
Research Article
Austin J Orthopade & Rheumatol. 2015; 2(3): 1024.
The Effect of Serum from Acute Traumatic Brain or Spinal Cord Injury Patients on the Growth of Bone Marrow-Derived Mesenchymal Stem Cells (Atcc-USA)
Khallaf FG1 and Kehinde EO2*
1Department of Orthopaedic Surgery, Jahra Hospital, Kuwait
2Department of Surgery, Kuwait University, Kuwait
*Corresponding author: Khallaf FG, Department of Orthopaedic Surgery, Jahra Hospital, Ministry of Health, Kuwait
Received: September 27, 2015; Accepted: December 18, 2015; Published: December 23, 2015
Abstract
Accelerated osteogenesis associated with traumatic brain injury BTI or spinal cord injury SCI is inconclusive and its cause remains obscured. The purpose of this study was to ensure a clinical evidence of its presence and to reveal the possible underlying mechanism. Healing indicators of diaphyseal femoral fractures in 20 patients with BTI and 20 patients with SCI were compared to 20 patients with femoral fracture only. The effect of sera of blood samples withdrawn from these patients on cell count proliferation rate of bone marrowderived Mesenchymal Stem Cells MSCs (ATCC-USA) were measured and compared to sera from 20 patients with BTI only, 20 patients with SCI only, and a control group. The results showed that femoral fractures with BTI or SCI heal more expectedly, faster with exuberant callus (p<0.001) and showed statistically significant increased cell count and growth rate of MSCs with sera from BTI and SCI patients with or without femoral fractures, 82.34%, 83.90%, 81.46%, and 81.50% respectively versus 52.96% in the control and 59.77% in patients with femoral fractures only (p<0.005). These results suggested enhancement of fracture-healing secondary to TBI and SCI due to the presence of factors in the serum that have a mitogenic effect on MSCs.
Keywords: Traumatic brain injury; Spinal cord injury; Long bone fractures; Acceleration of bone healing; Undifferentiated mesenchymal stem cells
Abbreviations
TBI: Traumatic Brain Injury; SCI: Spinal Cord Injury; MSCs: Undifferentiated Mesenchymal Stem Cells; ATCC: American Type Culture Collection; SPSS: Statistical Package for the Social Sciences; GCS: Glasgow Coma Scale; RTA: Road Traffic Accident
Introduction
There is some clinical evidence to suggest that fractures of long bones heal more rapidly in patients with severe head injury or acute traumatic spinal cord injury. The mechanism underlying this orthopedic phenomenon is not well understood. Early clinical reports that researched the correlation between accelerated bone healing and acute traumatic nervous tissue damage in head or spinal cord injuries were inconclusive and demonstrated no evidence of accelerated union or increased callus formation [1-10].
The current understanding of bone healing event showed that the process involves the participation of orchestra of many growth factors and cytokine molecules and cells, primarily Undifferentiated Mesenchymal Stem Cells (MSCs) and blood inflammatory cells to induce formation of union callus at the fracture site [11-25].
The primary objective of this prospective controlled study was to ensure the accelerating effect of severe acute traumatic head injuries and spinal cord injuries on the healing of concomitant diaphyseal femoral fractures and the secondary objective was to test the effect of sera taken from patients with severe head or spinal cord injuries with concomitant long bone femoral diaphyseal on the growth rate of bone marrow derived mesenchymal stem cells on stem cells culture to elucidate the mechanism of accelerated bone healing in such patients.
Patients and Methods
Recruited patients in this current study, were non-smokers, between 18 and 60 years old, and had no history of chronic illness or systemic diseases. Patients on permanent medications and therapy for chronic disease such as diabetes mellitus, ischemic heart diseases, chronic renal failure, or endocrine diseases, or patients on corticosteroids for bronchial asthma, rheumatoid arthritis, other inflammatory arthritis, and autoimmune diseases were excluded from the study
The patients were divided into five groups: Group A consisted of 20 patients with acute severe post-traumatic head (brain) injuries who were admitted to the Intensive Care Unit (ICU) with a Glasgow Coma Scale (GCS) of 8 or less(to define severe injury), Group B consisted of 20 patients with severe head injury and concomitant long bone diaphyseal femoral fractures, Group C consisted of 20 patients with acute post- traumatic spinal cord injuries with complete quadriplegia or paraplegia, Group D consisted of 20 patients with spinal cord injury and femoral shaft fractures, and Group E consisted of 20 patients with femoral diaphyseal fractures only. All femoral fractures in patients of group (B), (D), and (E) were treated surgically, by closed static reamed intra-medullary locking nail and followed -up weekly for three month and then, every three weeks for another three months (end-point of the study of fracture union, delayed union, or non-union), and every two months for another six to eight months. The patients’ biodata and characteristics of injuries of all groups were shown in (Table 1).
Groups
A
B
C
D
E
No. recruited
patients
20
20
20
20
20
Mean Age Years
28.5
29.6
32.7
29.5
30
Age Range Years
18-42
22-41
18-4 9
22-42
21-42
Sex
M
16
17
16
18
16
F
4
3
4
2
4
Mean (Range)
GCS*
6
7
15
15
15
5-8
5-8
0
0
0
Cause of Injury
RTA**
20
20
12
17
17
Fall from
height
0
0
8
3
3
Type of injury
Head injury
yes
yes
0
0
0
Spine injury
Quadriplegia
0
0
yes
9
yes
8
0
Paraplegia
0
0
11
12
0
No of femoral shaft
fracture
0
21
0
21
20
Status of Patient
Alive / Dead
20/0
20/0
20/0
20/0
20/0
Table 1: Patients’ biodata and characteristics of injuries.
Assessment of radiological healing of fractures is difficult and controversial, but mostly, radiological union is defined by the presence of bridging callus, disappearance of fracture line or the continuity of cortex in at least in three of the four bone cortices appear in the antero-posterior and the lateral X-ray views, so a score of 3-4 points of basically bridging callus defines fracture union. The healing of femoral shaft fractures in this study has been followed up by radiological assessment of the fracture in antero-posterior and lateral X-ray views weekly and once the plain X-ray showed fracture union according to the aforementioned radiological criteria, we use CT to assess the maximal amount of union callus formed at the fracture site. Delayed union was defined as absence of radiological union criteria 3 months after the occurrence of the femoral fracture, while non-union was defined as no bridging callus and radiologically visible fracture line 6 months after the injury with atrophic or hypertrophic fracture ends. The healing rate of femoral fracture was defined as the maximal thickness of union bridging callus in millimeters as observed in CT scan, divided by the time to healing in weeks. Time to radiological union, the maximal thickness of the amount of union callus formed, and healing rate of fractures were compared in three groups of patients: (B), (D), and (E).
Blood samples were withdrawn from the injured patients of all groups from (A) to (E) at one week from the time of injury. 10 ml of blood was withdrawn only once, to test its effect and response with an in vitro cell assay.
Methods
1) Blood samples were processed by centrifugation and separation of the sera which were preserved at -850C.
2) Cell cultures of bone Marrow-Derived Mesenchymal Stem Cells (BMDMSC) from ATCC, USA, were established as follows:
Components
1) 1 vial of Mesenchymal cells (1x106)
2) Mesenchymal stem cell Basal Medium
3) Supplements: FBS, rh FGF-b, rh IGF-1, L-alanyl-L-Glutamine, Penicillin Streptomycin
4) Reagents for Subculture: DPbs, Trypsin-EDTA, Trypsin neutralizing solution
Preparation of Complete Growth Medium
1. Obtain 1 mesenchymal stem cell growth kit from the freezer. Make sure the caps are tight.
2. Decontaminate the surfaces of all growth kit and basal medium with 70% ethanol or methanol.
3. Thaw the components of the growth kit prior to adding to the basal medium.
4. Take one bottle of basal medium.
5. Transfer the indicated volume of the kit component to the basal medium.
6. Swirl gently to assure homogenous solution.
7. Store at 2-8oC in the dark.
Procedure
1. Mesenchymal stem cells were grown until they were confluent using basal medium with supplements.
2. Cells were then trypsinized and counted the viable cells.
3. Aliquoted 10000cells/ml to each 21 flasks.
4. To 5000 per ml of BMDMSC cells growing in small tissue culture flasks, 100 μl of sterile serum from the following groups of patients, were added: group A of brain injury only, group B of brain injury and long bone fracture, group C of spinal cord injury only, group D of spinal cord injury and long bone fracture, group E of long bone fracture only, and one flask remains without serum as control.
5. Place the seeded flask in the 5% CO2 incubator at 370c for 72 hours.
6. Cells were trypsinized and viable cells counted using Vi-Cell XR cell viability analyzer (Beckman Coulter).
7. After 72 hours, growth inhibition or stimulating effect of serum from different patients’ groups on BMDMSC in culture was assessed by counting the number of cells.
8. The experiment was repeated per category of patient, 20 times, using serum from 20 different patients per category.
9. The mean ± SD of the viable cell count in patients of each group was determined for the five groups and the results were compared between the groups and statistically analyzed.
Post- operative rehabilitation and follow up
Patients in the three groups (B), (D), (E) were subjected to intensive program of physiotherapy including continuous passive motion CPM exercises whether in comatose, group (B) patients or in paralyzed, group (D) patients or patients with long bone fractures only, group (E). Patients with head injury, when they became awake were allowed to be mobilized on a wheel chair. Patients with spinal cord injury were also allowed to be mobilized on a wheel chair when all their fractures were fixed, while patients in group (E) actively exercised their limbs and were allowed to walk partial or full weight bearing with crutches or walker, once their femoral or tibial fractures were fixed.
Statistical analysis
Results were analysed with statistical package for the social sciences SPSS for Windows (Version 16). Means and standard deviations were determined. Mean scores between the two groups of patients were compared using chi square and the Student t-test. P value < 0.05 was considered statistically significant.
Results
The biodata of patients in the study are shown in (Table 1). 20 patients have been recruited in group (A). The mean age in this group was 28.5, range (18-42) years. The patients were 16 (80%) males and 4 (20%) females. The accidents in which, these patients were involved were high-velocity road traffic accidents RTA. 11 (55%) patients in this group had associated chest injuries and 4 (20%) had abdominal injuries. The mean GCS in the patients of this group was 6/15, range (5-8)/15. Four (20%) patients required neurosurgical procedures such as craniotomy, evacuation of hematoma or elevation of depressed skull fractures. The findings of head CT scan in the patients of this group have been mentioned in (Table 2).
CT scan head findings
Group A
Group B
No of
patients
%
No of patients
%
Skull fractures
17
85
18
90
Cranial bones only
16
80
15
75
Facial bones only
1
5
3
15
Cranial and facial fractures
1
5
3
15
Fracture base of the skull
0
0
0
0
Subdural hematoma
16
80
15
75
Subarachnoid haemorrhage
8
40
9
45
Diffuse brain oedema
15
75
17
85
Lobe and intra-cerebral hemorrhagic contusion
12
60
13
65
Midline shift
3
15
3
15
Extra-dural hematoma
5
25
4
20
Pneumocephalus
3
15
4
20
Impending conization
1
5
0
0
Diffuse axonal brain injury
3
15
3
15
Intra-ventricular haemorrhage
3
15
2
10
Table 2: Findings on CT of the skull in groups A and B patients.
20 patients were included in group (B) and had a mean age of 29.6 (range 22-41) years. There were 17 (85%) males and 3 (15%) females in this group. All patients in this group have been involved in RTA (Table 1). 9 (45%) patients in this group had associated chest injuries and 5 (25%) had abdominal injuries. The mean GCS in group (B) patients was 7/15, with a range of (5-8)/15. Five (25%) patients required neurosurgical procedures. The findings of head CT scan in the patients of this group have been mentioned in (Table 2). 21 closed diaphyseal femoral fractures were in the 20 patients in group (B), 11 (55%) of them were comminuted. These fractures were treated by closed or open reduction and internal fixation by static reamed interlocking intra medullary nail after a mean time of 7 and range of (5-9) days and were followed up for mean of 14 and range of (12-18) months.
The mean time to union in this group was 6.8 (range 4-17) weeks. There were no cases of non-union of the diaphyseal femoral fractures in this group. The mean maximal thickness of union bridging callus was 27 (range 8-48) mm. The mean healing rate of fractures of long bones in this group of patients was 4 (range 2.2-9.4) mm/week, as shown in (Table 4 & Figure 1).
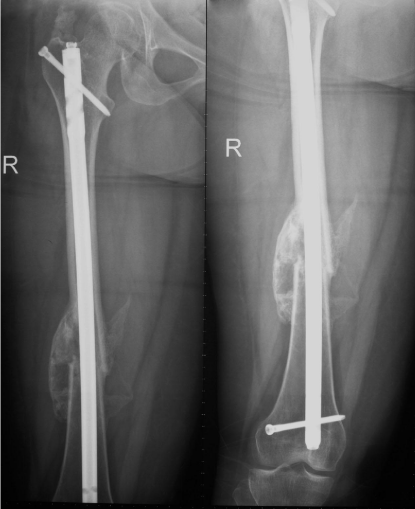
Figure 1: Showing x-ray of femur with accelerated fracture healing and
abundant callus formation 5.4 weeks post-injury in a patient with severe head
injury and long bone femoral diaphyseal fracture group (A) patient.
20 patients have been recruited in group (C). The mean age of the patients in this group was 32.7 range (18-49) years. These 20 patients included 16 (80%) males and 4 (20%) females. All patients have been involved in high velocity accidents, 12 patients (60%) have been involved in RTA and 8 patients (40%) in falling from height accidents. 7 (35%) patients in this group had associated chest injuries and one (5%) had abdominal injuries. Cervical spine injuries of fracture-dislocation with complete quadriplegia have occurred in 9 patients (45%) of this group and burst vertebral body fracture or fracture-subluxation of dorso-lumbar spine and complete paraplegia have occurred in the remaining 11 patients (55%). The details of spine injuries seen in this group were shown in (Table 3). In all group (C) patients spine surgery procedures of open reduction, decompression, cage, plate fixation, trans-pedicular screws posterior fixation, and or fusion have been done in cervical and dorso- lumbar spine injuries.
Type of spinal injury
Group C No of patients
Group D No of patients
Cervical spine injuries
9
8
Hangman fracture of C2
1
C4-C5 fracture dislocation
3
2
C5-C6 fracture dislocation
3
2
C6-C7 fracture dislocation
1
2
C7-T1 fracture dislocation
3
2
Unstable burst fracture
1
1
compression fracture
1
1
fracture pedicle or lamina
traumatic disc protrusion
1
Dorso-lumber spine injuries
11
12
Unstable burst fracture DV
6
5
Unstable burst fracture LV
3
5
Stable compression fracture DV
1
2
Stable compression fracture LV
2
2
fracture-dislocation
2
2
Table 3: Types of spine injuries seen in groups C and D patients.
20 patients were included in group (D) and had a mean age of 29.5 (range 22-42) years. There were 18 (90%) males and 2 (10%) females in this group. 17 (85%) of patients have been involved in RTA and 3 (15%) in falls from height accidents, as shown in (Table 1). 5 (25%) patients in this group had associated chest injuries and 2 (10%) had abdominal injuries. In this group, 8 (40%) patients had cervical spine injuries and complete quadriplegia and 12 (60%) patients sustained dorso-lumbar spine injuries and complete paraplegia. The details of spine injuries seen in this group were shown in (Table 3). Spinal surgery procedures of open reduction, decompression, transpedicular screws posterior fixation, and/or fusion were performed in the thirteen patients of this group with dorso- lumbar spine injuries and in the eight patients with cervical spine injuries. Different procedures of reduction and plate fixation, anterior corpectomy, and/ or fusion with instrumentation, were carried out.
21 closed diaphyseal femoral fractures were in the 20 patients in group (D), 11(52.4%) of them were comminuted. These fractures were treated by closed or open reduction and internal fixation by static reamed interlocking intramedullary nail after a mean time of 5 and range of (3-7) days and were followed up for mean of 14 and range of (12-17) months.
The mean time to union in of the diaphyseal femoral fractures in group D) was 6.2 (range 3-7.7) weeks. There were no cases of nonunion of the femoral fractures in this group. The mean maximal thickness of union bridging callus was 29 (range10-48) mm. The mean healing rate was 4.7 (range 2.6-7.5) mm/week, as shown, in (Table 4 & Figure 2).
Patients group
No of patients
finished
follow-up
No of long bone femoral shaft fracture
No of fractures
non-union
Mean (range) of healing time in weeks
Mean (range) of maximal
thickness
of union callus in mm
Mean
(range) of
healing
rate in mm/week
B
20
21
0
6.81
(4-17)
273
(4-48 )
45
(2.2-9.4)
D
20
21
0
6.22
(3-7.7)
294
(10-48)
4.76
(2.6-7.5 )
B+D
40
42
0
6.5
(3-17)
28
(4-48)
4.4
(2.2-9.4)
E
20
20
3
(15 % )
22.41,2
(13-41)
8.13,4
(2- 16)
0.365,6
(0.11- 1)
1p = 0.001, 2p = 0.01, 3p = 0.0005, 4p =0.001, 5p =0.001, 6p= 0.005
Table 4: Comparison of healing indicators of femoral fractures in patients in groups B, D, and E.
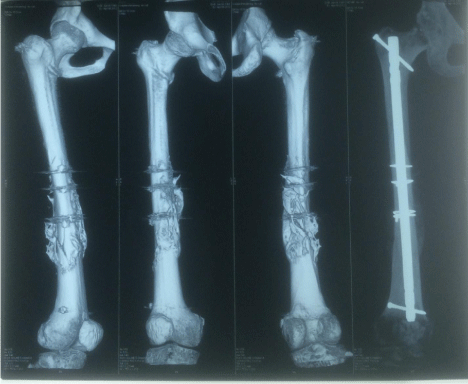
Figure 2: 3D CT scan and X-ray of femur with accelerated union of diaphyseal
fracture with abundant callus formation 5 weeks post-surgery and 5.8 weeks
post-injury in a group (B) patient with cervical spine fracture-dislocation and
quadriplegia.
20 patients were included in group (E) and had a mean age of 30 (range 21-42) years. There were 16 (80%) males and 4 (20%) females in this group. The type of accident was high energy in all patients, RTA in 17 (85%) and falling from height in 3 (15%), as shown in (Table 1). Only one patient (5%) in this group developed associated chest and abdominal injuries.
20 closed diaphyseal femoral fractures were in the 20 patients in group (E), 8 (40%) of them were comminuted. These fractures were treated by closed or open reduction and internal fixation by static reamed interlocking intramedullary nail after a mean time of 7 and range of (3-11) days and were followed up for mean of 17 and range of (14-20) months.
Among the 20 femoral fractures in the 20 patients in this group, 17 (85%) fractures united and 3 (15%) had delayed union, with union occurring 32 to 41weeks after the fractures occurred. Three (15%) fractures ended up by atrophic nonunion and required secondary procedures, as shown in figure. Two (10%) of these non-united femoral fractures developed metal failure of broken nails with the osseous failure of union. The mean healing time in this group of patients was 22.4 (range 13-41) weeks. The mean maximal thickness of callus in the united fractures in this group was 8 (range 2-16) mm. The mean healing rate was 0.36 (range0.11 - 1) mm/week, as shown in (Table 4 & Figure 3).
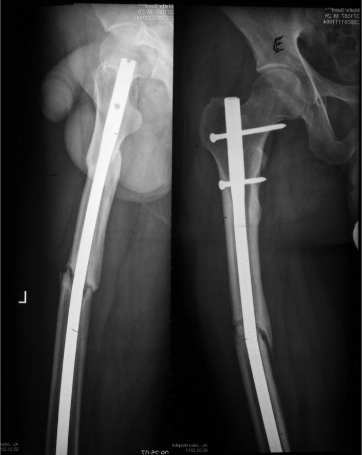
Figure 3: X-ray of fracture of the femur with osseous failure of atrophic
nonunion and metal failure of broken nail 28 weeks post-injury in a group (E)
patient with long bone fracture only.
Among the 20 femoral fractures in the 20 patients in this group, 17 (85%) fractures united and 3 (15%) had delayed union, with union occurring 32 to 41weeks after the fractures occurred. Three (15%) fractures ended up by atrophic nonunion and required secondary procedures, as shown in figure. Two (10%) of these non-united femoral fractures developed metal failure of broken nails with the osseous failure of union. The mean healing time in this group of patients was 22.4 (range 13-41) weeks. The mean maximal thickness of callus in the united fractures in this group was 8 (range 2-16) mm. The mean healing rate was 0.36 (range0.11 - 1) mm/week, as shown in (Table 4 & Figure 3).
Measuring the cell count of cell line of bone marrow-derived Mesenchymal Stem Cells Mscs (ATCC-USA) treated with control and patients’ sera from different groups after 72 hrs incubation showed high statistically significant cell count and growth and viability rate in patients with severe head injury with or without long bone fractures groups (A) and (B) and patients with spinal cord injuries with or without long bone fractures groups (C) and (D) in comparison to the control and to the effect of the sera from long bone fracture only group (E). The mean growth rate in groups (A), (B), (C), and (D) was 82.34%, 83.90%, 81.46%, and 81.50% respectively versus 52.96% in the control and 59.77% in group (E) with long bone fractures only (p<0.005), as shown in (Table 5 & Figure 4).
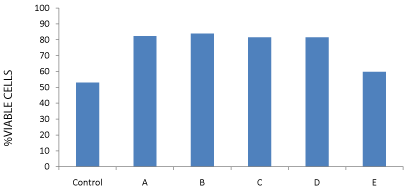
Figure 4: % cell growth viability chart of cell line MSCs treated with control
and all groups A to E patients sera after 72 hrs incubation.
Table 5 : Summary of Cell count of cell line MSCs treated with control and patient samples after 72 hrs incubation
Mean ± SD
Groups
Total no. of cells/ml
Viable cells/ml
% viable cells
Control (n=20)
1.66X105±7.7 X 104
8.7x104±2.25x104
52.961,2,3,4,5
A ( n=20)
1.17x105±1.5x104
9.7x104±1.5x104
82.341
B( n=20)
1.8x105±2.34x104
9.3x104±2.13x104
83.92
C( n=20)
1.15x105±1.79 x 104
9.4 x104±1.44 x 104
81.463
D( n=20)
1.44x105±2.88x 104
1.17x105±1.86x104
81.54
E( n=20)
1.27x105±2.43x104
1.0x104±1.91x104
59.775
1P = 0.001, 2P = 0.01, 3P = 0.005, 4P = 0.001, 5P= 0.005
Control: Healthy subjects; A: Brain injury only; B: Brain injury + long bone fracture; C: Spinal cord injury only; D: Spinal cord injury + long bone injury; E: long bone fracture only
Table 5: Summary of Cell count of cell line MSCs treated with control and patient samples after 72 hrs incubation
Moreover, we found a positive correlation between fracture union time and thickness of union callus on one side and proliferation of bone marrow-derived mesenchymal stem cells in the tissue cultures on the other side. On the contrary, we found no correlation between the severity of Glasgow Coma Scale (GCS) with either the acceleration of fracture healing or the percentage of proliferation and viability of cells in the MSCs tissue cultures.
Discussion
The current study is a prospective controlled study that compared the time of union, amount of union callus, and rate of healing of 42 diaphyseal femoral fractures in 40 patients associated with severe central nerve tissue damage (21 fractures in 20 patients with associated severe head injury and 21 fractures in 20 patients with spinal cord injury) to 20 femoral shaft fractures in 20 patients without head or spinal cord injuries with matching of the variables of age, type of accident, type of fracture, the fractured long bone, associated injuries, and method of skeletal stabilization . Furthermore, in this study we excluded patients with chronic illness or systemic diseases, thus further reducing confounders that may affect fracture healing.
From the results of the two groups of patients with diaphyseal femoral fractures associated with head injury in group (B) and with spinal cord injury in group (D) compared to the group of patients with only femoral fractures group (E), we observed that fracture union occurred faster over a short period of time in groups (B) and (D) compared to group (E). Femoral fractures in group (B) and (D) patients united within mean of 6.5 (range 4-17) weeks compared to mean of 22.4 (range 13-41) weeks in group (E) patients, a statistically significant difference (p<0.001).
Another important finding of our study is that diaphyseal femoral fractures in head or spinal cord injury patients healed with more exuberant and florid callus formation compared with patients with femoral fractures only. The mean thickness of callus in groups (B) and (D) was 28 (range 4-48) mm, compared to 8 (range 2-16) mm in group (E) (p<0.001). Accordingly, the mean healing rate was also statistically significantly faster in groups (B) and (D) compared to group (E) {4.4 (range 2.2-9.4) mm/week versus 0.38 (range 0.11-1) mm/week}.
The study also showed that femoral fractures in patients with severe head injury or spinal cord injury all united and healed without a single case of nonunion or delayed union. However, 3 (15%) femoral fractures in 3 patients of group (E) had atrophic nonunion, two (10%) of them developed metal failure of broken nails. Furthermore, 3 (15%) femoral fractures in group (E) had delayed union which united in 38, 39, and 41 weeks post-injury.
One of the points of weakness in this study was the determination of the exact time of union whether clinical or radiological and the controversies of definition of radiological union, that may require to be exact, to have daily X-ray which is impossible for very overt reasons. Another point of weakness was the significance of the meaning of the healing rate as a healing indicator which may give a delusive impression that fracture healing is consistent through the whole process of osteogenesis, for which we do not have any proof. The third point of weakness was our dependence, basically on the bridging callus as radiological criterion among other criteria to assess union, but this may be justified by our method of fractures treatment, using interlocking intramedullary fixation, which mostly lead to secondary fracture union with bridging callus formation and unlike if we would have treated these fractures by open anatomical reduction and internal fixation by Compression Dynamic Plates CDP, which mostly, may lead in this case to primary bone healing with radiological continuity of the cortex at the fracture site with no callus formation.
Some articles in the literature were found to support the results of the current study. Newman et al., (1987) performed a retrospective study and demonstrated an unusually rapid healing of 13 closed long bone fractures in patients with concomitant severe head injuries [26]. Giannoudis et al., (2006) performed a study that included 17 patients with head injury and associated femoral shaft fractures and 50 patients without head injury (25 treated with reamed and 25 with unreamed nailing technique), and these authors reported a significantly shorter mean time to fracture union in patients with head injury than either the reamed or the unreamed nailing groups without head injury [27]. Yang et al., (2012), performed a retrospective study and compared the healing of femoral shaft fractures in 20 patients with associated Traumatic Brain Injury (TBI) to 54 patients without brain injury, and these authors confirmed that an injury to the brain may be associated with accelerated healing and enhanced callus formation in femoral shaft fractures [28].
Investigating the underlying mechanism of this accelerated osteogenesis, the results of the current study showed a high statistically significant proliferation and growth rate of cell line of bone marrow-derived Mesenchymal Stem Cells MSCs (ATCCUSA) treated with control and patients’ sera from different groups after 72 hrs incubation in patients with severe head injury or spinal cord injury with or without long bone fractures groups (A) to (D) in comparison to the control and to the effect of the sera from long bone fracture only group (E). The mean growth rate in groups with central nervous tissue damage from (A) to (D) (C), was 82.3%, versus 52.96% in the control and 59.77% in group (E) with long bone fractures only (p<0.005). These results indicate that sera from severe head injury or spinal cord injury patients with or without long bone fractures are mitogenic in vitro and induce a statistically significant proliferation of the cell line of bone marrow-derived mesenchymal stem cells MSCs in the stem cell tissue culture. We understand that the in vitro mitogenic effect of these sera may be due to increased levels of growth factors and cytokines in the blood of the patients with severe head injury or spinal cord injury patients with or without long bone fractures or due to humoral substance, which has been produced in the damaged brain or spinal cord and crossed the blood brain barrier to peripheral circulation to enhance MSCs proliferation and differentiation in abundance into osteoblasts and chondrocytes at the fracture site to induce acceleration of fracture healing.
The results of in- vitro studies of the effect of serum from patients with a traumatic brain injury on cultured cells have not been uniformly conclusive. Although few studies documented activation of osteoblasts on exposure to serum from patients with a brain or spinal cord injury, that finding was not substantiated in other subsequent studies and no substance or protein has been identified as the causative agent [11-25].
Despite the current lack of conclusive results, a study by Binder et al. found strong evidence that patients with a traumatic brain injury possess a humoral mechanism for accelerated fracture healing by demonstrating a mitogenic effect of the serum taken from those patients on cultured osteoblasts [29].
Cadosch et al., for a better understanding of the pathophysiological mechanisms that lead to the formation of heterotopic ossification in patients with a traumatic brain injury, investigated whether cells from skeletal muscle adopt an osteoblastic phenotype in response to serum from thirteen patients with severe traumatic brain injury and concluded that serum from patients with severe traumatic brain injury supports the osteoblastic differentiation of cells derived from human skeletal muscle and accelerates proliferation of these cells [30].
Boes and colleagues proposed an influence of unknown factors released by injured brain tissue, which exert their proliferative effect specific to mesenchymal stem cells. In their in vitro analysis, they showed that the serum of rats with a fracture and concomitant TBI stimulated a multipotent Mesenchymal Stem Cell Line (C3H10Tcells) to proliferate at a significantly higher level, resulting in a 76% increase in cells in the fracture/TBI group compared to the fractureonly group [30]. Cadosch and Gautschi investigated a human fetal osteoblastic mesenchymal stem cell line (hFOB1.19 cells) in an early stage of its differentiation. They saw that the cerebrospinal fluid of patients with a traumatic brain injury had an osteoinductive potential and therefore they expected that any osteoinductive factor in the serum of patients with a traumatic brain injury would have a stimulating effect on the hFOB1 cells in vitro. They also observed an increased proliferation rate of osteoblasts exposed to sera from patients with TBI during the first week after injury [31-34]. Kurer et al. reported similar effect in patients with spinal cord injury and heterotopic ossification. However, there is no evidence that the invitro culture changes, which the serum of patients produce can lead to clinically significant changes in fracture healing or fracture methods of management [35].
Conclusion
We conclude according to the results of this study that union of diaphyseal femoral fractures is ensured, augmented and accelerated in patients with concomitant acute post-traumatic head or spinal cord injuries which may be due to enhancement of MSCs proliferation and differentiation into osteoblasts and chondrocytes to accelerate bone healing based on the in vitro mitogenic effect of sera taken from these patients on the cell line of bone marrow-derived mesenchymal stem cells MSCs cultures as shown in the study. Growth factors, cytokines, and damaged brain or spinal cord releasing humoral substance may the underlying cause of this mitogenic effect which needs further research to reveal it.
Acknowledgement
This study article was a part of a project, which was funded “Fully” by Kuwait Foundation for the Advancement of Sciences, KFAS under project code: 2010/1302/04.
The authors of this article are grateful to the sincere effort of Mr. Sherif Khallaf who made this piece of work possible.
References
- Garland DE, Dowling V. Forearm fractures in the head-injured adult. Clin Orthop Relat Res. 1983; 190-196.
- Garland DE, O'Hollaren RM. Fractures and dislocations about the elbow in the head-injured adult. Clin Orthop Relat Res. 1982; 38-41.
- Garland DE, Toder L. Fractures of the tibial diaphysis in adults with head injuries. Clin Orthop Relat Res. 1980; 198-202.
- Garland DE, Rhoades ME. Orthopedic management of brain-injured adults. Part II. Clin Orthop Relat Res. 1978; 111-122.
- Garland DE, Rothi B, Waters RL. Femoral fractures in head-injuries adults. Clin Orthop Relat Res. 1982; 219-225.
- Garland DE. A clinical perspective on common forms of acquired heterotopic ossification. Clin Orthop Relat Res. 1991; 13-29.
- Roberts P. Heterotopic ossification complicating paralysis of intracranial origin. J Bone Joint Surg [Br]. 1968; 50:70-77.
- van Kuijk AA, Geurts AC, van Kuppevelt HJ. Neurogenic heterotopic ossification in spinal cord injury. Spinal Cord. 2002; 40: 313-326.
- Trentz OA, Handschin AE, Bestmann L, Hoerstrup SP, Trentz OL, Platz A. Influence of brain injury on early posttraumatic bone metabolism. Crit Care Med. 2005; 33: 399-406.
- Smith R. Head injury, fracture healing and callus. J Bone Joint Surg Br. 1987; 69: 518-520.
- Nakahara H, Dennis JE, Bruder SP, Haynesworth SE, Lennon DP, Caplan AI. In vitro differentiation of bone and hypertrophic cartilage from periosteal-derived cells. Exp Cell Res. 1991; 195: 492-503.
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991; 9: 641-650.
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284: 143-147.
- Arthur A, Zannettino A, Gronthos S. The therapeutic applications of multipotential mesenchymal/stromal stem cells in skeletal tissue repair. J Cell Physiol. 2009; 218: 237-245.
- Granero-Moltó F, Weis JA, Miga MI, Landis B, Myers TJ, O'Rear L, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009; 27: 1887-1898.
- Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003; 88: 873-884.
- Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 2009; 24: 274-282.
- Hayashi O, Katsube Y, Hirose M, Ohgushi H, Ito H. Comparison of osteogenic ability of rat mesenchymal stem cells from bone marrow, periosteum, and adipose tissue. Calcif Tissue Int. 2008; 82: 238-247.
- Rahn BA, Gallinaro P, Baltensperger A, Perren SM. Primary bone healing. An experimental study in the rabbit. J Bone Joint Surg Am. 1971; 53: 783-786.
- Taguchi K, Ogawa R, Migita M, Hanawa H, Ito H, Orimo H. The role of bone marrow-derived cells in bone fracture repair in a green fluorescent protein chimeric mouse model. Biochem Biophys Res Commun. 2005; 331:31-36.
- Chen G, Deng C, Li YP. TGF-î and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012; 8: 272-288.
- Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009; 4: 206-216.
- Yasuhara S, Yasunaga Y, Hisatome T, Ishikawa M, Yamasaki T, Tabata Y, et al. Efficacy of bone marrow mononuclear cells to promote bone regeneration compared with isolated CD34+ cells from the same volume of aspirate. Artif Organs. 2010; 34: 594-599.
- Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001; 19: 180-192.
- Gautschi OP, Cadosch D, Frey SP, Skirving AP, Filgueira L, Zellweger R. Serum-mediated osteogenic effect in traumatic brain-injured patients. ANZ J Surg. 2009; 79: 449-455.
- Newman RJ, Stone MH, Mukherjee SK. Accelerated fracture union in association with severe head injury. Injury. 1987; 18: 241-246.
- Giannoudis PV, Mushtaq S, Harwood P, Kambhampati S, Dimoutsos M, Stavrou Z, et al. Accelerated bone healing and excessive callus formation in patients with femoral fracture and head injury. Injury. 2006; 37: S18-24.
- Yang TY, Wang TC, Tsai YH, Huang KC. The effects of an injury to the brain on bone healing and callus formation in young adults with fractures of the femoral shaft. J Bone Joint Surg Br. 2012; 94: 227-230.
- Bidner SM, Rubins IM, Desjardins JV, Zukor DJ, Goltzman D. Evidence for a humoral mechanism for enhanced osteogenesis after head injury. J Bone Joint Surg Am. 1990; 72: 1144-1149.
- Cadosch D, Toffoli AM, Gautschi OP, Frey SP, Zellweger R, Skirving AP, et al. Serum after traumatic brain injury increases proliferation and supports expression of osteoblast markers in muscle cells. J Bone Joint Surg [Am]. 2010; 92: 645-653.
- Boes M, Kain M, Kakar S, Nicholls F, Cullinane D, Gerstenfeld L, et al. Osteogenic effects of traumatic brain injury on experimental fracture-healing. J Bone Joint Surg Am. 2006; 88: 738-743.
- Cadosch D, Gautschi OP, Thyer M, Song S, Skirving AP, Filgueira L, et al. Humoral factors enhance fracture-healing and callus formation in patients with traumatic brain injury. J Bone Joint Surg Am. 2009; 91: 282-288.
- Cadosch D, Toffoli AM, Gautschi OP, Frey SP, Zellweger R, Skirving AP, et al. Serum after traumatic brain injury increases proliferation and supports expression of osteoblast markers in muscle cells. J Bone Joint Surg Am. 2010; 92: 645-653.
- Gautschi OP, Toffoli AM, Joesbury KA, Skirving AP, Filgueira L, Zellweger R. Osteoinductive effect of cerebrospinal fluid from brain-injured patients. J Neurotrauma. 2007; 24: 154-162.
- Kurer MH, Khoker MA, Dandona P. Human osteoblast stimulation by sera from paraplegic patients with heterotopic ossification. Paraplegia. 1992; 30: 165-168.