Abstract
Siderophores are low molecular weight (200-2000D) metal-chelating agents that can form complexes with iron and other essential elements. Siderophores are classified into three main classes: hydroxamate, phenolate and catecholate, and carboxylate. Natural and artificial siderophores have been widely used in a wide variety of environmental and medical applications. Siderophores have been utilized as antimicrobial agents or delivery carriers for antibiotics in the treatment of gram-negative resistant bacterial infections. They are also used as anticancer agents, antimalarial agents, and biosensors.
Keywords: Anti-microbial; Anti-cancer; Anti-malarial; Siderophores; Vaccines
Introduction
Iron plays a crucial role in biochemical processes of all microorganisms, plants, and animals. Although, iron is abundant in the earth’s crust, it sometimes has limited bioavailability in aerobic environments [1]. As a result, the living microorganisms and plants have developed strategies to absorb iron from the surrounding environment, such as soil and marine water. One of those strategies is biosynthesis of siderophores [2]. Siderophores are metal-chelating agents with masses ranging between 200 and 2000D that form complexes with iron and other essential elements, such as Mn, Co and Ni from the environment and render them bioavailable for microbial cells [2,3].
Siderophores are classified into three main classes: hydroxamates, catecholates and phenolates, and carboxylates, based on their chemical structures [4,5]. Hydroxamate siderophores have N-hydroxyamide (hydroxamate) functional group (-C(=O)-N(OH)-R), such as shizokinen, rhizobactin, as shown in Figure 1. Agrobactin, aminochelin and protochelin are examples of catecholate-based siderophores that are shown in Figure 2 [6].
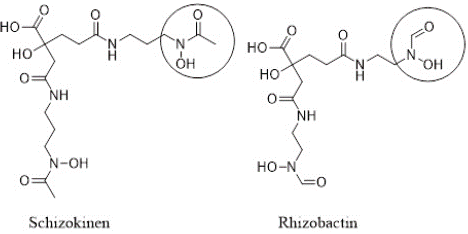
Figure 1: Examples of hydroxamate siderophores with encircled hydroxamate functional group.
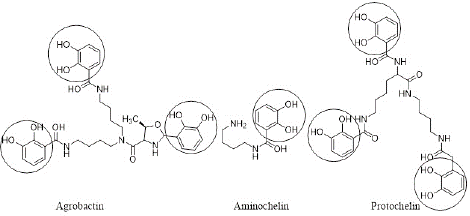
Figure 2: Examples of catecholate siderophores with encircled catecholate functional group.
Rhizoferrin is an example of carboxylate-type siderophore, which is produced by the fungus Rhizopus microspores. The structure of rhizoferrin is shown in Figure 3 [7,8]. Some siderophores are of mixed type, such as rhizobactin that has both hydroxamate and carboxylate functional groups (Figure 1).
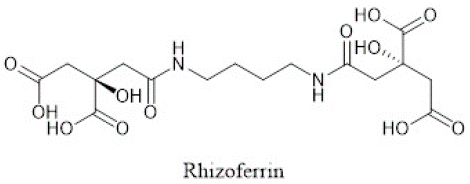
Figure 3: Structure of rhizoferrin, an example of carboxylate-type siderophores.
Microbial and plant siderophores, in addition to structurally related synthesized siderophores have been widely employed in many applications, including agriculture and medicine. In this review, we will discuss the medical applications of siderophores, such as anti-microbial therapy, chemotherapy, biosensors and vaccines [9].
Medical Applications of Siderophores
Siderophores as Antimicrobial Agents
Multi-drug bacterial resistance is one of the most challenging health issues that results in an urgent need for development of new antibiotics. Siderophores have attracted much attention as an alternative to overcome bacterial resistance. For example, Cefiderocol is a cephalosporin antibiotic-catecholate siderophore conjugate that displays a significant antimicrobial activity against cephalosporin-resistant Enterobacteriaceae (Figure 4) [10].
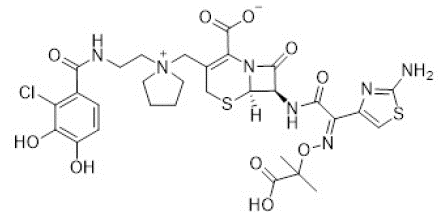
Figure 4: Structure of cefiderocol.
One of the main factors that contributes to antibiotic resistance in gram-negative bacteria is inability of antibiotics to diffuse through the outer bacterial cell membrane [11]. Interestingly, siderophores have been utilized as carriers to deliver antibiotics through the outer bacterial membrane via iron-uptake pathways [12,13]. This strategy to overcome bacterial resistance is called “Trojan Horse” strategy [11]. For example, Peukert et al. reported the conjugation of daptomycin antibiotic to tetrapodal 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic amide (DOTAM) siderophore (Figure 5). It was observed that daptomycin-DOTAM conjugate has an enhanced uptake and significant antimicrobial effect against multidrug-resistant Acinetobacter baumannii [14].
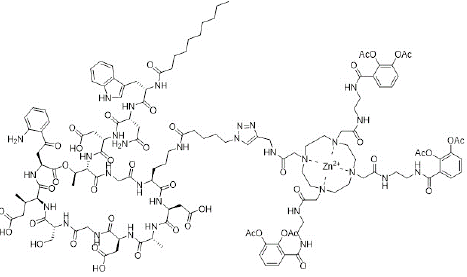
Figure 5: Structure of daptomycin-DOTAM conjugate.
Siderophores as Anticancer Agents
Iron plays a crucial role in the growth of tumor tissues. Consequently, the ability of siderophores to scavenge iron from cellular environment has been harnessed to inhibit the proliferation of cancer cells, and tumor growth [15]. It was reported that fungal hydroxamate siderophores produced by Aspergillus spp. exhibit a significant does-dependent inhibition effect on the growth and proliferation of hepatocellular carcinoma cell line HepG2 [16]. Enterobactin, a bacterial catecholate siderophore shown in Figure 6 was also reported to exhibit a significant cytotoxic effect on monocyte-related tumor cell lines [17].
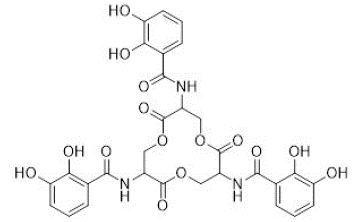
Figure 6: Structure of enterobactin.
Siderophores as Antimalarial Agents and Vaccines
Malaria is caused by Plasmodium parasites that are transmitted by mosquito bites. Iron is essential for several parasite’s metabolic processes, such as DNA synthesis that is catalyzed by iron-dependent ribonucleotide reductase [18]. Interestingly, siderophores that deprive the parasite from iron can be utilized in treatment of malaria. For example, desferrioxamine B (DFO-B) or Desferal®, a natural siderophore produced by Streptomyces sp. was reported to inhibit several iron-dependent metabolic processes of P. falciparum [19]. Also, artificial siderophores have been developed for treatment of malaria, such as DFO-ozonide conjugates [20].
Siderophores can also trigger production of antibodies when combined with carrier proteins. For example, it was demonstrated that enterobactin conjugated to cholera toxin subunit B evolves an immune response, leading to reduction in the severity of colitis caused by E. coli in mouse models with Inflammatory Bowel Diseases (IBD) [21].
Siderophores as Biosensors
Siderophores have high affinities to form complexes with a wide range of metal ions, such as Fe(III), Cu(II) and Mn(II). They can be utilized to detect metals in biological media. In addition, siderophores can be employed in medical diagnosis and detection of pathogens as a result of their high affinity for bacterial cell membrane receptors [22].
Conclusion
Siderophores have been a multidisciplinary endeavor that attracts attention of researchers to mitigate some global concerns and threatening health issues, such as cancer and antibiotic resistance. Siderophores have been an intriguing approach with an outstanding potential activity in the medical field. Nevertheless, other prospects of medical use of siderophores should be considered. For example, the iron-chelating efficiency of siderophores can be harnessed in treatment of iron overload diseases, such as hemochromatosis, in which excess iron is absorbed from food and stored in body organs, such as liver and heart, causing liver diseases and heart problems. The lead-absorption efficiency of siderophores can also be evaluated to overcome lead poisoning in children. Furthermore, the ability of siderophores to scavenge iron that affects the lipid degradation process can be considered for treatment of arteriosclerosis. In a response to high demand for siderophores in the medical field, researchers have been developing new techniques for extraction of natural siderophores from plants, fungi, and marine water, in addition to synthesis of structurally related artificial siderophores for more effective incorporation of siderophores into medical practice.
Author Statements
Conflicts of Interest: The authors declare no conflict of interest.
References
- Hider RC, Kong X. Chemistry and biology of siderophores. Natural product reports. 2010; 27: 637-657.
- Bellenger JP, Wichard T, Kustka AB, Kraepiel AML. Uptake of molybdenum and vanadium by a nitrogen-fixing soil bacterium using siderophores. Nature Geoscience. 2008; 1: 243-246.
- Braud A, Jézéquel K, Bazot S, Lebeau T. Enhanced phytoextraction of an agricultural Cr-and Pb-contaminated soil by bioaugmentation with siderophore-producing bacteria. Chemosphere. 2009; 74: 280-286.
- Ustiatik R, Nuraini Y, Handayanto E. Siderophore production of the Hg-resistant endophytic bacteria isolated from local grass in the Hg-contaminated soil. Journal of Ecological Engineering. 2021; 22: 129-138.
- Butler A, Theisen RM. Iron (III)–siderophore coordination chemistry: Reactivity of marine siderophores. Coordination chemistry reviews. 2010; 254: 288-296.
- Timofeeva AM, Galyamova MR, Sedykh SE. Bacterial siderophores: Classification, biosynthesis, perspectives of use in agriculture. Plants. 2022; 11: 3065.
- Carrano CJ, Drechsel H, Kaiser D, Jung G, Matzanke B, Winkelmann G, et al. Coordination chemistry of the carboxylate type siderophore rhizoferrin: the iron (III) complex and its metal analogs. Inorganic chemistry. 1996; 35: 6429-6436.
- Drechsel H, Metzger J, Freund S, Jung G, Boelaert JR, Winkelmann G. Rhizoferrin—a novel siderophore from the fungus Rhizopus microsporus var. rhizopodiformis. Biology of metals. 1991; 4: 238-243.
- Passari AK, Ruiz-Villafán B, Cruz-Bautista R, Díaz-Domínguez V, Rodríguez-Sanoja R, Sanchez S. Opportunities and challenges of microbial siderophores in the medical field. Applied Microbiology and Biotechnology. 2023; 107: 6751-6759.
- Syed YY. Correction to: Cefiderocol: A Review in Serious Gram-Negative Bacterial Infections. Drugs. 2021; 81: 1559-1571.
- Fan D, Fang Q. Siderophores for medical applications: imaging, sensors, and therapeutics. International Journal of Pharmaceutics. 2021; 597: 120306.
- Carver PL. Metals in medicine: The therapeutic use of metal ions in the clinic. Essential Metals in Medicine: Therapeutic Use and Toxicity of Metal Ions in the Clinic. 2019; 19: 2-11.
- Negash KH, Norris JK, Hodgkinson JT. Siderophore–antibiotic conjugate design: New drugs for bad bugs?. Molecules. 2019; 24: 3314.
- Peukert C, Rox K, Karge B, Hotop SK, Bronstrup M. Synthesis and Characterization of DOTAM-Based Sideromycins for Bacterial Imaging and Antimicrobial Therapy. ACS Infectious Diseases. 2023; 9: 330-341.
- Gokarn K, Sarangdhar V, Pal RB. Effect of microbial siderophores on mammalian non-malignant and malignant cell lines. BMC complementary and alternative medicine. 2017; 17: 145.
- Khan A, Singh P, Chaudhary A, Haque R, Singh P, Mishra AK, et al. Induction of iron stress in hepatocellular carcinoma cell lines by siderophore of Aspergillus nidulans towards promising anticancer effect. Biological Trace Element Research. 2022; 200: 3594-3607.
- Saha P, San Yeoh B, Xiao X, Golonka RM, Kumarasamy S, Vijay-Kumar M. Enterobactin, an iron chelating bacterial siderophore, arrests cancer cell proliferation. Biochemical pharmacology. 2019; 168: 71-81.
- Munro JB, Silva JC. Ribonucleotide reductase as a target to control apicomplexan diseases. Current issues in molecular biology. 2012; 14: 9-26.
- Rehan M, Barakat H, Almami IS, Qureshi KA, Alsohim AS. Production and Potential Genetic Pathways of Three Different Siderophore Types in Streptomyces tricolor Strain HM10. Fermentation. 2022; 8: 346.
- Tiwari R, Checkley L, Ferdig MT, Vennerstrom JL, Miller MJ. Synthesis and antimalarial activity of amide and ester conjugates of siderophores and ozonides. Biometals. 2023; 36: 315-320.
- Gerner RR, Hossain S, Sargun A, Siada K, Norton GJ, Zheng T, et al. Siderophore immunization restricted colonization of adherent-invasive Escherichia coli and ameliorated experimental colitis. Mbio. 2022; 13: e02184-22.
- Singh P, Khan A, Kumar R, Kumar R, Singh VK, Srivastava A. Recent developments in siderotyping: procedure and application. World Journal of Microbiology and Biotechnology. 2020; 36: 178.
