
Case Report
Austin J Orthopade & Rheumatol. 2016; 3(1): 1026.
Acyclovir Extravasation: Case Report and Review of the Literature
Lau BC* and Lee NH
Department of Orthopaedic Surgery, University of California San Francisco Medical Center, USA
*Corresponding author: Lau BC, Department of Orthopaedic Surgery, University of California San Francisco Medical Center, 400 Parnassus Avenue, San Francisco, CA 94143, USA
Received: April 06, 2016; Accepted: May 02, 2016; Published: May 05, 2016
Abstract
Background: Infiltration and extravasation of solutions is a common, under-reported problem with potentially severe morbidity. We report a case of intravenous extravasation of acyclovir and discuss our approach and management of this incident.
Methods: We describe a case of acyclovir extravasation and provide a literature review on the general management of extravasation injuries. PubMed articles in the English Language were used for this review. Informed consent was obtained from all individual participants included in the study.
Results: Close observation and extremity elevation with cold compresses was an adequate treatment for our patient. At final follow-up, the patient had some residual soft tissue swelling but no functional deficits.
Conclusion: Knowledge of general management principles and tailoring treatment based on properties of the solution, amount of solution extravasated, and the overall clinical picture will optimize outcomes of this common and potential sentinel event.
Keywords: Extravasation of intravenous; Herpes simplex virus; Human immunodeficiency virus
Introduction
An inadvertent extravasation of intravenous (IV medication occurs in 0.1% to 6/5% of hospital patients, but the actual incidence is likely higher due to inconsistent documentation and reporting [1]. At first these injuries may appear innocuous, but there is a risk of severe and progressive tissue dysfunction ranging from persistent tissue edema and fibrosis to delayed tissue necrosis [1]. These changes can be irreversible. A devastating potential sequela of an unmanaged intravenous infiltration or extravasation is the development of compartment syndrome and its subsequent morbidity. Rapid cessation of the offending agent and prompt management based on the identification of the infused solution is critical to prevent further morbidity.
Acyclovir is a highly potent inhibitor of Herpes Simplex Virus (HSV), type 1 and 2, and varicella zoster virus [2]. Its use has increased due to a greater number of immunocompromised patients, particularly Human Immunodeficiency Virus (HIV) and oncology patients treated with chemotherapy, that require prophylactic or treatment doses for HSV-related diseases [3]. The management of acyclovir extravasation is not clearly defined in the literature.
We present a case report of an IV extravasation of acyclovir and review the literature on management of IV extravasations.
Case Report
A 55-year-old African male police officer with HIV visiting from Kenya presented to the hospital with 2 weeks of acute worsening of baseline action tremors of bilateral upper extremities and an ataxic gait. He also endorsed 2 weeks of diplopia and photophobia. He was only alert and oriented to self on initial presentation. The patient was admitted to the neurology service for further evaluation with concern for underlying ischemia, infection, or an inflammatory process. The patient was started empirically on 700mg IV q8 hour acyclovir through an IV catheter placed on the dorsum of his right hand for possible herpes simplex encephalitis.
The orthopaedic hand service was consulted approximately 2 hours following extravasation of 150mL of IV acyclovir into the dorsum of the right hand on hospital day 2. Examination of the right hand demonstrated diffuse swelling that was firm but compressible. No apparent skin breakdown or necrosis was noted (Figure 1A & 1B).The patient denied pain with passive stretch of his digits. The patient was unable to fully flex his fingers secondary to the swelling but was able to be passively flexed to a complete grip. His two-point discrimination was 3mm in all digits and the thumb and capillary refill was less than 2 seconds in all digits with a 2+ radial pulse. The right upper extremity was placed in a skyhook (hand placed in a stockinette which was tied to an IV pole to keep the upper extremity elevated) and his hand compartments were evaluated every 2 hours for the first 24 hours. The hand was wrapped in webril and cold compresses applied 4 times a day for 15 minute intervals. The patient was consented for possible emergent surgery in case his symptoms acutely worsened.
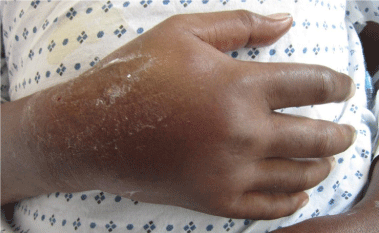
Figure 1a: AP view of the right hand after cessation of the acyclovir
extravasation.
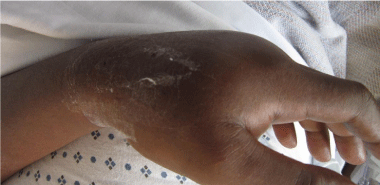
Figure 1b: Lateral view.
The patient underwent 48 hours of close observation with compartment checks, cold compresses, and strict elevation. The patient’s work up for ischemia, infection and inflammation was unrevealing. He had a negative brain MRI for an ischemic event and pan-infectious serology tests of serum and cerebrospinal fluid and rheumatologic antibodies were all negative. The patient was found to have a cerebrospinal fluid HIV viral load which was elevated in relation to his serum viral load which was consistent with viral escape. The patient’s tremor and gait improved with a change of his anti-retroviral medications and the addition of propranolol. Three days after IV extravasation the patient was discharged home. At discharge, the patient’s swelling had significantly decreased and his right hand motor function returned to full strength (Figure 2A, B & C). The patient returned to clinic for a follow up at 1 month after initial evaluation with mild residual swelling but was completely asymptomatic with full strength and grip (Figure 3A, B & C). He subsequently returned to Kenya.
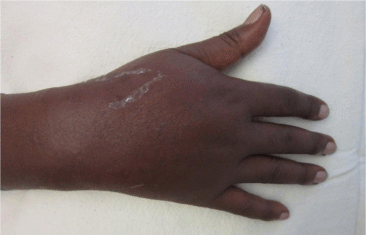
Figure 2a: AP view of the right hand 24 hours after acyclovir extravasation.
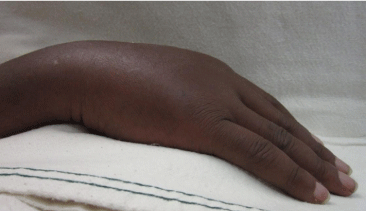
Figure 2b: Lateral view.
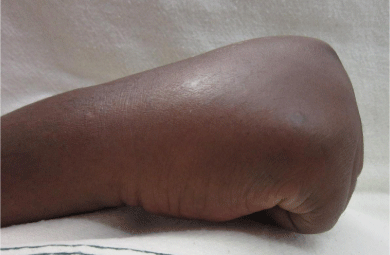
Figure 2c: Full composite grip.
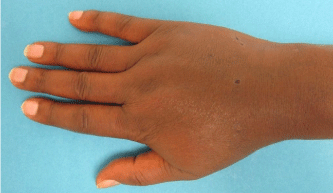
Figure 3a: AP view of the right hand 1 month after acyclovir extravasation.
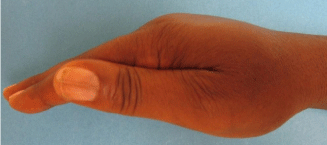
Figure 3b: Lateral view.
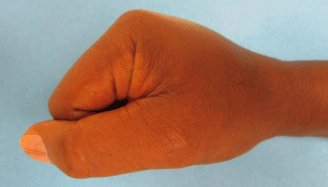
Figure 3c: Full composite grip.
vesicant solutions into surrounding tissues [4]. Infiltration is the spread of intravenous non-vesicant fluid into surrounding tissues [4]. These two terms are often used interchangeably but they are distinct entities in the literature. Vesicant medications are agents that have the potential to cause blistering, sloughing of the skin, and varying degrees of localized tissue damage when they inadvertently leak into the tissue [5]. Non-vesicant infusions, such as normal saline, do not impair or destroy the tissue when they infiltrate and have less potential for tissue necrosis [5]. Infiltrations and extravasations are essentially indistinguishable externally and follow similar treatment algorithms [4].
Extravasation of intravenous medications initially present as local swelling, erythema, and pain but can lead to serious injury including tissue necrosis and compartment syndrome and are considered a medical emergency and necessitate immediate treatment [1]. Common sites of extravasation injuries include the dorsum of the hand, forearm, antecubital fossa, and the dorsum of the foot because these are areas of the body where the skin and subcutaneous tissue are thinnest. The thin tissue makes these sites desirable targets for IV access but also the most susceptible to injury with extravasation [3].
The initial management of extravasation of intravenous medications is still controversial due to the paucity of evidence in the literature and the vast array of extravasated solutions [6]. Common recommendations include immediately stopping the infusion, drawing back on the intravenous catheter to aspirate any
remaining fluid, avoid pressure on the site, elevate the affected limb for 24 hours, and application of cold or warm compresses [6]. The specific medication should be identified to determine its vesicant or non-vesicant property and to check whether an antidote is available. If available, a decision should be made whether the amount of fluid and clinical appearance of the extremity warrants administering an antidote to prevent tissue necrosis. The patient should be counseled that immediate surgical intervention may be necessary [6].
The risk of extravasation injury is dependent on the amount of drug extravasated and the amount of subcutaneous tissue absorbing it [1]. The accumulated fluid exerts mechanical pressure which obstructs capillary blood flow and compromises oxygenation [1]. The amount of fluid that may lead to compartment syndrome depends on individual patient characteristics including elasticity of the subcutaneous tissue, fragility of vessels (e.g. children or elderly), as well as the osmotic properties of the medication. Clinicians must weigh patient specific factors with the location of the extravasation, physical exam findings, and the properties of the medication.
The mechanism of tissue damage by extravasated vesicant medications is contingent on its osmolarity, pH, and mechanism of action [7]. Studies suggest that hyperosmolality (>600 mOsm; normal range 285-310), extreme acidic or basic pH (<5 or >9), cytotoxic, and vasoconstrictive medications are associated with a higher risk of subsequent leakage and tissue damage [7]. A readily accessible institutional protocol for management of IV extravasation of high risk solutions is suggested.
There is a paucity of evidence-based interventions for vesicant extravasations. One method that has been employed includes administration of antidotes. The use of antidotes remains controversial but if appropriately administered may be helpful in vesicant solution extravasations [4]. Several commonly used antidotes for IV extravasations are Dimethyl Sulfoxide (DMSO) 99%, Hyaluronidase, and Phentolamine, (Table 1) [4,8].The preferred route of antidote injection has been through the infiltrated catheter, with the thought that the antidote will follow the path of the extravasated fluid [4]. Some vesicant medications have consensus recommendations for antidotes that can be administered (Table 2) [7]. An extensive practice guideline and summary recommendations is available through the Westmead Children’s Hospital policy, procedure and practice page [8].
Antidote
Mechanism of Action
Directions
Dimethyl sulfoxide 99% solution (DMSO)
- Enhances skin permeability to facilitate systemic absorption of drug.
- Apply topically to affected area
- Also has free radical scavenging properties.
- Allow to air dry
- 4 times a day for 7-14 days
Hyaluronidase
- Enzyme that temporarily decreases viscosity of hyaluronic acid, the ground substance or intracellular cement of tissues
- Must be applied within 60 minutes of extravasation
- Increased permeability facilitates rapid absorption
- Administered subcutanously or intradermally
Phentolamine
- Alpha-adrenoceptor blocker (vasodilator)
- Injected with fine hypodermic needed into area of extravasation
- Reverses vascoconstriction
Antidote
Mechanism of Action
Directions
Dimethyl sulfoxide 99% solution (DMSO)
- Enhances skin permeability to facilitate systemic absorption of drug.
- Apply topically to affected area
- Also has free radical scavenging properties.
- Allow to air dry
- 4 times a day for 7-14 days
Hyaluronidase
- Enzyme that temporarily decreases viscosity of hyaluronic acid, the ground substance or intracellular cement of tissues
- Must be applied within 60 minutes of extravasation
- Increased permeability facilitates rapid absorption
- Administered subcutanously or intradermally
Phentolamine
- Alpha-adrenoceptor blocker (vasodilator)
- Injected with fine hypodermic needed into area of extravasation
- Reverses vascoconstriction
*Modified with permission from IV Extravasation Management of Children’s Hospital of Westermead25
Table 1: Antidotes with mechanism of action and directions.
Medication
Antidote
Actinomycin D
DMSO*
Amsacrine
DMSO*
Epirubicin
DMSO*
Contrast
5-20mL-Hyaluronidase; if >20mL surgical drainage within 6 hours should be considered
Phenytoin
Hyaluronidase
Total Parental Nutrition
Hyaluronidase
Chemotherapeutic agents (Vinblastine, Vincristine, Vinorelbine)
Hyaluronidase
Vasopressors (Adrenaline, Dobutamine, Dopamine, Metaraminol, Noradrenaline)
Phentolamine
*DMSO: Dimethyl Sulfoxide 99% Solution
Modified with permission from IV Extravasation management of children’s hospital of westermead25
Table 2: Medications with consensus recommended antidotes.
Several other interventions have been attempted for extravasation of intravenous medications including infiltration with an agent to dilute the extravasated agent or manual extraction [7]. One of the more common interventions is the use of cold or warm compresses. Cold compresses are thought to prevent spread of extravasated solution by local vasoconstriction and allows local vascular and lymphatic systems to disperse the agent. Warm compresses are thought to assist with dispersion and decreasing local drug concentrations by local vasodilation [9]. For isotonic agents, such as acyclovir, the treatment strategy should be cold compresses to localize agents while hypertonic and/or vasoconstrictive solutions (e.g. epinephrine) are treated with warm compresses to prevent local ischemia [9]. For extravasations with known vesicant medications, a pressure dressing on the site should be avoided because it can disperse the medication and increase the tissue contact area [4].
Acyclovir is an acyclic purine nucleoside analog, which is a potent inhibitor of DNA replication of herpes simplex virus [2,7]. It is effective against herpes simplex encephalitis and other herpes related diseases [2,3,7]. It is considered a vesicant and has a pH of 11 and an osmolality of 278 mOsm/kg [7]. The literature on acyclovir extravasation is sparse. There are 2 documented case reports in the literature regarding extravasation of IV acyclovir [9,10]. De Souza et al reported a case of a 51 year-old insulin dependent diabetic man who presented with cellulitis and lymphatic edema over the prior site of extravasation 3 months after the incident [9]. Sarika et al reported the case of a 14-year old girl who was undergoing treatment with IV acyclovir for acute lymphoblastic leukemia. The infusion extravasated and a local bullous cutaneous eruption occurred approximately 10 cm distal to the site of venipuncture. It was unclear if the eruption was due to the local extravasation or a more systemic immunoallergic mechanism [10]. The bullous eruption subsided in 8 hours and completely resolved with a residual scar in 24 hours [10].
Acyclovir is known to be selective for HSV and relatively nontoxic to normal cells and has an osmolality (278 mOsm/kg) similar to human plasma (285-295 mOsm/kg)2. The tissue toxicity is thought to occur from the alkalinity of the solution resulting in a local chemical inflammation [9]. In previous case reports, treatment was nonoperative with either compression garments and physical therapy [9] or observation [10]. In our case, the patient was managed with arm elevation in a skyhook, frequent neurovascular evaluation, and cold compresses with the plan to proceed with surgical intervention if his condition acutely worsened. No tissue breakdown or subsequent infection was observed. His follow-up was limited to one clinic visit because he was visiting from Kenya and further follow-up could not be established. Nonoperative management of extravasation of intravenous acyclovir is a reasonable approach if tissue perfusion is not acutely compromised. Patients should be educated that there may be potential late complications such as persistent lymphatic edema, cellulitis, and tissue fibrosis and outpatient follow-up is recommended.
In summary, infiltrations and extravasations intravenous medications have a potential for devastating morbidity. These potentially sentinel events require prompt recognition and management by the healthcare team. The patient should be immediately evaluated for acute tissue compromise and compartment syndrome. Clinical decisions for nonoperative treatment, antidote infusions, or operative intervention should be based on the patient’s physical examination findings and the properties and amount of the extravasated solution. With mild symptoms, nonoperative modalities can be effective which includes elevation of the affected extremity in a skyhook, cold or warm compresses depending on properties of the medication, and close monitoring with a low threshold for operative intervention if there are worsening acute changes. Pressure dressings with vesicant extravasations should be avoided to prevent further spread. It is important to provide anticipatory guidance about the possible need for emergent surgical intervention and to have close follow-up after discharge for evaluation of late complications.
References
- Kumar RJ, Pegg SP, Kimble RM. Management of extravasation injuries. ANZ J Surg. 2001; 71: 285-289.
- Gnann JW JR. Barton NH, Whitely RJ. “Acyclovir: mechanism of action, pharmacokinetics, safety and clinical applications”. Pharmacotherapy. 1983; 3: 275-283.
- Tan IL, McArthur JC, Venkatesan A, Nath A. Atypical manifestations and poor outcome of herpes simplex encephalitis in the immunocompromised. Neurology. 2012; 79: 2125-2132.
- Hadaway L. Infiltration and extravasation. Am J Nurs. 2007; 107: 64-72.
- Schulmeister L. Extravasation management: clinical update. Semin Oncol Nurs. 2011; 27: 82-90.
- Wickham R, Engelking C, Sauerland C, Corbi D. Vesicant extravasation part II: Evidence-based management and continuing controversies. Oncol nurs forum. 2006; 33: 1143-1150.
- Le A, Patel S. Extravasation of Noncytotoxic Drugs: A Review of the Literature. Ann pharmacother. 2014; 48: 870-886.
- Westmead Children’s Hospital Policy, procedure and practice: IV Extravasation Management. 2012.
- De Souza BA, Shibu M. Painless acyclovir extravasation injury in a diabetic. Br J Plast Surg. 2002; 55: 264.
- Sarica A. Bullous Cutaneous Eruption due to Extravasation of Acyclovir in an Adolescent with Acute Lymphoblastic Leukemia. Turk J Haematol. 2012; 29: 109-110.