
Case Report
J Pediatri Endocrinol. 2024; 9(1): 1064.
Resistance To Thyroid Hormone Receptor Alpha Due to Heterozygous Pathogenic Variant in THRA Gene: A Case Report from India
Abhishek Kulkarni, MD, PDCC1*; Devika Desai, MD2; Joewin Monteiro, MD3; Poorvi Agrawal, MD4
1Senior Consultant, Department Coordinator & Postdoctoral Fellowship Program Director, Department of Pediatric & Adolescent Endocrinology, SRCC Children’s Hospital, India
2Clinical Associate, Department of Pediatric & Adolescent Endocrinology, SRCC Children’s Hospital, India
3Clinical & Research Fellow, Department of Pediatric & Adolescent Endocrinology, SRCC Children’s Hospital, India
4Assistant Professor, Department of Pediatrics, Topiwala National Medical College & B.Y.L Nair Charitable Hospital, India
*Corresponding author: Abhishek Kulkarni, MD, PDCC Visiting Fellowship, RCPCH, London, Senior Consultant, Department Coordinator & Postdoctoral Fellowship Program Director, Department of Pediatric & Adolescent Endocrinology, SRCC Children’s Hospital, Mumbai, India. Email: cpedndc@gmail.com
Received: April 08, 2024 Accepted: May 02, 2024 Published: May 09, 2024
Abstract
Background: Thyroid Hormone Receptor Alpha (THRA) gene mutation is a thyroid hormone resistance syndrome characterized by non-responsiveness of target tissues to the active form of TH (T3).
Clinical Description: We describe a heterozygous missense variant in exon 8 of THRA gene detected in a 2-month-old female with clinical phenotype of hypothyroidism, low free thyroxine, elevated free triiodothyronine & normal levels of thyroid stimulating hormone.
Management & Outcome: Clinical improvement in linear growth, motor development domain, hypotonia & constipation were noted with levothyroxine therapy but insufficiencies in cognitive and fine motor skills may remain.
Conclusion: THRA gene mutation should be considered in patients with features of clinical hypothyroidism and elevated free T3, decreased/ normal free thyroxine & normal thyroid-stimulating hormone levels.
Keywords: THRA gene, Resistance to thyroid hormone receptor a, hypothyroidism
Thyroxine hormone acts at peripheral tissues via specific nuclear receptors which is a complex to two subparts part A and B, which are transcribed by the TH receptor a (THRA) gene & TH Receptor β (THRB) gene respectively. THRA gene, located on chromosome 17q11.2, transcribes two isoforms of THRA receptors - TRa1 & TRa2, which are the nuclear receptors for target tissues namely, bones, skeletal and non-skeletal muscles, gastrointestinal system and brain [1]. Unresponsiveness to the hormones can lead to a clinical spectrum of disorder named as Resistance to Thyroid Hormone (RTH) & two subgroups of inheritable RTH have been described; RTH β and RTH a [2]. The first case of THRA mutation was published in 2012 in a young girl with growth retardation [1].
Clinical Case Description
A 2-month-old female born full term was referred to us with a presumptive diagnosis of central hypothyroidism (FT4 < -2SD, normal TSH levels) & normal serum cortisol and IGFBP3 levels. Enquiry revealed absence of social smile attainment. Family history was non-contributory except for presence of 3rd degree consanguinity among the parents. On examination her vitals were normal and anthropometric assessment suggested length & occipitofrontal circumference at the 1st (52 cm) & 50th centile (38 cm) for age on the WHO growth chart respectively. She had pallor, broad face and nasal bridge, macroglossia (Figure 1), umbilical hernia and axial hypotonia and there was history of infrequent passage of stools.
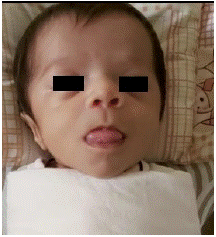
Figure 1: The clinical picture describing macroglossia, flat face and flat nasal bridge.
Further evaluation suggested presence of normocytic normochromic anaemia, higher FT3 (> + 2SD) & low rT3 (< -2SD), low FT4 (< -2SD), normal TSH and elevated creatinine kinase CK levels (Table 1). Based on the clinical profile and the abnormal biochemical profile, thyroid hormone resistance with mutation in Thyroid Hormone Resistance alfa (THRA) was suspected.
Reference range
Pre-Thyroxine
1 month on treatment
3 months on treatment
6 months on treatment
Thyroxine T4 (ng/dl)
(Free)1.09-1.35
0.72
1.02
1.27
1.41
Triiodothyronine T3
(Free) (pg/ml)3.65-4.5
5.97
5.83
5.67
5.75
Reverse T3 (ng/dl)
10-24
11
-
-
32
Thyroid-stimulating hormone
(mIU/l)1.9-4.5
3.11
1.12
0.47
0.34
Creatinine Kinase (CK)(U/l)
26-192
389
259
200
191
Table 1: Serial Thyroid function tests pre-treatment and post treatment.
Management & Outcome
L-Thyroxine supplementation in a dose of 25 mcg/day was initiated & periodic titration of the thyroxine dose, initially at 4 weeks & then at 8-12 weeks interval was done to ensure that the optimal FT4 levels (+ 1SD to + 2SD) were reached (Table 1). Clinical exome analysis was sent which yielded a heterozygous missense variant c.871G>A;p.Gly291Ser in exon 8 of THRA gene (transcript ID NM_003250.6) which was confirmed by sanger sequencing (Figure 2). The variant has been reported earlier as known pathogenic in ClinVar database [3]. Parental analysis for the variant turned out to be negative, making our variant a de-novo one.
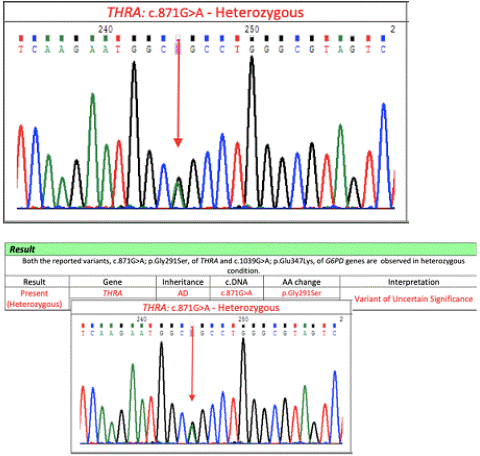
Figure 2: Chromatogram of sanger analysis performed for the detected variant.
Catch up in length to just above the 3rd centile (length 62 cm), improvement in axial tone albeit not total, improvement in the stool frequency, normalisation of FT4 & rT3, persistently elevated FT3, suppressed TSH and decrease in CK levels have been noted over the 6 months duration of follow up subsequently on thyroxine, though the results of evaluations using Bayley Scale of Infant and Toddler Development (BSID) 4 indicate neurodevelopmental delay.
Discussion with Review of Literature
The spectrum of Resistance to Thyroid Hormones (RTH) characterized by non-responsiveness of peripheral tissues to T3, comprises of THRA and THRB mutations, of which the latter is widely studied whereas the first patient with THRA mutation was reported in 2012 [1]. To the best of our knowledge, this is the first report of such case from Indian subcontinent. The clinical as well as biochemical profile of the two mutations are quite different from another, albeit the spectrum of severity ranging from mild to severe. Patients with THRB present with goitre, resting tachycardia, short stature, Attention Deficit Disorder (ADD) and depict increased free T3, free T4 and normal/ elevated TSH [4]. The plausible explanation being differential expression of THRA and THRB in tissues, where tissues expressing a malfunctioning receptor will manifest an organ specific clinical finding. THRA (TRa1, TRa2 isoforms) is expressed in the muscles (skeletal and non-skeletal), gastrointestinal system, bones and neurological system (but not in pituitary gland or hypothalamus). And thus, even though the peripheral tissues are in a functional hypothyroid state, THRA variants do not depict the expected TSH rise [5].
Since 2012, when first clinical case due to THRA mutation was described, almost 25 different pathogenic variants, namely frameshift, missense and premature stop codons have been identified [5]. TRa receptor, when binds to T3 undergoes a structural change and induces transcription of target genes in peripheral tissues. When variation in the gene occurs, the mutant THRA receptor fails to bind to T3 and exerts a dominant negative effect in heterozygous state [2].
The age at diagnosis varies widely in children in a cohort of 27 patients reported by Erbas et al, youngest being 9 months and oldest being 12 years [6]. Our patient was diagnosed at a very early age of 2 months and follow up of this patient on treatment with thyroxine could provide worthwhile insights on the effect of treatment on clinical manifestations. Myriad of clinical manifestations such as delayed milestones, cognitive delay, growth failure, macroglossia, changes in bone ossification, and constipation have to date been reported in THRA patients, similar to cases of long standing untreated hypothyroid patients [5]. Growth retardation has been consistently reported in untreated cases, which is caused by deficit in appendicular lower limb length with a normal trunk height [1]. However, a spectrum of clinical presentation, with normal stature also being reported in a cohort of patients, irrespective of their treatment status or age at diagnosis [6]. A plausible genotype phenotype correlation has been suggested by the authors where all the patients having normal stature harboured missense variants of the gene [6]. Our patient showed catch up in length from just at 1st centile at diagnosis to above 3rd centile in the duration of 6 months follow up on treatment with L-thyroxine, though further follow up is required to arrive at a definitive inference. Significant improvement in height (from -2.4 SDS to -1.8 SDS) in a 4 year old patient with missense variant has been reported by Korkmaz et al during his 2.5 year period of follow up on treatment [8]. Macrocephaly is one of the characteristic features of THRA mutation, though our patient’s head circumference was around the 50th centile for age [2,5-7]. The report of first identified patient in 2012 uses the term relative macrocephaly with no mention of exact SDS [1]. A report of a sporadic case published from China reports normal head circumference, though exact mechanism is yet to be determined [9]. Reports of some cases support the association of phenotype and genotype correlation, with intellectual disability and development delay being more associated in cases with nonsense mutations [7]. Normocytic normochromic anaemia as in our case is a common characteristic in THRA patients [8,10]. THRA gene regulate the expression of erythroid cell series in the bone marrow, accounting for normocytic anaemia in the THRA mutants. Increased Creatine Kinase levels similar to those observed in long standing untreated hypothyroidism is also known with THRA variants. The expression of THRA affecting the colonic function accounts for the clinical finding of constipation [10].
Thus, although a constellation of clinical findings that is characteristic to THRA variants is described in aforementioned reports, there is high variability in presentation. More cases need to be diagnosed and studies along with further characterization of mutations to understand the variability of phenotype and establish a genotype-phenotype correlation.
Since the pathogenesis of this disorder lies at nuclear receptor level, the ideal therapeutic agent would be the one that can mimic TRa receptor functions. However, treatment with levo-thyroxine has been attempted to raise the circulating levels of thyroxine hormone. The previous reports do describe clinical improvement in motor development domain, nerve conduction, hypotonia, constipation, decrease in CK levels and increase in IGF-1 levels when treated with levothyroxine therapy in THRA cases but insufficiencies in cognitive and fine motor skills may remain [2,6]. Anaemia remains refractory to the treatment and variable reports of effect on growth has been reported [6]. While limited benefit has been reported by most studies [1,2,5], improvement in linear growth has also been reported [6].
Conclusion
THRA mutations may be more common than currently thought. THRA gene mutation should therefore be considered in patients with features of clinical hypothyroidism and elevated free T3, decreased/ normal free thyroxine & normal thyroid-stimulating hormone levels. The diagnosis can easily be missed when only TSH and free T4 are analysed, since these may be normal. When THRA mutations are suspected, serum T3 and rT3 should be measured as well.
Lessons Learnt
Using free T3 assays in conventional neonatal thyroid screening programs especially in patients where free T4 values are relatively lower could help differentiate central hypothyroidism from rarer forms of thyroid hormone insensitivity. Clinical & biochemical suspicion of thyroid hormone insensitivity must be validated by pertinent genetic testing. Thyroxine treatment alleviates hormone resistance in patients with mutations in the THRA gene albeit partially possibly ameliorating the phenotype.
Author Statements
Conflicts of Interest
There are no conflicts of interest.
References
- Bochukova E, Schoenmakers N, Agostini M, Schoenmakers E, Rajanayagam O, Keogh JM, et al. A mutation in the thyroid hormone receptor alpha gene. N Engl J Med. 2012; 366: 243-9.
- Van Mullem AA, Visser TJ, Peeters RP. Clinical consequences of mutations in thyroid hormone receptor-a1. European thyroid journal. 2014; 3: 17-24
- https://www.ncbi.nlm.nih.gov/clinvar/variation/1186100/.
- Singh BK, Yen PM. A clinician’s guide to understanding resistance to thyroid hormone due to receptor mutations in the TRa and TRβ isoforms. Clin Diabetes Endocrinol. 2017; 3: 8.
- Van Gucht AL, Meima ME, Zwaveling-Soonawala N, Visser WE, Fliers E, Wennink JM, et al. Resistance to thyroid hormone alpha in an 18-month-old girl: clinical, therapeutic, and molecular characteristics. Thyroid. 2016; 26: 338-46.
- Erbas IM, Demir K. The Clinical Spectrum of Resistance to Thyroid Hormone Alpha in Children and Adults. Journal of Clinical Research in Pediatric Endocrinology. 2021; 13: 1.
- Tylki-Szymanska A, Acuna-Hidalgo R, Krajewska-Walasek M, Lecka-Ambroziak A, Steehouwer M, Gilissen C, et al. Thyroid hormone resistance syndrome due to mutations in the thyroid hormone receptor a gene (THRA). Journal of medical genetics. 2015; 52: 312-6.
- Korkmaz O, Ozen S, Ozdemir TR, Goksen D, Darcan S. A novel thyroid hormone receptor alpha gene mutation, clinic characteristics, and follow-up findings in a patient with thyroid hormone resistance. Hormones. 2019; 18: 223-7.
- Sun H, Wu H, Xie R, Wang F, Chen T, Chen X, et al. New Case of Thyroid Hormone Resistance a Caused by a Mutation of THRA/TR a 1. Journal of the Endocrine Society. 2019; 3: 665-9.
- Demir K, van Gucht AL, Büyükinan M, Çatli G, Ayhan Y, Bas VN, et al. Diverse genotypes and phenotypes of three novel thyroid hormone receptor-a mutations. The Journal of Clinical Endocrinology & Metabolism. 2016; 101: 2945-54.