three times as likely to be at risk for fibrosis when compared to females. This finding can partially be explained by age as age became not significant in our multivariate analysis when gender was held constant suggesting that though females were older, the effects of the menopausal loss of estrogen had not caught up yet to effects of MetS on males. Together these factors again suggest that there is a significant interplay between environment, gender, and genetics that play a role in the development of not only NAFLD but NAFLD related fibrosis [43-47].
In fact, in regards to environment, we found that those who resided in a rural area were over five times more likely to develop fibrosis. Rural residency is a similar finding from a recent study where they too found patients with NASH cirrhosis were most likely to have come from a rural area [48]. Notably in our study, the provinces of Cuba were distinctly different in their sociodemographic make up where the Western region was mostly white, female with 2.8% residing in a rural area while the Eastern region was more likely to be Mestizo, either male or female with almost 20% residing in a rural area, and the Central region was most likely to be white, female with no one residing in a rural area suggesting that the Eastern region may require more focused interventions.
However, despite these differences, a recent survey on risk factors and non-communicable diseases in Cubans [49], found that the rural and urban populations were similar in demographics, personal habits, and anthropometrics with only a few nutritional habits reported as different (ex. rural residents had higher consumption of saturated fats 23.1% vs. 9.7%; urban resident used more salt and consumed less fruit and vegetables). On the other hand, the report also noted that Cubans in general displayed several unhealthy eating habits associated with the development of fatty liver [50,51] which include an insufficient consumption of fruits and vegetables, use of saturated fats while cooking, not eating breakfast, and adding salt to meals after they were prepared [49]. Which, when these points are taken together, suggests that diet plays a role in the development of NAFLD for all Cubans but still does not fully explain the impact of rural residency.
Another possible explanation may be related to access to healthcare such that those who live in rural areas lack access to care. However, it is important to note that since 1984 Cuba has had a Family Doctor Program that guarantees free access to healthcare through the family doctor and nurse teams that are located in neighborhoods across the country (including rural areas). Despite the availability of these clinics, rural residents may still have to travel long distances to seek care especially for subspecialist services such as gastroenterology, endocrinology, hepatology, etc. As such, it is probable that travel may be a barrier to seeking care early in the course of disease. This fact is compounded by NAFLD being considered a “silent” disease and a lack of risk perception such that only when symptoms of advanced liver disease are present do residents seek medical care [52-54]. Therefore, we suggest that Cuba’s national chronic non-communicable disease program [52,53] could be enhanced by incorporating active and early screening for NAFLD in high risk groups such as those with MetS and those residing in rural areas which is of utmost importance as cirrhosis is the ninth cause of death in Cuba [20]. Additional interventions are also needed to increase both awareness of NAFLD and its outcomes and to reverse its disease progression [54-56].
Alarmingly, though, were the values of eGFR detected in this study. The median eGFR reported for the Cuban population is 110.8mL/min/1.73m² (95% confidence interval: 108.9-112.7 mL/ min/1.73m²) with almost 95% of the entire population having an eGFR greater than 60mL/min/1.73m2 [49] However, among patients with NAFLD in our study, the mean eGFR at baseline was of 81.5mL/ min/1.73m2 where over 67% of those with NAFLD fell into CKD stages 2 and 3. Though the patients in this study share common metabolic risk factors associated with CKD which may partially explain these results, the impaired renal function may also be associated with more severe disease related to NAFLD [57] as NAFLD has been found to be independently associated with an increased risk of advanced CKD development even after stratification by age, gender and preexisting comorbidities [58]. Additionally, a recent study found that improvement in liver histology from lifestyle modification (diet and exercise) was independently associated with improved kidney function [15]. Although, we were not able to determine urine protein excretion in order to meet the complete definition of chronic kidney disease, in particular for stage 1 or 2, we do suggest that efforts must be undertaken to address this apparent interaction of renal impairment, MetS, and those with NAFLD in Cuba [59-61].
Limitations inherent to the study design need to be acknowledged. Since our study patients were recruited from hospitals and a tertiary referral center, a natural study selection bias may have occurred whereby our study findings may not be a representation of all patients with NAFLD in Cuba. In addition, a proportion of eligible patients with a probable diagnosis of NAFLD were not included because they did not consent to participate or did not complete the data required for the study, so we suggest that there is an underestimation of the frequency of NAFLD. Our NAFLD diagnosis was made only based on sonography due its inexpensiveness and non-invasiveness. However, despite the selection bias that may have occurred, sonography is the most widely used non-invasive method at this time for the diagnosis of NAFLD so any discrepancy would be found across all study populations. Fibrosis was also determined using non-invasive methods so there is a potential for misclassification bias, but since several studies have demonstrated the deleterious effect of multiple components of the metabolic syndrome on increasing the risk for severe liver disease [62-64], we feel our fibrosis results are fairly accurate. Finally, we were unable to report the impact of both dietary and environmental factors on the development of steatosis which may also help to explain the association of rural residency with the presence of more advanced fibrosis cases. We suggest that further natural history studies on NAFLD consider collecting dietary and environmental factors that have been reported to be associated with NAFLD [63,64].
Summary
Our multi-center study of a diverse population highlights the higher clinical impairment of NAFLD patient with features of metabolic syndrome to include an increased risk of fibrosis which is important as NAFLD will become the leading aetiology of chronic liver disease in Cuba in the near future due the growing prevalence of diabetes, obesity and hypertension. However, perhaps most importantly, we found that living in a rural area was the strongest predictor for the development of NAFLD related fibrosis suggesting that an environment located further away from health care and the lack of risk perception may play a large role in fibrosis development. In addition, over two thirds of those with NAFLD have indications of renal disease. Taken together, health policy must focus to determine how best to provide early screening of NAFLD, particularly of higher risk groups and identify the reasons for delayed diagnosis especially to for those in rural areas.
Acknowledgement
The authors acknowledge Armando Borrego and Rosaura Pichs for their technical support and collaboration with the statistical analyses.
Ethics Approval Statement
The study was approved by the Institutional Review Board of all the participating hospitals.
Patient Consent Statement
Patient informed consent was obtained prior to study enrollment.
Authorship Statements
Marlen I. Castellanos-Fernandez: Conceptualization, Methodology, Resources, Supervision, Validation, Visualization and Writing-original draft; Maria Stepanova: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization and writing-review editing; Linda Henry and Mirtha E. Infante-Velázquez: Supervision and writing-review editing. Eduardo Crespo-Ramírez, Sergio del Valle-Díaz, Javier Díaz Elias, Lorenzo Santaló-Rodríguez, Sahili Corrales-Alonso, Ignacio Morales- Martínez, Elisa Cedeño-Ramírez, Teresita Pérez-González, Sila M González-Suero, Caridad Ruenes-Domech, Eduardo Barreto Suárez: Investigation, Methodology and writing-original draft; Zaily Dorta Guridi and Enrique Arus Soler: Supervision and writing-original draft.
References
- Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018; 67: 328-357.
- Byrne CD, Targher G. NAFLD: a multisystem disease. Journal of hepatology. 2015; 62: S47-64.
- Danford CJ, Lai M. NAFLD: a multisystem disease that requires a multidisciplinary approach. Frontline gastroenterology. 2019; 10: 328-329.
- Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005; 42: 44-52.
- Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nature reviews Gastroenterology & hepatology. 2018; 15: 11-20.
- Kim D, Li AA, Gadiparthi C, Khan MA, Cholankeril G, Glenn JS, et al. Changing Trends in Etiology-Based Annual Mortality From Chronic Liver Disease, From 2007 Through 2016. Gastroenterology. 2018; 155: 1154-1163.
- Kallwitz ER, Daviglus ML, Allison MA, Emory KT, Zhao L, Kuniholm MH, et al. Prevalence of suspected nonalcoholic fatty liver disease in Hispanic/Latino individuals differs by heritage. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2015; 13: 569-576.
- Saab S, Manne V, Nieto J, Schwimmer JB, Chalasani NP. Nonalcoholic Fatty Liver Disease in Latinos. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2016; 14: 5-12.
- Arshad T, Golabi P, Henry L, Younossi ZM. Epidemiology of Non-alcoholic Fatty Liver Disease in North America. Current pharmaceutical design. 2020; 26: 993-997.
- Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011; 140: 124-131.
- Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clinical Gastroenterology and Hepatology. 2011; 9: 524-530.
- Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clinical Gastroenterology and Hepatology. 2012; 10: 646-650.
- Golabi P, Otgonsuren M, de Avila L, Sayiner M, Rafiq N, Younossi ZM. Components of metabolic syndrome increase the risk of mortality in nonalcoholic fatty liver disease (NAFLD). Medicine. 2018; 97: e0214.
- Vilar Gomez E, Rodriguez De Miranda A, Gra Oramas B, Arus Soler E, Llanio Navarro R, Calzadilla Bertot L, et al. Clinical trial: a nutritional supplement Viusid, in combination with diet and exercise, in patients with nonalcoholic fatty liver disease. Alimentary pharmacology & therapeutics. 2009; 30: 999- 1009.
- Vilar-Gomez E, Calzadilla-Bertot L, Friedman S, Gra-Oramas B, Gonzalez- Fabian L, Villa-Jimenez O, et al. Improvement in liver histology due to lifestyle modification is independently associated with improved kidney function in patients with non-alcoholic steatohepatitis. Alimentary pharmacology & therapeutics. 2017; 45: 332-344.
- Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015; 149: 367-378.
- Marcheco-Teruel B, Parra EJ, Fuentes-Smith E, Salas A, Buttenschøn HN, Demontis D, et al. Cuba: exploring the history of admixture and the genetic basis of pigmentation using autosomal and uniparental markers. PLoS Genet. 2014; 10: e1004488.
- Fortes-Lima C, Bybjerg-Grauholm J, Marin-Padrón LC, Gomez-Cabezas EJ, Bækvad-Hansen M, Hansen CS, et al. Exploring Cuba’s population structure and demographic history using genome-wide data. Scientific reports. 2018; 8: 1-13.
- Cintado A, Companioni O, Nazabal M, Camacho H, Ferrer A, De Cossio ME, et al. Admixture estimates for the population of Havana City. Annals of human biology. 2009; 36: 350-360.
- MINSAP. Anuario estadistico de salud 2019. La Habana: ECIMED. 2020.
- Suárez AR, Sánchez MED. La obesidad en Cuba. Una mirada a su evolución en diferentes grupos poblacionales. Revista Cubana de Alimentación y Nutrición. 2013; 23: 12.
- Jiménez Acosta S, Díaz Sánchez ME, García Roche RG, Bonet Gorbea M, Wong Ordóñez I. Cambios en el estado nutricional de la población cubana adulta de diferentes regiones de Cuba. Revista Cubana de Higiene y Epidemiología. 2012; 50: 4-13.
- NIAAA N. Rethinking drinking. 2020.
- Expert Panel on Detection E, Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001; 285: 2486-2497.
- Expert Committee on the D, Classification of Diabetes M. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes care. 2003; 26: S5-20.
- Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Followup report on the diagnosis of diabetes mellitus. Diabetes care. 2003; 26: 3160-3167.
- ONEI. Censo de poblacion y vivienda 2012: ONEI. 2012.
- WHO. Global Strategy on Diet, Physical Activity and Health. 2020.
- Carey RM, Whelton PK. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension Guideline. Annals of internal medicine. 2018; 168: 351-358.
- Díaz Sánchez M. Manual de técnicas antropométricas para estudios nutricionales. INHA Instituto de Nutrición e Higiene de los Alimentos La Habana. 2005.
- American Diabetes A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes care. 2019; 42: S13-S28.
- Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/ NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2019; 73: 3168-3209.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999; 130: 461-470.
- Va/Dod Clinical Practice Guideline. Management of Chronic Kidney Disease. 2019.
- Younossi ZM, Noureddin M, Bernstein D, Kwo P, Russo M, Shiffman ML, Younes Z, Abdelmalek M. Role of Noninvasive Tests in Clinical Gastroenterology Practices to Identify Patients With Nonalcoholic Steatohepatitis at High Risk of Adverse Outcomes: Expert Panel Recommendations. Am J Gastroenterol. 2021 Feb 1;116(2):254-262. doi: 10.14309/ajg.0000000000001054. PMID: 33284184.
- Castera L, Friedrich-Rust M, Loomba R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019; 156: 1264-1281.
- del Busto Mesa A, Cabrera Rego JO, Guanche Valenciano O. Cintura hipertrigliceridémica y enfermedad por hígado graso no alcohólico en pacientes hipertensos. Revista Cubana de Medicina. 2017; 56: 4-14.
- Rivas Vázquez D, Miguel Soca PE, Llorente Columbié Y, Marrero Ramírez GM. Comportamiento clínico epidemiológico del síndrome metabólico en pacientes adultos. Revista Cubana de Medicina General Integral. 2015; 31: 259-269.
- Canciano Chirino E, Iglesia Reyes CK, de Armas Romero I, Fandiño González L, Iglesia Reyes ME, Río Ponciano O. Hígado graso no alcohólico como marcador de calidad de vida en mujeres de edad mediana. Revista Cubana de obstetricia y ginecología. 2011; 37: 533-540.
- Hernández Tamayo M, Miguel Soca P, Marrero Hidalgo M, Pérez López L, Peña Pérez I, Rivas Estévez M. Comportamiento de variables clínicas, antropométricas y de laboratorio en pacientes con síndrome metabólico. Medisur. 2011; 9: 102-109.
- Ye ZL, Guo WQ, Li L. Sex-Based Differences in the Association Between Nonalcoholic Fatty Liver Disease and Mortality. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2019; 17: 211-212.
- Huffman FG, Whisner S, Zarini GG, Nath S. Waist circumference and BMI in relation to serum High Sensitivity C-Reactive Protein (hs-CRP) in Cuban Americans with and without type 2 diabetes. International journal of environmental research and public health. 2010; 7: 842-852.
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016; 64: 73-84.
- Le MH, Devaki P, Ha NB, Jun DW, Te HS, Cheung RC, et al. Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PloS one. 2017; 12: e0173499.
- Loomba R, Sanyal AJ. The global NAFLD epidemic. Nature reviews Gastroenterology & hepatology. 2013; 10: 686-690.
- Povsic M, Wong OY, Perry R, Bottomley J. A Structured Literature Review of the Epidemiology and Disease Burden of Non-Alcoholic Steatohepatitis (NASH). Advances in therapy. 2019; 36: 1574-1594.
- Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. Journal of hepatology. 2019; 71: 793- 801.
- Patel K MP, Mundil D, Mumm D, Marandi S, Power S, et al. Oral Abstracts. Hepatology. 2020; 72: 63A-64A.
- Bonet M VP, Chiang M, García R, Suárez R, Arcia N. III Encuesta Nacional de factores de riesgo y actividades preventivas de Enfermedades no transmisibles. Cuba 2010-2011. La Habana. 2015.
- Younossi ZM, Corey KE, Lim JK. AGA Clinical Practice Update on Lifestyle Modification Using Diet and Exercise to Achieve Weight Loss in the Management of Nonalcoholic Fatty Liver Disease (NAFLD): Expert Review. Gastroenterology [Internet]. 2020.
- Michel NMM, Rodríguez CP, Suero SMG, Fernández MIC. Aspectos nutricionales y dietéticos en pacientes con hepatopatía grasa no alcohólica en un centro de atención terciaria. Archivos Cubanos de Gastroenterología. 2021; 1.
- Varona P, Bonet M, Garcia R, Chang M, Suarez R, National-Provincial Coordinating G. Implementation of chronic disease risk factor surveillance in 12 Cuban municipalities. MEDICC review. 2014; 16: 43-47.
- Landrove-Rodriguez O, Morejón-Giraldoni A, Venero-Fernández S, Suarez- Medina R, Almaguer-López M, Pallarols-Mariño E, et al. Non-communicable diseases: risk factors and actions for their prevention and control in Cuba. Pan American Journal of Public Health. 2018; 42: e23.
- Cleveland ER, Ning H, Vos MB, Lewis CE, Rinella ME, Carr JJ, et al. Low Awareness of Nonalcoholic Fatty Liver Disease in a Population-Based Cohort Sample: the CARDIA Study. Journal of general internal medicine. 2019; 34: 2772-2778.
- Singh A, Dhaliwal AS, Singh S, Kumar A, Lopez R, Gupta M, et al. Awareness of nonalcoholic fatty liver disease is increasing but remains very low in a representative US cohort. Digestive Diseases and Sciences. 2020; 65: 978- 986.
- Wieland AC, Mettler P, McDermott MT, Crane LA, Cicutto LC, Bambha KM. Low awareness of nonalcoholic fatty liver disease among patients at high metabolic risk. Journal of clinical gastroenterology. 2015; 49: e6-e10.
- JA CC, JF P-OD. Prevalence and incidence of chronic kidney disease in Cuba. Clinical Nephrology. 2020; 93: 68-71.
- Park H, Dawwas GK, Liu X, Nguyen MH. Nonalcoholic fatty liver disease increases risk of incident advanced chronic kidney disease: a propensitymatched cohort study. Journal of internal medicine. 2019; 286: 711-722.
- Almaguer M, Herrera R, Alfonzo J, Magrans C, Manalich R, Martinez A, et al. Chronic kidney disease in Cuba: epidemiological studies, integral medical care, and strategies for prevention. Renal failure. 2006; 28: 671-676.
- Herrera Valdes R, Almaguer Lopez M, Chipi Cabrera JA, Perez-Oliva Diaz JF, Landrove Rodriguez O, Marmol Sonora A. Prevalence and incidence of chronic kidney disease in Cuba. Clin Nephrol. 2020; 93: 68-71.
- Almaguer M, Herrera R, Alfonso J, Magrans C, Manalich R, Martinez A. Primary health care strategies for the prevention of end-stage renal disease in Cuba. Kidney international Supplement. 2005: S4-10.
- Petta S, Eslam M, Valenti L, Bugianesi E, Barbara M, Camma C, et al. Metabolic syndrome and severity of fibrosis in nonalcoholic fatty liver disease: An age-dependent risk profiling study. Liver international: official journal of the International Association for the Study of the Liver. 2017; 37: 1389-1396.
- Aberg F, Helenius-Hietala J, Puukka P, Farkkila M, Jula A. Interaction between alcohol consumption and metabolic syndrome in predicting severe liver disease in the general population. Hepatology. 2018; 67: 2141-2149.
- Wong RJ, Liu B, Bhuket T. Significant burden of nonalcoholic fatty liver disease with advanced fibrosis in the US: a cross-sectional analysis of 2011-2014 National Health and Nutrition Examination Survey. Alimentary pharmacology & therapeutics. 2017; 46: 974-980.