
Review Article
Ann Obes Disord. 2016; 1(1):1001.
Drug Therapy for Obesity with Anti-Aging Genes Modification
Martins IJ1,2,3*
¹School of Medical Sciences, Edith Cowan University, Australia
²School of Psychiatry and Clinical Neurosciences, The University of Western Australia, Australia
³Mccusker Alzheimer’s Research Foundation, Hollywood Medical Centre, Australia
*Corresponding author: Martins IJ, School of Medical Sciences, Edith Cowan University, 270 Joondalup Drive, Joondalup, Western Australia 6027, Australia
Received: March 01, 2016; Accepted: March 14, 2016; Published: March 16, 2016
Abstract
Nutritional regulation and drug therapy has been the focus of the current obesity epidemic in various countries in the world. Epigenetics is the major mechanism for the development of insulin resistance and obesity with unhealthy diets, oxidative stress and environmental factors relevant to alterations in gene expression with effects on mitochondrial biogenesis, adipose tissue lipid metabolism and energy expenditure. Anti-aging genes are involved in the regulation of adipogenesis with increased sensitivity to anti-aging gene dysfunction associated with adipocyte-neuron interactions compared to other cells. Unhealthy diets downregulate adipocyte anti-aging genes associated with the development of Non Alcoholic Fatty Liver Disease (NAFLD) with relevance to regulation of drug metabolism and delayed pharmacokinetics in the body. Evaluation by different methods and techniques to quantify and characterize adiposity has been undertaken to obtain a better understanding of adipocyte metabolism in obesity but adipocyte analysis is now required to determine adipose tissue anti-aging gene expression. Effective drug treatment programs cannot be determined in individuals with obesity with defective adipocyte tissue gene expression. New drug development needs to be carefully interpreted in relation to nutritional intake with drug safety concerns/adverse effects relevant to adipogenesis and NAFLD in obesity.
Keywords: Obesity; Metabolism; Drugs; Diet; Anti-aging genes; NAFLD
Introduction
The susceptibility of humans to obesity is far higher compared with other species and in man favours the deposition of fat. Amongst mammals, humans have been reported to have the highest levels of fat than any other species and genes and environmental factors such as exercise, drugs and diets may predispose humans to obesity. In the world obesity has more than doubled since 1980 and in 2014 more than 1.9 billion adults (18 years and older) were overweight with 600 million adults were obese [1]. In the USA obesity trends and rates indicate that more than a third of adults (34.9 percent) were obese as of 2011 to 2012 [2]. More than two-thirds of adults were overweight or obese (68.6 percent) with17 percent of children obese and 31.8 percent were either overweight or obese.
In particular, intra-abdominal adipose tissue referred to as visceral fat has been associated with the metabolic syndrome and obesity. Visceral fat is more metabolically active than peripheral fat with the waist-to-hip ratio that identifies patients with excess visceral adiposity. Women with a waist-to-hip ratio >0.8 and men with a ratio >1.0 are considered to have excess central adiposity that confers risk for developing the metabolic syndrome and Non Alcoholic Fatty Liver Disease (NAFLD). Morbid obesity is classified as a BMI of >35 kg/m2 and severe obesity>40 kg/m2. Adiposity is the body fat tissue content and its increase is measured by Body Mass Index (BMI). Obese individuals are defined as having a BMI of >30 (BMI = weight in kg/m2) [height in m] whereas overweights are defined as having a BMI from 25 - 30 kg/m2 and ideal lean individuals to have a BMI of 25 kg/m2. The role of adipose tissue and the induction of obesity early in life are of critical understanding to the development of hyperinsulinemia which may lead to diabetes and other morbid diseases such coronary artery disease and NAFLD [3].
Interests in anti-obese drugs as the major intervention for weight loss has been an important strategy for the treatment of obesity. Drugs that have been developed for the treatment of obesity are either involved in the reduction of energy intake or the increase of energy expenditure and their beneficial effects may include improvements in glycemic control or psychiatric illness. Interests in the use of obesity drugs have increased since safety and use of the drugs for continued weight loss (reduced visceral fat stores) have become of concern to medical authorities in Western communities [4-9]. Anti-obesity drugs that improve insulin resistance, dyslipidemia and the metabolic syndrome have not been appropriate to lifestyles, diet and health conditions and drug effectiveness have been variable without weight loss. Long term treatment of obesity with the use of anti-obese drugs particularly centrally active agents has not been achieved with safety concerns and withdrawals due to adverse effects that were never predicted from clinical trials and drug development. Removal of several of these drugs from the market has allowed the introduction of other drugs such as gut based hormone treatments which target many pathways involved in the regulation of energy balance (Contrave TM or Empatic TM).
Epigenetic modifications involve anti-aging genes with increased risk for adipogenesis
The understanding of genetic factors involved in the risk for obesity has identified genes that are closely linked to obesity related diseases [10-13]. A single gene effect versus multiple genes effect may indicate either the interaction unique to various environments that regulate abnormal molecular or cellular events responsible for obesity with several hypotheses proposed in relation to the development of obesity [14]. The understanding of the development of adipogenesis has been the focus of various research groups with unhealthy diets that alter DNA methylation (epigenetic), genes and transcription factors important to the world-wide obesity epidemic with increased risk for adiposity [3,15-17]. Alterations in 400 genes, single gene disorders and variants are involved in obesity with particular genes (e.g. leptin, melanocortin/Melanocortin 4 receptor, ghrelin, neuromedin β, Peroxisome Proliferator Activated Receptor (PPAR), and mitochondrial uncoupling proteins) involved in the behaviour, metabolism, energy expenditure, taste and appetite of the organism.
The search for specific genes that are sensitive to nutritional regulation, oxidative stress, inflammation, endocrine disease, lipid/ glucose metabolism and insulin resistance has been the focus of the current obesity epidemic in various developed countries. Epigenetics is now considered as an important mechanism for the development of obesity and can result from changes in cellular chromatin structure without alterations in DNA sequence, including DNA methylation, histone modifications and chromatin remodelling. Epigenetic modifications in adipocytes are more sensitive compared to other cells and are altered by unhealthy diets with increased oxidative stress or environmental factors that have the ability to change adipocyte gene expression with effects on mitochondrial apoptosis, lipid metabolism and energy expenditure. Epigenetic modifications that involve the anti-aging genes in the adipose tissue have become important and are closely involved with adiponectin release associated with the regulation of hepatic lipid metabolism and the induction of NAFLD [18].
The increased susceptibility to fat deposition in humans is far higher compared with other species and indicate that in man the susceptibility to down regulation of the anti-aging genes by Western diets and lifestyle alterations (stress, heavy workloads) that alter the metabolism of dietary fat in the liver with the development of adipogenesis [19]. The connections between the anti-aging gene Sirtuin 1 (Sirt 1) and other anti-aging genes such as Klotho, p66Shc (longevity protein) and Fork head box proteins (FOXO1/ FOXO3a) have been associated with appetite regulation, mitochondrial apoptosis and the accelerated aging process with these anti-aging genes involved in the regulation of glucose, lipid and amyloid beta metabolism that are important to NAFLD and obesity [20]. These anti-aging genes have become important and may supersede the effects of DNA methylation (epigenetic), genes and transcription factors important to the world-wide obesity epidemic with increased risk for adiposity. The involvement of the anti-aging gene Sirt 1 in the regulation of adipogenesis has been shown [21-23] with adipocyte dysfunction associated with excess transfer of fat to liver with NAFLD [18,24,25] (Figure 1).
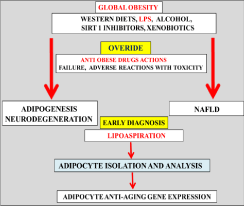
Figure 1: Anti-aging genes are involved in the regulation of adipogenesis and
NAFLD with increased sensitivity to anti-aging gene dysfunction and delayed
drug metabolism in the body. Evaluation by different methods and techniques
to quantify and characterize adiposity now require adipocyte tissue anti-aging
gene expression and effective drug programs for global obesity cannot be
undertaken without healthy diets that activate Sirt 1 regulation of adipose
tissue and neuron interactions involved in adipose tissue lipid metabolism.
In adipose tissue gene expression profiles of Klotho, p66Shc (longevity protein) and Fork head box proteins (FOXO1/ FOXO3a) have been completed and indicate down regulation of these genes are related to mitochondrial apoptosis, adipogenesis and adipocyte differentiation [26-38]. The anti-aging gene Sirt 1 is central to the down regulation of these anti-aging genes via its role as a NAD+ histone deacetylase and p53 transcriptional dysregulation of these genes [18,20]. Bacterial Lipopolysaccarides (LPS) have been shown to induce insulin resistance, adipocyte dysfunction with transport of LPS lipoproteins to adipose tissue [39-41]. LPS has been shown to repress Sirt 1 by defective transcriptional regulation [42] and its role in obesity has been clearly documented [39-41]. Diets that are high in fat stimulate LPS absorption [42] and LPS in dietary lipoproteins induce NAFLD and obesity. The role of Sirt 1 and LPS with relevance to adipocyte dysfunction is also relevant to neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease [43- 45]. LPS interferes with Sirt 1 regulation of neuropeptide Y [20] with relevance to the hypothalamus and adipose tissue connections (Figure 1) associated with disturbed energy metabolism in the adipose tissue [46,47].
Methods of visceral fat measurement, adipocyte analysis and drug treatment programs
The critical evaluation of different methods and techniques to quantify and characterize adiposity has been discussed to obtain a better understanding of fat metabolism in obesity. These methods include anthropometric techniques, bioelectrical impedance analysis, dual energy X-ray absorptiometry, and air displacement plethysmography, Ultrasound, CT and MRI. The quantitative assessment of intra-abdominal adipose tissue is by CT and MRI and represents a direct method of assessing visceral fat deposition in both adult with other methods of visceral fat assessment associated with imprecision or poor reliability [48]. In the global obesity epidemic a direct diagnosis of adipocyte dysfunction needs to be made early in life to determine that adipogenesis relevant to defective adipocyte and fat metabolism has advanced that cannot be characterized by methods of visceral fat assessment. Analysis of adipocytes by flow cytometry as well as isolation of adipocytes by flow sorting (Figure 1) has been completed [49,50] that now can allow anti-aging gene expression analysis of adipocytes [51] from individuals at various ages.
Long term treatment of obesity with the use of anti-obese drugs may not have been successful with over nutrition and LPS involved in the corruption of effective anti-obese drug treatment programs [42] and withdrawals due to these adverse effects were never predicted from clinical trials and drug development. The anti-aging gene expression (down regulation) of adipocytes may indicate the presence of LPS or Sirt 1 inhibitors in adipose tissues. The consumption of Western diets/ alcohol completely downregulate Sirt 1 with adipocyte isolation and analysis required for early diagnosis of adipogenesis defects. Effective anti-obese drug treatment programs cannot be determined in individuals in the developing/developed world until plasma analysis of LPS, Sirt 1 inhibitors [52], fatty acids such as butyric acid, palmitic acid (Sirt 1 inhibitors) [3,20] and xenobiotics [53] can be determined to assess effect on adipose tissue gene expression in man. The effective anti-obese drugs in clinical trials and new drug development need to be carefully interpreted in relation to safety concerns/adverse effects with relevance to Sirt 1 inhibitors that may be routinely consumed in various diets by obese individuals.
Conclusion
In global communities humans are more susceptible to adiposity compared with other species with the increased development of overweight individuals, NAFLD and obesity. Drugs that improve insulin resistance, dyslipidemia and the metabolic syndrome with long term treatment have not been successful with removal of various anti-obese drugs from the market. Defective anti-aging genes are linked to mitochondrial apoptosis in obesity and indicate that these genes are associated with defective hepatic drug clearance and metabolism. Lipoaspirates from obese individuals allow assessment of anti-aging genes relevant to mitochondrial biogenesis and effective drug therapy will be determined by non consumption of inhibitors of anti-aging genes (drugs) and consumption of healthy low calorie diets that activate adipocyte anti-aging genes.
Acknowledgement
This work was supported by grants from Edith Cowan University, the McCusker Alzheimer’s Research Foundation and the National Health and Medical Research Council.
References
- WHO. Obesity and overweight. Fact sheet N°311. 2015.
- Obesity Rates and Trends: The State of Obesity. 2015.
- Martins IJ. Induction of NAFLD with Increased Risk of Obesity and Chronic Diseases in Developed Countries. OJEMD. 2014; 4: 90-110.
- Adan RA. Mechanisms underlying current and future anti-obesity drugs. Trends Neurosci. 2013; 36: 133-140.
- Derosa G, Maffioli P. Anti-obesity drugs: a review about their effects and their safety. Expert Opin Drug Saf. 2012; 11: 459-471.
- Elangbam CS. Review paper: Current strategies in the development of anti-obesity drugs and their safety concerns. Vet Pathol. 2009; 46: 10-24.
- Li MF, Cheung BM. Rise and fall of anti-obesity drugs. World J Diabetes. 2011; 2: 19-23.
- Rankin W, Wittert G. Anti-obesity drugs. Curr Opin Lipidol. 2015; 26: 536-543.
- Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014; 311: 74-86.
- Lyon HN, Hirschhorn JN. Genetics of common forms of obesity: a brief overview. Am J Clin Nutr. 2005; 82: 215S-217S.
- Walley AJ, Blakemore AI, Froguel P. Genetics of obesity and the prediction of risk for health. Hum Mol Genet. 2006; 152: R124-130.
- O'Rahilly S, Farooqi IS. Genetics of obesity. Philos Trans R Soc Lond B Biol Sci. 2006; 361: 1095-1105.
- Mutch DM, Clément K. Unraveling the genetics of human obesity. PLoS Genet. 2006; 2: e188.
- Samaras T, Elrick H. An alternative hypothesis to the obesity epidemic: obesity is due to increased maternal body size, birth size, growth rate, and height. Med Hypotheses. 2005; 65: 676-682.
- Herrera BM, Keildson S, Lindgren CM. Genetics and epigenetics of obesity. Maturitas. 2011; 69: 41-49.
- Choi SW, Friso S. Epigenetics: A New Bridge between Nutrition and Health. Adv Nutr. 2010; 1: 8-16.
- Chase K, Sharma RP. Epigenetic developmental programs and adipogenesis: implications for psychotropic induced obesity. Epigenetics. 2013; 8:1133-1140.
- Martins IJ. Unhealthy Nutrigenomic Diets Accelerate NAFLD and Adiposity in Global communities. J Mol Genet Med. 2015; 9: 1-8.
- Lowe CE, O'Rahilly S, Rochford JJ. Adipogenesis at a glance. J Cell Sci. 2011; 124: 2681-2686.
- Martins IJ. Anti-Aging Genes Improve Appetite Regulation and Reverse Cell Senescence and Apoptosis in Global Populations. AAR. 2016; 5: 9-26.
- Mayoral R, Osborn O, McNelis J, Johnson AM, Oh da Y, Izquierdo CL, et al. Adipocyte SIRT1 knockout promotes PPAR? Activity, adipogenesis and insulin sensitivity in chronic-HFD and obesity. Mol Metab. 2015; 4: 378-391.
- Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, et al. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008; 22: 2941-2952.
- Chakrabarti P, English T, Karki S, Qiang L, Tao R, Kim J, et al. SIRT1 controls lipolysis in adipocytes via FOXO1-mediated expression of ATGL. J Lipid Res. 2011; 52: 1693-1701.
- Eguchi A, Feldstein AE. Adipocyte cell death, fatty liver disease and associated metabolic disorders. Dig Dis. 2014; 32: 579-585.
- Alkhouri N, Gornicka A, Berk MP, Thapaliya S, Dixon LJ, Kashyap S, et al. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J Biol Chem. 2010; 285: 3428-3438.
- Hagenbuchner J, Ausserlechner MJ. Mitochondria and FOXO3: Breath or die. Front Physiol. 2013; 4: 147.
- Cheng Z, Guo S, Copps K, Dong X, Kollipara R, Rodgers JT, et al. Foxo1 integrates insulin signaling with mitochondrial function in the liver. Nat Med. 2009; 15: 1307-1311.
- Orsini F, Migliaccio E, Moroni M, Contursi C, Raker VA, Piccini D, et al. The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. J Biol Chem. 2004; 279: 25689-25695.
- Trinei M, Migliaccio E, Bernardi P, Paolucci F, Pelicci P, Giorgio M. p66Shc, mitochondria, and the generation of reactive oxygen species. Methods Enzymol. 2013; 528: 99-110.
- Murata M, Miwa Y, Sato I. Expression of respiratory chain enzyme mRNA and the morphological properties of mitochondria in the masseter muscles of klotho mutant mice. Okajimas Folia Anat Jpn. 2009; 86: 93-103.
- Suomalainen A, Elo JM, Pietiläinen KH, Hakonen AH, Sevastianova K, Korpela M, et al. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: a diagnostic study. Lancet Neurol. 2011; 10: 806-818.
- Martins IJ. Diet and Nutrition Reverse Type 3 Diabetes and Accelerated Aging Linked to Global Chronic Diseases. Journal of Diabetes Research and Therapy. 2016; 2: 1-6.
- Munekata K, Sakamoto K. Fork head transcription factor Foxo1 is essential for adipocyte differentiation. In Vitro Cell Dev Biol Anim. 2009; 45: 642-651.
- Nakae J, Kitamura T, Kitamura Y, Biggs WH, Arden KC, Accili D. The fork head transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell. 2003; 4: 119-129.
- Razzaque MS. The role of Klotho in energy metabolism. Nat Rev Endocrinol. 2012; 8: 579-587.
- Chihara Y, Rakugi H, Ishikawa K, Ikushima M, Maekawa Y, Ohta J, et al. Klotho protein promotes adipocyte differentiation. Endocrinology. 2006; 147: 3835-3842.
- Berniakovich I, Trinei M, Stendardo M, Migliaccio E, Minucci S, Bernardi P, et al. p66Shc-generated oxidative signal promotes fat accumulation. J Biol Chem. 2008; 283: 34283-34293.
- Ciciliot S, Albiero M, Menegazzo L, Poncina N, Scattolini V, Danesi A, et al. p66Shc deletion or deficiency protects from obesity but not metabolic dysfunction in mice and humans. Diabetologia. 2015; 58: 2352-2360.
- Hersoug LG, Møller P, Loft S. Gut microbiota-derived lipopolysaccharide uptake and trafficking to adipose tissue: implications for inflammation and obesity. Obes Rev. 2015.
- Liang H, Hussey SE, Sanchez-Avila A, Tantiwong P, Musi N. Effect of lipopolysaccharide on inflammation and insulin action in human muscle. PLoS One. 2013; 8: e63983.
- Frayn KN. Adipose tissue and the insulin resistance syndrome. Proc Nutr Soc. 2001; 60: 375-380.
- Martins, IJ. Overnutrition Determines LPS Regulation of Mycotoxin Induced Neurotoxicity in Neurodegenerative Diseases. Int J Mol Sci. 2015; 16; 29554-29573.
- Martins IJ. LPS Regulates Apolipoprotein E and Aß Interactions with Effects on Acute Phase Proteins and Amyloidosis. AAR. 2015; 4: 69-77.
- Martins IJ. Unhealthy Diets Determine Benign or Toxic Amyloid Beta States and Promote Brain Amyloid Beta Aggregation. Austin J Clin Neurol. 2015; 2: 1060-1066.
- Martins IJ. Diabetes and Cholesterol Dyshomeostasis Involve Abnormal a-Synuclein and Amyloid Beta Transport in Neurodegenerative Diseases. Austin Alzheimers J Parkinsons Dis. 2015; 2: 1020-1028.
- Turtzo LC, Lane MD. NPY and neuron-adipocyte interactions in the regulation of metabolism. EXS. 2006; 95: 133-141.
- Zhang W, Cline MA, Gilbert ER. Hypothalamus-adipose tissue crosstalk: neuropeptide Y and the regulation of energy metabolism. Nutr Metab (Lond). 2014; 11: 27.
- Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis.Br J Radiol. 2012; 85: 1-10.
- Lee JH, Kirkham JC, McCormack MC, Medina MA, Nicholls AM, Randolph MA, et al. A novel approach to adipocyte analysis. Plast Reconstr Surg. 2012; 129: 380-387.
- Majka SM, Miller HL, Helm KM, Acosta AS, Childs CR, Kong R, et al. Analysis and isolation of adipocytes by flow cytometry. Methods Enzymol. 2014; 537: 281-289.
- Maeda K, Okubo K, Shimomura I, Mizuno K, Matsuzawa Y, Matsubara K. Analysis of an expression profile of genes in the human adipose tissue. Gene. 1997; 190: 227-235.
- Villalba JM, Alcaín FJ. Sirtuin activators and inhibitors. Biofactors. 2012; 38: 349-359.
- Martins IJ. Increased Risk for Obesity and Diabetes with Neurodegeneration in Developing Countries. J Mol Genet Med. 2013; 1: 1-8.