
Review Article
Austin J Pharmacol Ther. 2022; 10(2).1163.
Progress of Hollow Materials in Diagnosis of Covid-19
Hussain A1,2, Shabbir S1,2 and Faizan M1,2*
1Beijing Key Laboratory of Ionic Liquids Clean Process, State Key Laboratory of Multiphase Complex System, Institute of Process Engineering Chinese Academy of Sciences, PR China
2University of Chinese Academy of Sciences, PR China
*Corresponding author: Muhammad Faizan, Beijing Key Laboratory of Ionic Liquids Clean Process, State Key Laboratory of Multiphase Complex System, Institute of Process Engineering Chinese Academy of Sciences, PR China
Received: August 08, 2022; Accepted: September 09, 2022; Published: September 16, 2022
Abstract
Since the outbreak of COVID-19 in Wuhan, China, it has dramatically changed the global geopolitics, economics, and even society standard norms. The present world scenario is changed regarding business, traveling, and education. Rapid global dissemination and the high mortality rate of coronaviruses are the greatest challenges for drug developers. It will be moving forward toward the identification and treatment of emerging coronaviruses with the aid of nanotechnology. The COVID-19 pandemic raised the question of researchers’ capability to manage this dilemma in a short period. In the present review, we described how hallow material could be developed as a pro-drug that shows an excellent therapeutic effect. Hollow nanoparticles that exploration of antiviral or diagnostic agents against emerging coronaviruses. Hollow nanomaterials in vaccine development are essential because hollow nanocomposites are suitable for mimicking viral structures and antigen delivery. A biosensor that generates a signal from a transducer for comparing and analyzing biological conjugates such as cell receptors, antibodies, RNA, DNA, and nucleic acids. Different biosensors, such as graphene-based biosensors, nanoplasmonic sensor chips, nanomaterial biosensors, electrochemical biosensors, dual modality biosensors, and optical biosensors, have several advantages, characteristics, and a wide range of applications, most remarkably in medical treatment and are used for monitoring and diagnosis. This review focuses on modern experimental studies to identify intelligent and innovative bio/nanomaterials and matrices for developing targeted and controlled drug release systems, nanosensors and nanovaccines to combat pathogenic viruses.
Keywords: COVID-19; Nanoparticles; Hollow materials; Diagnostic agents; Nano/bio-sensors; Pathogenic viruses
Introduction
Since the 1918 influenza pandemic, coronavirus has emerged as a severe threat to human life. To solve the current pandemic situation, the planet needs both viral diagnostics and an antiviral vaccine [1]. However, the ongoing research and development period required for them is very lengthy, and they must pass multiple stages before being approved for human use. It is critical to develop a comprehensive and long-term strategy for combating infections, especially respiratory viruses. Material science and nanotechnology have revolutionized the biomedical industry, bringing numerous benefits to diagnostic and therapeutic applications [2]. Hollow nanomaterials play an excellent role in a vital role in diagnostics and drug delivery. Hollow nanomaterial-based delivery systems have recently attracted researchers to address drug treatment challenges and viral infection treatment. Alternatively, hollow nanomaterials have a charge with a small surface area that can be transformed into effective antiviral agents [3]. This is the most effective method for scientific investigation and biomedical use. Using hollow nanosystems exacerbates the low bioavailability and dose limit of drugs. Recently, hollow nanomaterials have been used with different mechanisms for promising antiviral activities. Hollow nanomaterial properties, such as surface charges [4], surface area, and size, make them an excellent tool for viral diagnostics and treatment [5].
The length of hollow nanomaterials can be adjusted to the delivery site, maximizing the drug's efficacy. Large molecules and drug payloads could be accommodated on the surface area. The biological system forges a strong link with nanoscience by incorporating hollow nanomaterials with nanomedicine to detect and treat diseases. Furthermore, the role of hollow nanomaterials is essential in drug delivery management [6,7]. Researchers have already faced several pandemics and viral outbreaks in the past; for example, the swine flu pandemic in 2009 infected many more people than the Ebola virus Zika outbreaks [8]. The current antiviral treatment is not as effective due to some hurdles. Hollow nanomaterials have shown great antiviral activity [9]. The drug and food organizations and other administrations approved so many nanobased platforms for an antiviral vaccine. For instance, an intramuscular vaccine such as epaxial is used for hepatitis A virus [10]. Such vaccines have liposome vehicles of 150 nm size. Epaxial possesses intrinsic adjuvant properties and helps reduce toxicity. Furthermore, a virosome vaccine has been developing as Influvac Plus, another approved virosome vaccine that is used to prevent certain types of influenza. Nanomaterial-based techniques such as polysaccharide particles, liposomes, dendrimers, and cationic nanoemulsions have been utilized to improve the distribution and stability of mRNA-based vaccines [11]. Therefore, the development of such vaccines with the administration of nanotechnology for COVID-19 is highly favorable. Hollow nanomaterials in vaccine development are essential because hollow nanocomposites are suitable for mimicking viral structures and antigen delivery [12]. In comparison to traditional vaccine design, this approach has many advantages. The ongoing threat of outbreaks of acute respiratory infections, for example, Middle East respiratory syndrome coronavirus (MERS-CoV), is to develop a potent and safe vaccine strategy based on novel vaccine technology that is useful in preventive measures. A hollow nanoscale vaccine was developed for the delivery of STING agonists and viral antigen subunits in a virus-type configuration [13]. Herein, STING agonists show multiple advantages, such as local immune potentiation and pH-responsive release profiles, after being encapsulated into hollow nanomaterials. Following antigen conjugation, the nanoparticles resemble native virions morphologically [14]. The nanoparticles morphologically resemble native virions, making it easier to deliver STING agonists and antigens to draining immune cells and lymph nodes for immune system potentiation. The effectiveness of nanocomposite vaccinations can be immunized with MERS-CoV nanoparticles in mice and has been encouraged with the derivation of effective neutralization of antigen-specific and antibody T cell interactions [15,16]. A transgenic MERS-CoV mouse model showed that immunized mice that accumulated hollow nanoparticles of the MERS-CoV vaccine were cured from a fatal MERS-CoV problem in the absence of unwanted eosinophilic immunopathology [17]. Biocompatible hollow nanoparticles were described here, with significant therapeutic potential for the creation of novel adjuvants and subunit vaccine candidates, enabling the quick synthesis of safe, efficient, and effective vaccinations against novel viral infections [15].
In Wuhan china many COVID-19 cases were associated with the Huanan Seafood Market [18]. It considered an obvious candidate for the location of the initial zoonotic (that is, cross-species transmission) event. However, none of the animals from the market (including rabbits, snakes, stray cats, badgers and bamboo rats) tested positive for SARS-CoV-2 [19]. In addition, some of the early cases of COVID-19 in Wuhan were not epidemiologically linked to the market [20]. From the initial genomic judgements, it was clear that SARS-CoV-2 had a genomic group similar to SARS-CoV. The three three-dimensional structures of spike proteins of both viruses have similar same cell surface receptor in human Angiotensin-Converting Enzyme 2(ACE2) [18] that was very soon confirmed in vitro and using structural biology [21]. SARS-CoV-2 differs from SARS-CoV because, six amino acid positions in the Receptor-Binding Domain (RBD) of the spike protein that mediate the attachment of the SARSCoV and SARS-CoV-2 spike proteins to the human ACE2 receptor [22]. However, amino acids at five of the six positions differed between SARS-CoV and SARS-CoV-2, such differences caused SARS-CoV-2 to have a higher binding avidity to the human ACE2 receptor11, and may have contributed to the higher transmissibility of SARS-CoV-2 compared with SARS-CoV [23].
The comparison of alpha- and beta coronaviruses identifies two remarkable genomic features of SARS-CoV-2. First feature are, it can appears to beoptimized for binding to the human receptor ACE2, that can show on the basis of biochemical experiments [24,25] and structural studies [26-28]. Second feature are, the spike protein of SARSCoV-2 has a functional polybasic (furin) cleavage site at the S1–S2 boundary through the insertion of 12 nucleotides, which additionally led to the predicted acquisition of three O-linked glycans around the site [27].
The goal of this discussion is to review recent research on hollow nanoparticles in the search for antiviral or diagnostic medicines to combat developing coronaviruses. In this context, we also discussed the potential of hollow nanomaterial-based biosensors and vaccines. Furthermore, we explained the utilization of nanoparticles for the identification and treatment of coronaviruses because of their antiviral potential. Rapid global dissemination and the high mortality rate of coronaviruses are the greatest challenges for drug developers. It will be moving forward toward the identification and treatment of emerging coronaviruses with the aid of nanotechnology. We ascribed the three different aspects for the utilization of hallow nanoparticles as diagnosis the coronavirus via biosensors, combating the coronavirus, employing as nano drug and performed antiviral activity.
Mechanism of Actions SARS-CoV-2
1. Acter et al. described the stage of coronavirus infections, and its transcription/replication mode will be as follows:
2. As an intermediate host, people come in contact with environments that have SARS-CoV-2 virus germs.
3. In the initial phase, alveolar cells are affected due to the interaction of coronavirus cells with the lungs via the ACE2 enzyme with a spike (an exclusive glycoprotein surface is involved). Later, in the second stage, it makes its way to the host cell. In addition, the virus was detached. Consequently, the RNA genome intercalates with cytoplasmic cells and then connects to ribosomes (host cells). Eventually, it makes it to the host cell [29].
4. In regard to RNA transcription and replication, proteins that are not structurally rearranged and RdRp RdRp stand for as RNA-polymerase-RdRp Replicas the Transcriptase Complex (RTC) of multiple proteins, and RdRp produces genomic RNA (positivesense) descending viruses via subgenomics and transcripts of replication [30].
5. Figure 1 depicts the Human Coronaviruses (HCoVs), which are derived from genomic groups.
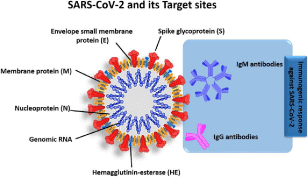
Figure 1: SARS-CoV-2 schematic structure and possible diagnostic targets: Reprint and permission from ref [31]. Copyright 2020 King Abdulaziz City for Science
and Technology.
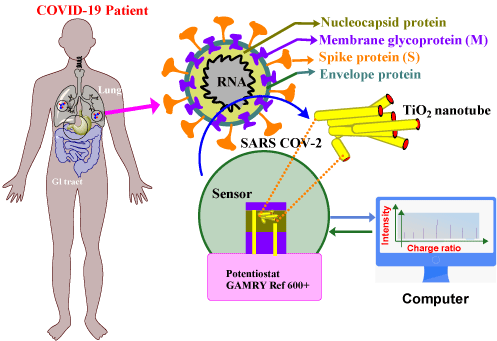
Figure 2: Co-functional TiO2 nanotube (Co-TNT)-based sensing platform for the detection of SARS-CoV-2. Reproduce with permission from ref [44]. Copyright
2020 MDPI.
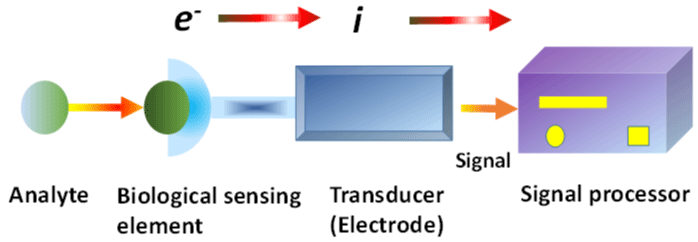
Figure 3: An electrochemical transducer biosensor. Reproduce with permission from ref [95]. Copyright 2010 The Royal Society of Chemistry.
Virus Detection Techniques
SARS-CoV-2 identification using real-time Reverse Transcription–Polymerase Chain Reaction (RT–PCR) is a vital step in COVID-19 management. Asymptomatic infected individuals whose biological shedding accidentally distributes the disease to the mature and comorbidities are tested to avoid infectious propagation across people and communities [32]. The first step in limiting the COVID-19 epidemic is accurate virus detection [33]. Serological testing is recommended for detecting viruses and determining prior infection that can be used for medicinal research. The identification of antibodies can be achieved from Enzyme-Linked Immunosorbent Assay (ELISA) [26], which is a qualitative approach for detecting Immunoglobulin M (IgM) or Immunoglobulin G(IgG) antibodies [34]. These studies detect an protected response to the spike protein(Sprotein) and could be useful in determining to protect against future pathological exposure as well as for contact outlining [35]. Such tests are crucial, and they cannot be exaggerated. It could be applied to epidemiological assessments as well as wide global therapeutic requirements [36]. In upcoming modern research, diagnostic tests might be developed to improve immunoassay specificity and sensitivity [35]. Such analysis will eventually demonstrate viral protection when infections occur [37]. Immunity induction against SARS-CoV-2 is the next step in COVID-19 control [37,38]. Instead of RT–PCR, hollow nanomaterial-based techniques provide speedy and effective viral detection.
Hollow nanoparticles can be used to extract viral RNA via coprecipitation and polyamine ester attached with 3-aminopropyl (triethoxysilane) to perform almost 50000 diagnosis tests [29]. A colorimetric test consisting of thiol modified with antisense oligonucleotides linked to gold nanoparticles was developed to identify the N-gene RNA found in SARS-CoV-2. This technique is used for rapid diagnosis and can be completed in less than ten minutes. The detection limit for RNA particles is 0.18+ngl1 [39]. A binding of S-receptor domain linked with fluorescent Quantum dot QDs was designed by quenching with ACE2-attached gold nanoperticles (AuNPs). As the ACE2 receptor is linked with the S-protein, the fluorescence probe is quenched with adjacent gold nanoparticles, allowing for the observation of binding events present in solution. Through cell-based tests, QD probes will also help in the validation and discovery of ACE2 receptor binding and SARS-CoV-2 S-protein inhibitors [30]. QDs serve as probes for the investigation of additional viral receptors [40]. This system can be used to distinguish recombinant proteins and neutralizing antibodies against virus diagnosis, such as SARS-CoV-2, and many others that recognize and enter cells through the S-receptor. Similarly, TB biomarkers have been detected from co-functional TiO2 Nanotubes Tunneling Nanotubes (Ni-TNTs) with a greater surface to volume ratio [41]. A sensing mechanism is based on the creation of a complex between the biomarker and Co owing to Co ion reduction and biomarker oxidation. Simultaneously, they predicted that by coupling the Sprike Receptor Binding Domain (S-RBD) protein with functional nanoparticles, they might identify SARS-CoV-2 or S-RBD. Table 1 describe the comparison between different methods based on nanomaterials.
Detection Method
Technique
Detection
Baseadvantages
Disadvantages
Ref
Serological detection
immunoblotting assay, neutralization assay,
viral protein
easy; low-cost
poor sensitivity; necessity for fresh reagents
Nanobased detection
nanobiosensors
viral protein/nucleic acid
very high selectivity and sensitivity; high stability; fast response; portable system
pH and temperature influence theselectivity and sensitivity of biosensor
basic detection
cell culture
infection test
broad spectrum; low-cost
difficulty in maintaining cell cultures;lengthy test
[42]
Molecular detection
polymerase chain reaction, reverse transcription polymerase chain reaction,
viral nucleic acid
high sensitivity; easy to set up
extremely liable contamination noteasy to quantitate results; high-skill operator required
[43]
Table 1: Advantages and Cons of Common Virus Detection Method.
The current study evaluated whether cobalt Tunneling nanotubes Co-TNTs have the ability to electrochemically diagnose the S-RBD protein of SARS-CoV-2 [44]. Anodization electrochemical technique was used to make TNTs because it is easy and cost-effective. To accomplish Co functionalization, an incipient wetting approach was applied [45]. They presented that cobalt-functional TNTs can detect the SARS-CoV-2 S-RBD protein in just 30 seconds using an amperometry electrochemical method [45].
Numerous biosensors have been created to detect viruses, e.g. influenza, human immunodeficiency virus, and other viral illnesses [46]. Graphene-based biosensors have a wide variety of applications; they are made up of hexagonal carbon ordered in a 2D layer. Graphene biosensors are particularly sensitive due to their high electrical conductivity, large surface area and high carrier mobility. To construct a graphene based biosensor for SARS-CoV-2 diagnosis, the coronavirus S antibody was immobilized at the graphene surface from 1-pyrene-butyric acid with ester linkage of N-hydroxysuccinimide [47]. Toroidal plasmonic metal sensors were designed to detect viral S-protein concentrations as low as femtomolar. Monoclonal antibodies conjugated to functionalize AuNPs were detected at a concentration of 4.2FM, according to the researchers (lower limit of detection). A polarized light beam at the terahertz frequency might conceivably modify the transmission spectra of a metal sensor. In Point-Of-Care (POC) testing circumstances requiring a quick and fast assay, metasensors can be useful [48]. A one-step optical S-protein specific nanoplasmonic resonance sensor that needs little sample preparation and delivers direct and rapid viral detection has been developed as a consequence of a recent study. The detection limit for this assay is 30 virus particles and completed in just 15 minutes. Such an assay is capable of quantifying the virus at concentrations lower than those found in regular nasopharyngeal swabs and viral saliva [49]. It was discovered that nanoplasmonic sensor chips had a very high specificity (>1,000:1) for detecting SARS-CoV-2 after determining the sensor's specificity to bind SARS-CoV-2 with SARSCoV, MERS-CoV, and vesicular stomatitis pseudoviruses [50]. With sensitive viral detection, an affordable and portable device such as smartphone application may identify SARS-CoV-2 in a single phase within 15 minutes [51]. The virus may be measured linearly between 0 and 107 viral particles per milliliter, making it helpful in hospitals, houses, and highway screening stations, where the detection limit is 370. Thirty-eight gold nanoparticle-based sensors paired with artificial intelligence can identify volatile organic chemicals linked to SARS-CoV-2 in exhaled breaths [52]. The test can identify viruses based on changes in the resistivity of the nanomaterial biosensor layer. Collectively, biosensors and other hollow nanomaterial-based detection approaches can be used to perform quick and portable SARS-CoV-2 tests. Nths by using a variety of nanomaterials and undertaking a more thorough cohort study [53]. AuNP-based sensors paired with artificial intelligence can identify volatile organic chemicals linked to SARS-CoV-2 in exhaled breaths. The test identifies viruses based on changes in the nanomaterial biosensor layer resistance. The employment of additional nanomaterials and a greater sample size characterize the Nths [54]. A clinical diagnostic sensor with a dual-function plasmonic photothermal effect and transduction via localized Surface Plasmon Resonance (SPR) has been created. On 2-D gold nanoislands, several experiments are carried out. Gold nanoislands include complementary DNA receptors, which further hybridize with SARS-CoV-2 nucleic acids [53] and the device may be stimulated at two different wavelengths. F1ab-COVID, RdRp-COVID, and SARS-CoV-2 Envelope (E) genes may all be detected using this biosensor. The 0.22 pg/mL detection limit of the dual-purpose localized SPR biosensor allows for the exact identification of SARS-CoV-2 sequences in a multigene mixture. The development of antibody detection assays is underway, although progress is modest [55]. Numerous studies have had small differences, yet considering the remarkable regularity with which scientific information is communicated, few have published conclusions that should be questioned. One of the biggest current concerns with immunodiagnostic procedures have deficiency of accuracy, which may be give incorrect positive antigen readings. They are extensively conserved among CoV species and have a role in autoimmune disorders by interacting with auto antibodies. Immunodiagnostic approaches work best 7–11 days after exposure, rendering them ineffective for identifying acute infections [56].
The combination of metagenomics detection and nucleic acid amplification techniques can provide clinicians and epidemiologists with new insights [57]. In the future, immunodiagnostic platforms based on S and N can be used in conjunction with nucleic acid amplification tests to significantly improve COVID-19 detection sensitivity at a low cost [58]. Future research into the creation of novel diagnostic platforms could be useful based on the tests' precision, specificity, and ease of use, as well as their ability to deliver results quickly and at a low cost. The benefits and disadvantages of continuous testing methods and their singular performance values are that outcomes should be close. In both clinical and nonclinical settings, data are analyzed before making decisions [59]. Additionally, it is important to consider alternative methods for forecasting potential outbreaks, such as low-budget mass pooling and metagenomics profiling. Efforts to improve coronavirus detection have been made to date, and a number of improved or novel methods have been developed. In practice, multiple strategies are typically combined to minimize the disadvantages of a single technique. In summary, we expect that with the rapid advancement of new technology and techniques, additional excellent and effective detection methods will be built in the future, providing scientists and clinicians with more options. Simultaneously, only by weighing the benefits and drawbacks of different detection assays for particular applications can we arrive at the most cost-effective and optimal solution [60].
Hollow Nanoparticles for Diagnostics
COVID-19 is clinically diagnosed using a combination of CT and RT–PCR results [59]. Apart from clinical settings, RT–PCR research accounts for the lion's share of testing for surveillance carried out on the job or classrooms. Since RT–PCR testing is so prevalent. By finding the flaws in research stage, potential detection methods can be improved [61]. Due to improper specimen collection timing, poor specimen consistency, and other factors, nucleic acid amplification tests can be difficult. The demands for qualified laboratory technicians is increasing, as well as lengthy wait times for results. RT–qPCR (quantitative PCR), considered the gold standard, takes 4–6 hours to complete without counting the time it takes to obtain the samples in the lab [62].
Additionally, the RT–PCR results are highly dependent on the type of sample used. Oropharyngeal swabs (32to 48 percent), nasopharyngeal swabs (63 percent), Broncho alveolar lavage fluid (79 to 93 percent), sputum (72–76 percent), and stool (72–76 percent) have significantly different rates of good sampling (29 percent) [62]. There may be a shortage of primers and other reagents necessary to conduct the experiments [63]. New platforms are being aggressively pursued in response to RT–PCR testing limitations. Due to its high selectivity and simplicity, RT–PCR is the most frequently used RNA detection tool. RT–PCR is commonly used in COVID-19 detection. However, this approach has several drawbacks, including low extraction performance, a lengthy procedure, and false positives due to contamination. Hollow nanomaterials have been employed to build viral detection technologies due to their huge surface area and ultrasmall size. Numerous hollow nanomaterials have been studied for their potential use in virus diagnosis, such as Quantum Dots (QDs), metal nanoparticles, silica nanoparticles, polymeric nanoparticles, and carbon nanotubes. The majority of these techniques are calorimetric, electrochemical, fluorescence, or optical in nature.
Qi et al. studied the applications and properties of hollow nano/ microstructures and described that the efficiency of additional shells was increased due to the involvement of more sites [64]. Hollow nanostructures have many benefits for sensor applications, such as shorter electron transfer paths, low densities, increased active sites, high permeability, and large communication areas between electrolytes and electrodes [65,66]. Hollow structures resist nanoparticle agglomeration due to their internal voids, enabling better volume adjustments to be accommodated in subsequent electrochemical measurements. Numerous previous studies have demonstrated the excellent catalytic activity of hollow nanomaterials in alkaline solutions for glucose detection [67-69]. Other nanomaterials can be used to adapt hollow nanomaterials with superior properties, such as wide surface area and strong electrical properties, to improved sensing properties—a necessary step in the battle against a viral pandemic. The first prerequisite is to detect the virus. The exploration of rapid and accurate testing techniques enables touch isolation and tracing of infected individuals, thus containing the virus's propagation. Specifically, SARS-CoV-2 is quickly transmitted via asymptomatic carriers [70].
A wide range of nanomaterials has been proposed for virus detection. These nanomaterials include metal, silica, polymeric nanoparticles, Quantum Dots (QDs), and carbon nanotubes (Table 2).
Nanoparticles (NPs)
Characteristics
Target viruses
biosensor type
Ref
Graphene oxide (GO)
size controllability of GO Nano sheets and changes in their oxidation level to detect specific viruse.
HIV, RotavirusHIV-1, HBV
Potentiometric/ optical/ electrochemical
[71.72]
Carbon nanotubes (CNTs)
It possess high selectivity and sensitivity due to their high surface area
HPV, HBV
electrochemical/ FET
[73,74]
zinc oxide (ZnO)
Plays a main role in special sensors known as mechano-chemicals
HIV
Electrochemical/ piezoelectric
[75]
quantum dots (QDs)
It have unique optical and electrical properties, having are powerful tools for providing rapid and sensitive virus detection and treatment of viral disease.
EBV, HIV, HBV, CoVs
optical/ electrochemical
[76]
copper NPs
Due to small size and high surface-to-volume ratio of copper NPs are easy to enable for detection of viruses.
IAV, HBV
electrochemical
[77,78]
silica NPs
Silica NP provided a platform important for bioanalytical studiessuch as antigen-antibodies, peptides and DNA
HBV, HPV
optical
[79,80]
Aluminum(AINPs)
AINPs is the most prominent and attractive feature for designing biosensors due to Nano porous morphology.
DENV, Ebola
electrochemical
[81,82]
Magnetic (MNPS)
MNPs are widely used in reusable biosensor platforms
CoVs, IAV, HBV
piezoelectric/ electrochemical
[83]
Gold(AuNPs)
AuNPs have been used widely for very sensitive detection of viral diseases due to theirunique optical and electrical properties
DENV, RVFV, KSHV, CoVs
optical/ electrochemical
[84,85]
Silver (AgNPs)
fluorescent properties of AgNPs introduce high sensitivity to optical-based biosensors
KSHV, CoVs, WNVinfluenza
optical/ electrochemica
[86]
Table 2: Summary of Representative Engineered Nanomaterials Employed As Biosensors for Virus Detection.
Biosensors
A biosensor is a system that generates a signal from a transducer for comparing and analyzing biological conjugates such as cell receptors, antibodies, RNA, DNA, and nucleic acids. The biosensor device was made up of four important components: a bioreceptor, a transducer, a biosensor, and a digitally controlled detector [87]. The second element is the transducer or detector; it operates by detecting the signal associated with a physicochemical shift induced by the interaction of the analyte and bioreceptor. It converts the signal to an evaluable and quantifiable form. A biosensor's final component is the reader system. It entails a display that is produced by software and hardware [88]. The development of a new class of diagnostic biosensors known as nanobiosensors was enabled by electrodes fabricated at the microscopic nanoscale and scale sensors. The biosensor characteristics were affected by continuous-material dimension reductions from large to small scales up to 100 nm [89]. However, it greatly expands the scope of their implications.
Because of the interaction of the sensor surface with the analyte, biosensors with a high surface-to-volume ratio are very effective in nanosized devices. With the help of natural elements, hollownanomaterial transducers have been improved to develop commonly used biosensors for disease diagnostics and biomolecule detection.
Such instruments can assess a patient's physiological status quickly and accurately. Conduct pesticide and water contamination analyses on food and environmental samples [90]. Patients' physiological status can be quickly assessed with such instruments. The interaction of analytes with surface sensors will become very effective due to the large surface volume ratio of nanosized devices [91]. Biosensors can be used to detect bacteria and viruses in food and water, which are potential disease sources. Depending on the type of biosensor, biologically sensitive components recognize and interact with the analyte [92]. Zhao et al. designed and built a compact microfluidic device at a cheaper rate. For in situ fast diagnosis of E. coli, a biosensor is developed with the support of a graphene monolayervia streptavid in antibody and AuNPs. The surface of the biosensor is used to collect the bacteria, and identification is carried out electronically [93]. These devices incorporate bioreceptor sensing elements that mimic in vivo molecular recognition phenomena [94].
The combination of biosensor characteristics and nanotechnologies is now seen as a candidate that could be considered to accelerate the production of rapid, susceptible and precise devices for real bacterial and viral detection. Nanobiosensors, therefore, use magnetic, optical, electrical, and chemical characteristics of pathogens and biomolecules to detect the materials [96,97]. Research into hollow nanomaterials and nanostructures (e.g., nanogels, polymer nanocomposites, plasmonic nanomaterials, metal nanoclusters, metal oxide NPs, and carbon nanotubes) has made a significant contribution to the production of biosensors [98,99]. They have been used to adjust electrode surfaces and increase critical characteristics, including sensitivity, selectivity, and reproductiveness, due to their high adsorption capability, compatibility of structures, and biocompatibility.
Nanomaterials are candidates that are important for the use of biosensing. Nanotechnological advances have encouraged the production of medical diagnostic tests and quicker, cheaper, more sensitive, and more precise devices. Hollow nanomaterial biosensors put together diverse fields, such as chemistry, molecular biology, material science, and biotechnology. They give extreme sensitivity so that some biosensors can now detect as low as a parasite per microliter of blood [100].
Electrochemical Biosensor
Electrochemical studies provide essential benefits, such as design simplicity, higher selectivity and sensitivity, lower power demand, low-price equipment, and ready incorporation into instruments for microfluidics compared to techniques used in the past. Interestingly, the investigation of electrochemical gadgets has exhibited excellent performance in medical apparatuses [101]. In the last few decades, the world has been facing serious virus outbreak diseases such as SARS-CoV-2, SARS-CoV-1, MERS-CoV, and Ebola. It will be demanding a fast test kit to help and prevent more pandemics within the congested population. From a care diagnostic perspective, a new platform with specialized instruments for cases such as COVID-19 is a future obstacle [102].
Working electrodes can be made from semi conductive ingredients ranging from metals to nonmetals, such as materials and carbon of different sizes, ranging from main part materials to hollow nanostructures. Materials, fabrication methods, and design methods are all factors to consider, and the selectivity of the electrode and detection limit output are influenced by the electrode's characteristics and structures. As biosensors, a variety of gold- and platinumbased electrodes are used [103-105]. Cutting or conventional micromanufacturing techniques were used to create a large metal area or thin-film metal electrode using screen printing techniques and physical vapor deposition [106,107]. Polymer electrodes (which offer longevity, biocompatibility, and adjustable electric conductivity) and ceramic electrodes (made of indium tin oxide, polysilicon, and titanium dioxide) have also been utilized to produce electrodes [108]. However, selecting materials for electrochemical sensors, especially those used to detect pathogens, requires expert knowledge. Critical aspects of electrochemical sensors, including coupling chemistry, double-layer capacitance and heterogeneous electron transfer rate bioreceptors, may be normed, and they require immobilization. Hollow nanoparticle expendability and malleability have attracted interest in biological sectors, with several reaching commercialization, particularly for viral detection [109].
These discoveries have opened up incredible prospects for nanotechnology to detect and diagnose viral infections. As these materials exhibit a definite characteristic that compared to their bulk counterparts and have dramatically increased the ratio of surface-volume nanomaterials, to create robust materials for COVID detection [110], these materials have dramatically increased the aforementioned properties to create robust materials for COVID detection. Signals have been magnified from two aptamers. Zhao et al. produced a reliable electrochemical technique for detecting Hepatitis B Virus (HBV) based on nanoparticles of Cu3 (PO4)²-AuNP [111]. The constructed sensor had excellent binding sites, a flexible shape, and biocompatibility. Lee et al. devised a label-free avian influenza viral detection approach using electrochemistry and a multifunctional DNA structure on a porous (p) AuNP-modified electrode [112-114].
DNA sequence that has been proposed as a three-way intersection/p AuNP-based detection method can be used for the detection of a number of targets as a useful biosensor for the detection of pathogen subtypes on a single platform or single target detection with a high degree of reliability using the dual detection method [115]. The existing diagnostic procedures for SARS-CoV-2 are both timeconsuming and expensive. However, some excellent and low-cost techniques are available for immediate use, such as the synthesis of a low-cost but sensitive electrochemical sensor such as Co-TNTs for rapid SARS-CoV-2 diagnosis via spike detection (Receptor-Binding Domain, RBD) available on the virus's surface [44].
A potentiost at was used to cobalt-functionalize the TNT platform connected for data collection, after which a cheaper, onestep electrochemical technique was used to prepare TNTs. This sensor can detect the S-RBD protein of SARS-CoV-2 even at low concentrations (14–1400 nM). Furthermore, this sensor exhibits a linear relationship with viral protein diagnosis over a broad concentration range. In approximately 30 seconds, the Co-TNT sensor can sense SARS-CoV-2 S-RBD protein in nasal secretions and saliva samples, which might be exploited to establish quick point-ofcare diagnostics for SARS-CoV-2 detection in nasal secretions and saliva samples [44]. Because of their great sensitivity, precision, and accuracy in identifying biomarkers, electrochemical biosensors are crucial for detecting biomolecules [116]. Electrochemical biosensors have been used to successfully detect viruses such as MERS-CoV. Lahyquah et al. discovered MERS-CoV using a carbon electrode array to enhance its capability with gold nanoparticles. A biosensor for detecting SARS-CoV-2 has been described [117], which uses AuNP-decorated Fluorine Tin Oxide (FTO) glass impregnated with nCovid-19 monoclonal antibody.
By integrating hollow nanomaterials with the electrode, the efficiency of electrochemical biosensors can be improved. As the electrode surface area-volume ratio grows, the electrochemical reaction rate improves, resulting in a larger electrode surface area to analyte fluid volume. Tuberculosis biomarkers have also been detected using Ni-TNTs with a greater surface-volume ratio [118,119].
The suggested sensing method is based on the creation of a complex between the biomarker and Co due to Co ion reduction and biomarker oxidation at a certain bias voltage. As biosensors, several metal-based electrodes consisting of platinum and gold are utilized [103,104,120]. Cutting or traditional microfabrication techniques such as physical vapor deposition and screen printing, for example, were used to fabricate a thin film electrode or a thick surface based on metal. Additionally, polymer electrodes and ceramic electrodes composed of indium tin oxide, polysilicon, and titanium dioxide have the advantages of durability and flexibility. The fabrication of electrodes has been based on biocompatibility and tunable electric conductivity [108,121]. However, selecting materials for electrochemical sensors, especially those used to detect pathogens, requires expert knowledge.
The most frequent carbon materials used in conventional carbon sensors are glassy carbon, carbon fibers, and pyrolytic graphite, with pyrolytic graphite, carbon fibers, and glassy carbon being the most common carbon materials used in traditional carbon sensors [122,123]. The majority of carbon-based hollow nanomaterials exhibit several advantageous properties, including increased electrocatalytic activity, adsorption biocompatibility, and a high electron transfer rate [124].
Sensor applications have been investigated using carbon nanotubes and graphene. Simple drop casting, substrate growth, code position with metal nanoparticles, and field-effect transistors can all be used to directly integrate these into biological sensors [125]. Using the electrochemical impedimetric technique, researchers recently suggested the aid of gold hollow nanotubes for label-free detection of Human Papilloma Virus (HPV) [126]. An external electric field was used to force the negatively charged DNA oligonucleotide into the desired orientation in this study. By regulating immobilization and hybridization of the sequence onto gold nanotube surfaces, the sensing responsiveness may be improved. Silver nanoparticles (Ag- NP) are simple to make and have strong biomolecular attraction. As a result, they are perfect for making electrochemical biosensors [127]. Further investigation allowed researchers to explore an electrochemical immunoassay to detect antibodies against Tick- Borne Encephalitis Virus (TBEV) [128].
Before covalently immobilizing the antigen onto the electrode surface, the gold and carbon composite electrode was thiolated and gluttonized. Because of graphene's excellent chemical, mechanical, thermal, and electrical capabilities, hollow nanostructures are useful. They are the most often employed biosensors for DNA detection, because they have higher affinity for single-stranded DNA due to p-p stacking and hydrophobic interactions [129]. To improve biocompatibility, selectivity and solubility, graphene can be employed to interact with a variety of biomolecules and tissues. Wang et al. devised a sandwich-type electrochemical immunoassay by using apoferritin-embedded Cu nanoparticles with graphene QDs [23]. To determine the amount of Cu emitted from the apoferritin cavity assembly, differential plus voltammetry was used. Due to vast surface area of graphene quantum points, the electric signals increased, effectively accommodating considerable antibody loading [130]. Cu-apoferritin nanoparticles here have improved the charge of an electro active sample to further enhance the signals. The approach could identify avian leuck with a detection limit of 115 TCID50/mL and the range of 102.08 to 104.50 TCID50/mL.
The electrode material used in electrochemical sensors is important because it may alter the sensor output in a variety of ways. The capacitance of the double layer (which affects S/N), the heterogeneous electron transfer rate (which affects sensitivity and reaction time), the presence of the coordination chemistry used to immobilize the receptors, and the proclivity of electrochemicalluminescent sensors for nonspecific binding and quenching are all factors to consider. Carbon is a popular electrode material that is used as a platform for sensor growth Fluorine-Doped Tin Oxide (FDTO) and silicon (oxide) [131-133].
Because the faradic electrode is now dependent on the active site of the electrode until the background current grows in proportion, increasing the active electrode region will improve the detection sensitivity. To increase the area, hollow nanomaterials form other receptors that can be immobilized to give a more possibly increasing sensitivity and dynamic range, e.g., nucleic acid capture rings or antibodies [134]. Serious respiratory diseases in humans, including MERS and SARS, that cause contemporary problems were detected by viruses. In particular, however, the COVID-19 virus SARS-CoV-2 [135]. To identify the coronavirus type virus MERS-CoV, an immune sensor using voltammetry detection was established.
An electrochemical sensor was developed using folding paper techniques by Crooks and coworkers [136] that detects a 30-base nucleotide associated with HBV DNA and has a limit of detection of 85 pM. Micrometer-scale particles are accommodated in a hollow tube, and an extremely advanced slip sheet enables the separate incubation phases to be conveniently staged in time [137]. By using silver nanoparticle labels, an overall amplification factor of 250,000 was achieved using two stages of amplification. Simultaneously, magnetic microbeads equipped with detention investigations can be pre concentrated at a detection electrode to approximately 25-fold amplify the signal [138]. Notably, the assay is carried out without the use of enzymes or antibodies, which increases its stability, speed, toughness, shelf life and temperature tolerance. Only one incubation step is required prior to detection.
Biosensors based on Field Effect Transistors (FETs) have several advantages and characteristics when used with various analyte concentrations. Biosensors based on FETs have a wide range of applications, most notably in medical treatment, monitoring, and diagnosis at the point of care [139-141]. Graphene is a new nanomaterial made up of 2-D carbon atom layers. This material is an ideal candidate for active sensing surfaces because of its high carrier mobility, superior electrical conductivity, wide surface area and ease of surface functionalization [142]. As a result, graphene base materials are regarded as the best material for improving the sensitivity of FET biosensors and are a prerequisite for preventing an influenza pandemic. A field-effect transistor based on a graphene (G-FET) configuration was used to detect biological substances. The high sensitivity and stability of biological objectives, including the bio-FET process and the virus gene of H5N1 influenza, allowed for rapid detection, which is used for a flow-through strategy and provides an admirable platform for label-free detection. Chan et al. improved its efficiency by employing reduced graphene oxide films on Si/SiO2 substrates. However, the Limit Of Detection (LOD) of influenza viruses was in the picomolar range, requiring the usage of a single-layer hexagonal carbon network [143,144]. Using a film of graphene-based material, Seo et al. produced an FET detector for the COVID-19 active virus that has antibodies functional with SARSCoV- 2 spike antibody (Figure 4) [47].
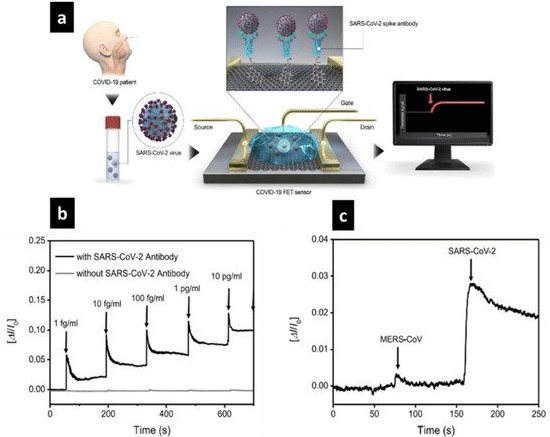
Figure 4: SARS-CoV-2 (spheres) attaches to antibodies (Y-shapes) in this schematic representation of the graphene-based field-effect transistor (FET) biosensor
mechanism and detection (a). COVID-19 FET reaction to SARS-CoV-2 spike protein in real time (b). MERS-CoV and SARS-CoV-2 are two distinct proteins that
bionanosensors respond to differently (c). Reprinted with permission from ref [145]. Copyright 2020 the American Chemical Society.
As a bioreceptor, the spike antibody SARS-CoV-2 was used. Moreover, it is an immunogenic transmembrane protein that selectively recognizes SARS-CoV-2. By incorporating a graphenebased FET biosensor into this biosensor, electrical characterization revealed an actual response to COVID-19 detection. S-protein, 1 fg/mL SARS-CoV-2 has good real-time detection. 4C Additionally, Figure 4 shows the biosensor's selectivity and sensitivity in distinguishing SARS-CoV-2 from other antigen proteins, such as MERS-CoV, and the ability of the surface to bind directly to the SARS-CoV-2 antigen of choice. The accuracy with which this biosensor identified the virus is impressive, and it could be used or updated to screen for other diseases in the future. A biosensor based on FET for the detection of SARS-CoV-2 in clinical samples [146]. Cultured virus, antigen protein, and COVID-19 patient nasopharyngeal swab specimens were used to calculate the sensor output. The S-protein SARS-CoV-2 may be detected at 1fg/mL in phosphate-buffered saline and 100 fg/ mL in clinical transported medium by using the FET device. The yield-effect transistor sensor successfully identified SARS-CoV-2 in culture media (LOD 1.6 101 pfu/mL [145].
Biosensing system-based FETs have several advantages over other currently available diagnostic techniques. This includes the capability to perform instantaneous and sensitive analyses with trace quantities of analytes [147,148]. Electrochemical biosensors are excellent for biomolecule sensing and can detect biomarkers with specificity high and sensitivity accuracy. Viruses have been successfully identified using electrochemical biosensors (such as MERS-CoV) [149]. Recently, gold nanoparticle-coated immobilized FTO glass was used to detect SARS-CoV-2 with a nCovid-19 monoclonal antibody [117]. The electrochemical biosensor functionality can be developed even further by hollow nanostructuring of the electrode, which increases the electrode surface area-volume ratio, resulting in a higher electrode surface area to analyte fluid volume ratio. Personalized medicine and digital health tracking have grown in popularity in recent years, and this great potential is now a reality thanks to considerable developments in skin-interfaced wearable sensors [150]. These sensors interact with the skin on multiple scales, from cellular to molecular, and can perform therapeutic and diagnostic activities with pinpoint accuracy, consistency, and speed. Electrochemical detection is potentially the most suitable technique for such sensor devices due to its low electric power consumption and ease of miniaturization [151].
Dual Modality Biosensors
Magnetic hollow nanoparticles with numerous uses are a new type of material with much potential in terms of increased functionality. It is difficult to create new nanocomposites without compromising magnetic activity or adding useful characteristics. An optically active Quantum Dot (QD) (core) enclosed within iron oxide (hollow shell) is the first electrochemical/fluorescence dual-modality probe in this study [152]. The encapsulation of QDs on the hollow shell structure preserves fluorescence with negligible quenching effects and good magnetic characteristics, paving the way for future fluorescence modality readout applications. Various viruses (such as Hepatitis E Virus-Like Particles (HEV-LPs) and Norovirus (Nov), were detected using a dual-modality sensor with Quantum dot -encapsulated magnetic hollow sphere nanoparticles (QD@MHS NPS) with highly integrated multimodal sensing and magnetic separation capability. In addition, the sensitivity of feces from HEV-infected monkeys was comparable to that of a gold-standard real-time quantitative Reverse Transcription–Polymerase Chain Reaction (RT–qPCR). This welldefined QD@MHS NP-based nanoplatform intelligently integrates dual-modality sensing and magnetic bioseparation, paving the door for efficient virus diagnostic point-of-care testing [152].
Each method of sensing has its own set of drawbacks; combining them into multimodal sensing can provide additional data [153- 155]. Fabricating hollow nanomaterials with multiple functional constituents that act as bifunctional markers is fascinating. Hybrid nanomaterials with a variety of synergistic properties can be created by combining magnetic nanoparticles with other active components. High-fluorescence inorganic fluorescent QDs are an appealing component widely used in high-throughput detection [156]. Coen capsulation in inorganic materials such as silica, template-based syntheses utilizing physical appendage or chemical bonding, or attachment of individual nanoparticles is the most common way to make magneto-fluorescent materials. Magnetic nanoparticles (core) and fluorescent QDs (shell) were assembled in the presence of a thin layer of silica to create colloidal structures. These nanoassemblies are rich in fluorophores and have a high magnetic content [157].
Rather than developing traditional single-mode sensors, the researchers in this study used CdSeTeS-QD@Fe2O3 hollow sphere nanoparticles (QD@MHS NPs) to create a dual-mode biosensor. Although hollow nanoparticles with magnetic and fluorescence properties have been used in a variety of bioimaging and drug delivery applications, more research is needed [158-159]. To our knowledge, their use in virus detection has yet to be demonstrated. With the recent increase in demand for virus sensing, a viable embodiment of sensitive and accurate strategies, particularly for the early stages of infection, is required, capable of rapidly providing a reliable and precise signal. The authors described how they used the synthesized QD@MHS NPs to develop a dual-modality biosensor for virus detection that incorporated electrochemical and fluorescence modalities to minimize false movement and self-verification. Although the target virus is present in basic sample analysis, several interferences indicate that magnetic bioseparation is required [152- 160]. The most widely used method, fluorescence, is used to obtain rapid sensing signals, and QD@MHS NPs have both magnetic and fluorescence properties. These structures provide a novel platform for separating targets from a complex biological matrix while also amplifying significant and reliable signals for clinical diagnosis [161]. Anti-monoclonal antibodies against HEV (anti-HEV-IgG) and NoV (anti-NoV-IgG) were conjugated to the surface of QD@MHS NPs, conferring HEV and NoV specificity, respectively.
Antibody-functionalized QD@MHS NPs, as shown in Figure 5 (A), can be used to capture the target virus from complex virus samples, forming virus/QD@MHS complexes as a result of the specific antigen-antibody reaction. Without sample pretreatment by applied virus/QD@MHS complexes, excess QD@MHS NPs, or an external magnetic field, the magnetic response of QD@MHS NPs was quickly separated and enriched. Virus/QD@MHS complexes and free QD@MHS NPs were redispersed in the new buffer after magnetic separation, and the vial was filled with antibody-labeled reduced graphene oxide-modified gold electrodes. The virus/QD@ MHS complexes bind to antibodies on the electrode surface, forming sandwich structures that are used to read electrochemical modality signals using an impedimetric response. The supernatant was used to read the fluorescence modality signal using the back-calculation method [162]. The structural merits of the QD@MHS NPs allowed for high fluorescence efficiency, and optimizing situations for their sensing parameters and sensor formulation were thoroughly investigated. The immunological interactions between the target virus and antibody molecules on the surface of QD@MHS NPs are the basis for this dual-modality sensor. An electrochemical signal is generated when antibody-conjugated QD@MHS NPs capture the virus and then bind it to an antibody-conjugated electrode. The change in the fluorescence signal was measured using unbound QD@MHS NPs with a diameter of 20 nm. Both the electrochemical impedance and fluorescence signal from the same sensing sample will provide two distinct signals due to the specific antigen-antibody reactions that occur as the virus concentration changes. The unique structure of QD@MHS NPs allows sensitive and precise biomolecule detection, which has important implications in disease diagnosis [152]. with electrochemical impedance and fluorescence as dual modalities (c). RE, WE, and CE denote reference, working, and counter electrodes, respectively. Reprinted with permission from ref [152]. Copyright. 2020 Elsevier B.V.
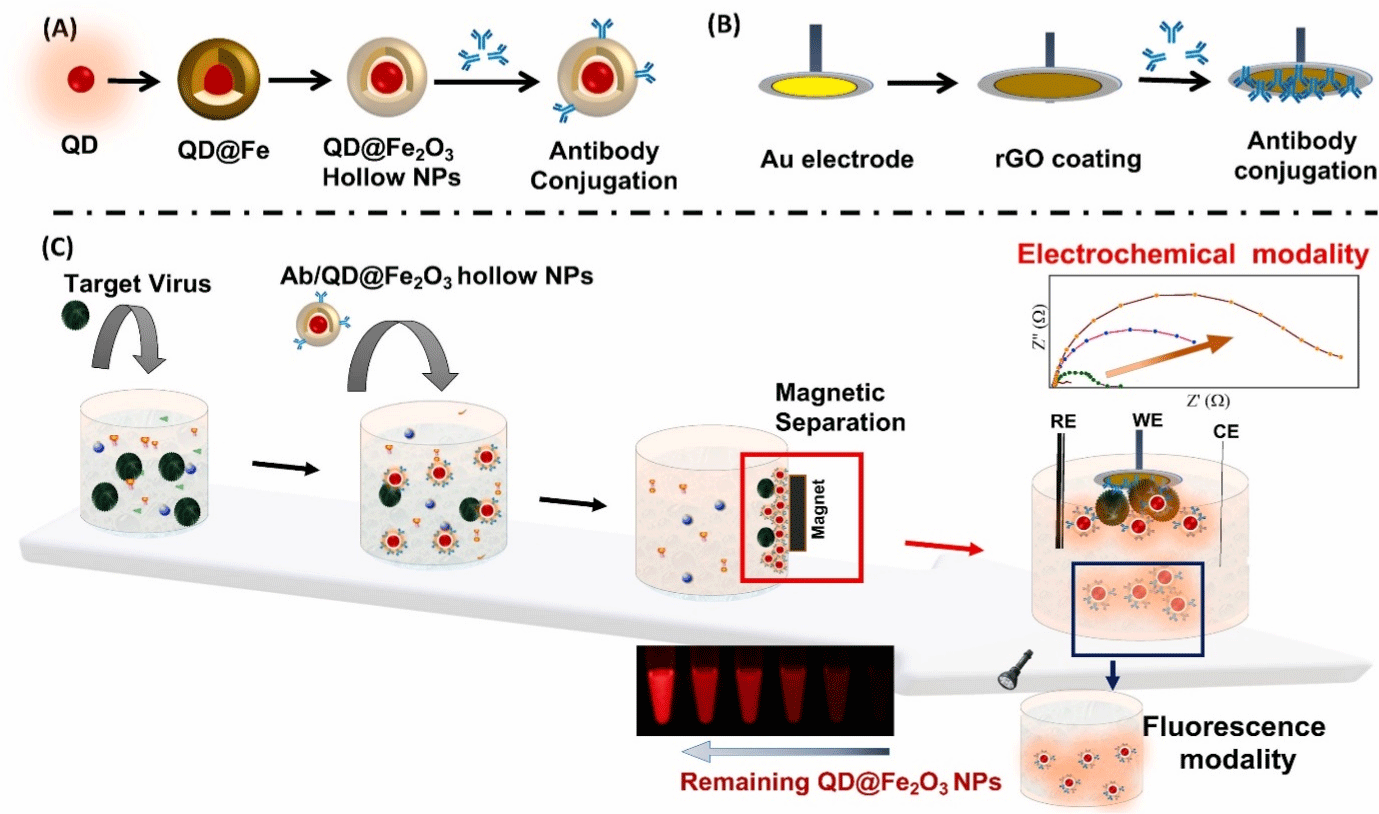
Figure 5: The fabrication process for QDs encapsulated within a hollow iron oxide sphere (QD@Fe2O3) is depicted schematically (a). An rGO-coated Au electrode
with antibody conjugation (b) and antigen-antibody reaction and magnetic separation are used to detect the target virus, with electrochemical impedance and
fluorescence as dual modalities (c). RE, WE, and CE denote reference, working, and counter electrodes, respectively. Reprinted with permission from ref [152].
Copyright. 2020 Elsevier B.V.
The interaction of the target virus with the Au rGO/antibody and QD@MHS NPs is governed by antibody conjugation. For the proof of concept, an anti-HEV antibody is conjugated to QD@MHS NPs, and an Au rGO electrode selectivity experiment is carried out in the presence of serum medium containing 10% diluted human serum with influenza virus, NoV, White Spot Syndrome Virus (WSSV), and Zika virus as other samples to confirm the specificity of the proposed sensor [163]. The signal attributed to the magnetic separation of the virus complex was not significantly changed by the sensors in buffer or 100% serum (as a negative control), effectively separating the interferences. Only the HEV-LP sample shows an increase in impedance, while the rest of the samples show no change [164].
All negative pieces have a slight increase in impedance from 0.5 to 5%, indicating the advanced dual-modality sensor's superior specificity. The fluorescence modality is also used to determine the selectivity of the developed HEV-LP sensor [165]. As a result, the number of nanoparticles does not change after separation, resulting in a minor difference in fluorescence. These findings suggest that the dual-modality sensor that was developed is well suited to detecting HEV-LP. A dual-modality sensor based on QD@MHS NPs has the following advantages over a traditional single-modality assay. This dual-mode sensor combines electrochemical impedance and fluorescence sensing into a single sensing system, allowing for more sensitive and accurate linear range sensing [166]. QD@MHS NPs are bioseparation probes made up of an optically active semiconductor QD core with enhanced fluorescence encapsulated within a hollow iron oxide sphere. The unique morphology of QD@MHS NPs prevents unwanted interactions within the nanoparticles, which could affect the fluorescence and magnetic properties. The magnetic property helps to separate the virus from the robust sample matrix, resulting in minimal background noise and possibly aiding in antiinterference solid ability and accuracy [152].
Furthermore, the sample volume required to integrated with dual-modality sensor is smaller than that required in two spatially separated electrochemical and fluorescence detectors [167]. Finally, the developed dual-modality sensor eliminates the need for timeconsuming sample preparation as well as sophisticated analytical equipment. Presenting a unique platform for significant applications in disease diagnostics. The current integrated dual-modality sensor recognizes and quantifies viruses using both electrochemical and fluorescence detection methods on a single nanocomplex. Because the sensor can generate two readouts for a specific antigen-antibody reaction at the QD@MHS NP surface, the integrated dual-modality design has the potential to improve detection reliability [168].
Optical Biosensors
One of the most promising biosensors for virus detection now available on the market is optical biosensors, which use Lab-On-a- Chip (LOC) techniques to increase nucleic acids for fluorescence examination [169]. Optical imaging, particularly single viral imaging, has the potential to be utilized to monitor and track virus reproduction, cell termination and contact, allowing for the development of faster and more systematic treatment alternatives. These imaging approaches are already being used in COVID-19 detection research [47,170]. However, more work is needed to transfer these innovations from the lab to the market. The plasmon principle is used in optical biosensors such as LSPR and SPR [171]. With plasmon detection, advanced surface chemistry approaches for viral strain detection provide a high degree of accuracy and a short reaction time. Researchers have devised a unique study strategy employing a biosensor that combines two distinct effects, visual and thermal, to create an optical sensor LSPR for the stability of viral RNA samples [172]. To artificially construct DNA receptor sequences, biosensors that complement RNA genome portions of the SARS-CoV-2 virus utilizing gold nanoparticles on a glass substratum are employed. After these precise orders were attached onto gold nanoislands, SARS-CoV-2 was successfully identified. Further refinement is required prior to application [170]. The creation of an optical fiber sensor based on Evanescent Wave Absorbance (EWA) for the quick and precise detection of COVID-19 was proven in a point-of-care situation. As a consequence of this study, two suggestions were made. The first proposal is to measure the host immune response, and the second is to identify viral cell surface proteins using suitable receptors. However, the host immune response was not a perfect prediction of the current COVID-19 virus, and other respiratory infections, such as SARS-CoV and MERS, can elicit comparable responses [173]. Using Localized Surface Plasmon Coupled Fluorescence (LSPCF), the studied an optical biosensor for SARS-CoV detection. The detection limit was 104-fold for 3 hours at a low concentration of COVID-19 (N) protein in blood (1 pg/mL). On the other hand, the purification and isolation process required time for sample preparation [174]. Using a SARS-CoV detection quantum dot-conjugated biosensor test chip, the diagnostic time was one hour after sample separation and washing. Researchers have created a sensitive molybdenum disulfide (MoS2) biosensor. These biosensors are inexpensive and simple to use. A fluorescent immunosensor-based biosensor with a sensitivity of 4.6/102 per mL was used as a coronavirus detector for Fluorescence Energy Resonance Transfer (FRET) [175].
Plasmonic effects have long been employed in biosensing, and a range of detection techniques have been developed, such as SPR and localized SPR have all been employed in biosensing for extensive time [176]. This was demonstrated in Lung Function Data (LFD) experiments that used laser excitation to increase the colorimetric signal of gold nanoparticles. Another advantage is that laser-reader systems may operate in normal LFD architecture and modes [176]. It is worth mentioning that LSPR can help researchers better grasp SARS-CoV-2 infection processes. For example, protein interactions with human receptors are known to be critical for viral survival in human cells. Experimental proof of docking-related phenomena can be obtained using SPR [177,178]. Another benefit of LSPR biosensors is their inexpensive development costs — a home-built LSPR system based on white-light extinction, for example, would cost $25000 to construct. This is a less expensive alternative to more complex Localized Surface Plasmon Resonance (LSPR) technology. The thermoplastic effect is created by absorbed light's non radiative relaxation in hollow nanomaterials, which creates more than localized heat energy that may be exploited as a source of localized heating for controlled thermal operations [179]. In dual-function biosensors, a similar technology was utilized to detect SARS-CoV-2 [170]. To make synthetic viral oligonucleotide sequences, a similar technique has already been employed by the Corman et al research group [180]. A synthetic receptor oligonucleotide such as RdRp SARS-CoV-2-C was used to incorporate the AuNI (Au nanoisland) sensing device into an LSPR detection system. When RdRp SARS-CoV-2 genes were introduced into the sensing chamber, hybridization became eight times faster because of the thermoplastic effect. (Figure 6) illustrates the results of applying LSPR responses to evaluate this dual-plasmonic device for viral nucleic acid detection. Before the LSPR biosensor can be utilized on COVID-19, a number of challenges must be solved, including the difficulties of producing strong and reproducible substrates.
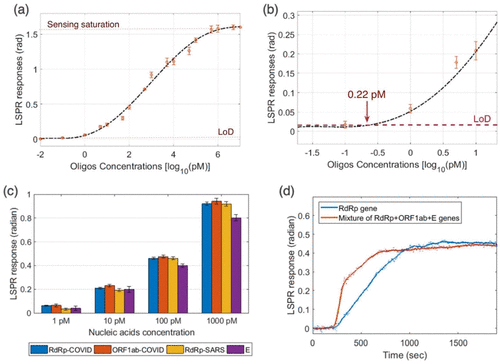
Figure 6: LSPR response versus RdRp conc. of SARS-CoV-2 (a); zoom of the low-conc. region of LSPR biosensor responses for different RdRp oligos conc. (b);
LSPR biosensor response for detection of other viruses, such as ORF1ab and E protein from SARS-CoV-2 and RdRp from SARS-CoV (c); the comparison of LSPR
biosensor response in single-analyte (d). Reprinted with permission from ref [181]. Copyright 2020 American Chemical Society.
Researchers have successfully developed an optical sensor for identifying the COVID-19 virus [182]. It is advantageous to determine the virus's existence in the surrounding environment. Optical sensors might be a viable option for measuring virus quantities in the air heavily inhabited and congested locations. Wang et al. investigated and decreased artificially produced airborne contaminants such as aerosols and nanoparticles. To increase detection safety, reliability, and sensitivity, researchers created an optical nanosensor based on LSPR technology [183]. An LSPR biosensor was created by surface functionalizing a succinimidyl ester group and attaching it 2-D Au nanoislands with an usual size of 40.2 nm on a glass substrate [184,185].
The visual and luminescent features of hollow nanoparticles, as well as the impacts of Surface Plasmons (SPs) and Luminescence Resonance Energy Transfer (LRET) are all factors in his optical nanobiosensor design principles (Figure 7). Biosensors can benefit from hollow nanoparticles' intrinsic luminescence or plasmonic optical absorption capabilities [162]. The acceptor's fluorescence signal would be recovered when employing LRET-based biosensors to target the analyte. The donor luminescence intensity decreases while the receptor luminescence intensity increases as energy is transmitted between the donor and acceptor via resonance interactions (Figure 7A and 2B). As a consequence of the energy transfer, the donor's life expectancy is lowered. Palladium, platinum and silver nanoparticles have been employed widely in the creation of sensing probes [186,187]. Noble metal nanostructures display extraordinary optical features via LSPR that have conduction band electrons due to their unique interaction with light [188].
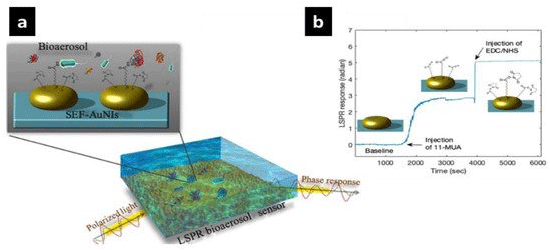
Figure 7: AuNI surface functionalization is used to detect bioaerosols (a). The in situ surface functionalization phase utilizes 11-mercaptoundecanoic (11-MUA)
and EDC/NHS activators (b). Reprinted with permission from ref [185]. Copyright 2020 American Chemical Society.
Through strong detectable light scattering, it also directs radiative or nonradiative attenuation. Photon energy is converted into thermal energy as a result of this. Both attenuation mechanisms have benefited diagnostics, bio imaging, and therapeutics. The LSPR approach was recently employed to identify SARS-CoV-2 (severe coronavirusrelated acute respiratory illness) [170,189].
Colloidal Gold Immunochromatographic Assay (GICA) is not suitable for early disease detection because the human antibody reacts to novel coronaviruses; in the case of influenza, the response time is relatively slow (approximately seven days after infection). Xia et al. created an application using smart phones to detect avian influenza virus on a point-of-care platform [190]. Following the capture of the antibody conjugation virus, a colorimetric reaction based on Au/AgNPs with a detection limit of 2.73% embryo infectious dose EDI50/mL can be used to detect the virus with the naked eye. This is a tenfold improvement over traditional fluorescent Enzyme-Linked Immunoassay (ELISA)-based detection. The smart phone imaging system's data-processing capabilities enhance the detection limit even further, decreasing it to 83103 EID50/mL [190]. Moitra et al. were able to visually detect nucleic acids in less than 10 minutes [39], and the N-gene (nucleocapsid phosphoprotein) of extracted RNA samples was detected using a colorimetric assay based on SARS-CoV-2 antisense (ASO)-modified AuNPs. In their method, four ASO sequences were chosen for their energy binding with the N-target in the presence of SARS-CoV-2 N-gene sequences and ASO arrangements were employed to cap AuNPs, resulting in complementary binding [191]. Color changes in the solution may be noticed with the naked eye [39]. Another researcher presented a paper-based biosensor that combines extraction of nucleic acids with amplification and optical detection to detect dengue virus. The biotinylated target DNA in the test would attach to the AuNP sensing samples and interact with streptavidin in the test zone, resulting in a visible red signal. By integrating agarose into the test strip, they were able to manage the flow of biomolecules for optimum interactions. When related to the complete test strip, the target identification sensitivity was considerably improved. However, using naked readings has drawbacks such as poor quantification and low analytical sensitivity [192].
Qiu et al. demonstrated a dual-functional Au nanoisland-based biosensor via thermoplastic heating. SPR imaging was utilized to identify RNA polymerase that is reliant on RNA (RdRp) (to produce signal amplification), and SARSCoV2 has a nearly comparable nucleic acid sequence with a detection limit of 0.22 pM [170]. A total of 2.26107 copies of the RdRp COVID series were identified in a 200 L analyte solution, which is greater than the SARS-CoV-2 viral load at onset (1106 copies/mL), considered fluorescent labeling to quickly and reliably identify the presence of SARS-CoV-2 [193]. Colorimetric indicators change color when enough viral DNA units are present. To identify anti-SARS-CoV-2 Immunoglobulin G (IgG) antibodies in human serum (LIFA), Chen et al. devised a lateral flow immunoassay [194]. On lanthanide-doped polystyrene nanoparticles, a goat antirabbit IgG control line and recombinant SARS-CoV-2 nucleocapsid phosphoprotein were functionally coated (LNPs). The test line was conjugated with MHIgG@LNPs and coupled with human anti-SARS-CoV-2 IgG antibodies in the flow experiment, resulting in a fluorescent signal change on the test line.
In the same way, rIgG@LNPs was combined with the control line material. To measure the anti-SARS-CoV-2 IgG concentration in human blood sample, a test line ratio is computed in the control fluorescence signal. In unprocessed blood samples, Li et al. devised a technique for identifying immunoglobulin M (IgM), the initial line of viral infection protection, and immunoglobulin G (IgG), longterm immunity antibodies, in less than 15 min [195]. The biophysical capacity of the SARS-CoV-2 virus to identify and attach to high-affinity Angiotensin-Converting Enzyme 2 (ACE2) is particularly intriguing [196,197]. SARS-CoV-2 spike anticorpus AuNPs, which have shown potential as a SARS-CoV-2 field-effect transistor detection technique, can be used to make LFIAs [198]. Antibody binding to SARS–CoV-2 has been discovered in recent investigations by Wu et al. and Chen et al. This study will contribute to the creation of optical biomarkers that can be connected to SARS-CoV-2 antibodies with success. The S-proteinin SARS-CoV-2 that interacts with the ACE2 receptor in host cells has been the subject of recent therapeutic investigations. The purpose of this research was to design and synthesize SARSCoV- 2-binding aptamers. If optical biomass sensors based on SARSCoV- 2 receptors were created and produced, COVID-19 might be evaluated more successfully [199].
Conclusion and Future Prospects
Despite the constraints caused by the absence of standardized assessment techniques, the purpose of this study is to offer complete overview of contemporary antiviral materials. In previous review publications on COVID-19 prevention, were discussed but we focused on medicines and techniques that inactivate SARS-CoV-2. Because of their capacity to infiltrate cells fast and interact with viruses to limit viral genome replication, hollow nanomaterials, particularly CoVs, can be regarded as good options against viral infection. It is vital to have a comprehensive and long-term plan for fighting infections, particularly respiratory viruses. The biological system forges a close relationship with nanoscience by merging hollow nanomaterials with nanomedicine to detect and cure disorders. Hollow nanoparticles are not only effective for viral diagnostics but also considered a therapeutic potential tool. It has the potential to reverse antiviral resistance, which poses a significant danger to currently available conventional therapies. Furthermore, by combining cutting-edge nanotechnology with standard antiviral medications, it is feasible to greatly boost the bioavailability of hollow nanoparticles while lowering their toxicity. Researchers have confronted various pandemics and viral outbreaks in the past, including the swine flu pandemic and Ebola virus Zika epidemics, which have impacted considerably more people. Researchers are currently focusing their efforts on high-level experimental investigations to produce unique and intelligent nano/ biomaterials and matrices for the creation of controlled release and targeted drug delivery systems. Currently, scientists are focusing their efforts on advanced experimental investigations to produce unique and intelligent nano/biomaterials and mediums for the creation of controlled release and targeted drug delivery systems. Researchers are currently focusing their efforts on high-level experimental investigations to produce unique and intelligent biomaterials and matrices for the creation of controlled release and targeted drug delivery systems. There is a need for more research into nanovaccines and nanosensors to combat pathogenic viruses, especially human and animal CoVs. Throughout history, many people have died as a result of numerous epidemics and pandemics. Meanwhile, crisis management power could be highly effective in managing current and future outbreaks by utilizing new solutions, intelligent management, and intelligent and modern technology, notably upcoming nanotechnologies. The major obstacles for medication researchers include global spread and the high fatality rate of coronaviruses. Many nanobased platforms for an antiviral vaccine have been approved by drug and food organizations, as well as other administrations; therefore, the development of such vaccines using nanotechnology for COVID-19 is extremely advantageous. Hollow nanomaterials are used with different mechanisms for promising antiviral activities due to their different characteristics, such as surface charges, surface area, and size, and make them an excellent tool for viral diagnostics and treatment. The continued danger of acute respiratory diseases such as MERS-CoV needs the development of a strong and safe vaccination approach based on innovative vaccine technology and efficient preventative measures.
Coronaviruses are classified as alpha, beta, gamma, delta, or omicron and have a variety of genetic architectures. People and animals are more affected by alpha- and beta-coronaviruses, which induce gastroenteritis in animals and respiratory problems in humans. Wide surface area, high electrical conductivity, and high carrier mobility have all been used to produce biosensors for the detection of viruses, including human immunodeficiency virus, influenza, and other viral infections. These tools may swiftly and reliably examine a patient's physiological condition. Rapid and portable SARS-CoV-2 diagnostic testing may be possible with biosensors and other hollow nanomaterial-based detection approaches. SARSCoV- 2 identification using RT–PCR is a vital step in COVID-19 management.
However, there are some disadvantages to this method, including poor extraction effectiveness, a long operation, and false positives owing to contamination. Hollow nanomaterials have been employed to build viral detection technologies due to their huge surface area and ultrasmall size. Metal nanoparticles, silica nanoparticles, QDs, polymeric nanoparticles, and carbon nanotubes are among the hollow nanomaterials that have been studied for their possible application in virus detection. Calorimetric, electrochemical, fluorescent, and optical techniques make up the bulk of these methods. Chemistry, molecular biology, material science, and biotechnology are all combined in hollow nanomaterial biosensors. They provide biosensors such high sensitivity that they can currently identify as few as a parasite per microliter of blood. Instead of RT–PCR, hollow nanomaterial-based techniques provide speedy and effective viral detection.
Hollow nanomaterials have antiviral biomedical applications due to their unique physical and chemical properties, such as superior biocompatibility, small sizes, good stability, unique structures, bioavailability, time-release control, large surface-to-volume ratios, and tunable surface load to encapsulate various drug types and improve solubility. Synthetic hollow nanoparticles have been shown to significantly improve vaccination safety and effectiveness. Antiviral coating materials are expected to include metal oxide nanostructures, titanium dioxide, carbon QDs, carbon nanotubes, and graphene bionanoparticles comprised of silicon, gold, graphene, and chitosan-capped silver nanoparticles. According to specialists, the coronavirus should be able to persist on varied surfaces for tens of hours to seven days. Face masks are comprised of nonwoven fabrics to minimize airborne transmission; an antiviral/antimicrobial coating adds another layer of protection against aggressive respiratory viruses. Because uncoated respiratory masks were shown to be the most infectious in studies of viral infectivity on coated and uncoated respiratory masks, a hybrid silica/silver nanocluster coating was sputter coated onto the top surface of disposable face masks to reduce SARS-CoV-2 virus transmission.
There are several potential for future studies based on the existing understanding of antiviral coating materials and prospective nanocoatings for stopping the transmission of the transferrable SARS-CoV-2 virus in response to the worldwide SARS-CoV-2 outbreak [47]. Surface contamination is currently removed using traditional disinfecting cleaning procedures; however, studies reveal that disinfection only gives short relief. Antiviral coatings have been shown to be extremely effective, but more research is clearly required [200]. New surface coating solutions based on current research and commercial goods can be produced when the world is upheaval, and it is unrealistic to supply a single solution for all sorts of surfaces [201].
Ionic liquids are fascinating chemical substances with applications in a wide range of fields. Ecologists, biochemists, and medical experts have paid close attention to the chemical and physical features of ionic liquids, as well as their remarkable biological activity [202]. The development and function of many drug carriers have been demonstrated to be influenced by ionic interactions [203]. We recommend using ionic liquid complexes for drug production and drug delivery systems because of their high loading efficiency, preservation of biomolecule activity, and simplicity of manufacture [204].
Compliance with Ethics Requirement
This article does not contain any studies with human or animal subjects.
Data Availability Statement
This work is a review article, and as such, readers will need to contact referenced authors to obtain additional information regarding the data presented.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Joshi VG, Dighe VD, Thakuria D, Malik YS, Kumar S. Multiple antigenic peptide (MAP): a synthetic peptide dendrimer for diagnostic, antiviral and vaccine strategies for emerging and re-emerging viral diseases. Indian Journal of Virology. 2013; 24: 312-320.
- Abbasi BH, Nazir M, Muhammad W, Hashmi SS, Abbasi R, Rahman L, et al. A Comparative Evaluation of the Antiproliferative Activity against HepG2 Liver Carcinoma Cells of Plant-Derived Silver Nanoparticles from Basil Extracts with Contrasting Anthocyanin Contents. Biomolecules. 2019; 9: 320.
- Hayat SMG, Darroudi M. Nanovaccine: A novel approach in immunization. Journal of Cellular Physiology. 2019; 234: 12530-12536.
- Yang X, Tian Z, Guo K, Lu T, Ji J, Hao S, et al. Preparation and mechanism of hydroxyapatite hollow microspheres with different surface charge by biomimetic method. Journal of Materials Science: Materials in Medicine. 2020; 31: 1-9.
- Nikaeen G, Abbaszadeh S, Yousefinejad S. Application of nanomaterials in treatment, anti-infection and detection of coronaviruses. Nanomedicine. 2020; 15: 1501-1512.
- Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta- Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects. Journal of Nanobiotechnology. 2018; 16.
- Ding J, Venkatesan R, Zhai Z, Muhammad W, Nakkala JR, Gao C. Microand nanoparticles-based immunoregulation of macrophages for tissue repair and regeneration. Colloids and surfaces. B, Biointerfaces. 2020; 192: 111075.
- Ogden N, P Abdelmalik, J Pulliam. Emerging Infections: Emerging infectious diseases: prediction and detection. Canada Communicable Disease Report. 2017; 43: 206-211.
- Cojocaru F, Botezat D, Gardikiotis I, Uritu C, Dodi G, Trandafir L, et al. Nanomaterials Designed for Antiviral Drug Delivery Transport across Biological Barriers. Pharmaceutics. 2020; 12: 171.
- Kanellos T, Sylvester ID, Howard CR, Russell PH. DNA is as effective as protein at inducing antibody in fish. Vaccine. 1999; 17: 965-972.
- Shin MD, Shukla S, Chung YH, Beiss V, Chan SK, Ortega-Rivera OA, et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nature Nanotechnology. 2020; 15: 646-655.
- Singh A, Misra R, Mohanty C, Sahoo SK. Applications of nanotechnology in vaccine delivery. International Journal of Green Nanotechnology: Biomedicine. 2010; 2: B25-B45.
- Vijayan V, Mohapatra A, Uthaman S, Park I. Recent Advances in Nanovaccines Using Biomimetic Immunomodulatory Materials. Pharmaceutics. 2019; 11: 534.
- Lin LC, Huang C, Yao B, Lin J, Agrawal A, Algaissi A, et al. Viromimetic STING Agonist-Loaded Hollow Polymeric Nanoparticles for Safe and Effective Vaccination against Middle East Respiratory Syndrome Coronavirus. Advanced Functional Materials. 2019; 29: 1807616.
- Lin LC, Huang C, Yao B, Lin J, Agrawal A, Algaissi A, et al. Viromimetic STING Agonist-Loaded Hollow Polymeric Nanoparticles for Safe and Effective Vaccination against Middle East Respiratory Syndrome Coronavirus. Advanced Functional Materials. 2019; 29: 1807616.
- Hasanzadeh A, Alamdaran M, Ahmadi S, Nourizadeh H, Bagherzadeh MA, Jahromi MAM, et al. Nanotechnology against COVID-19: Immunization, diagnostic and therapeutic studies. Journal of Controlled Release. 2021; 336: 354-374.
- Ranjbar S, Fatahi Y, Atyabi F. The quest for a better fight: How can nanomaterials address the current therapeutic and diagnostic obstacles in the fight against COVID-19?. Journal of Drug Delivery Science and Technology. 2021; 67: 102899.
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet (London, England). 2020; 395: 565-574.
- Organization WH. WHO-convened global study of origins of SARS-CoV-2: China part. 2021.
- Li Q, Med M, Guan X, Wu P, Wang X, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. New England journal of medicine. 2020; 382: 1199-1207.
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science (New York, N.y.). 2020; 367: 1260-1263.
- Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nature Medicine. 2020; 26: 450-452.
- Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. Journal of Virology. 2020; 94.
- Zhang J, Xie B, Hashimoto K. Current status of potential therapeutic candidates for the COVID-19 crisis. Brain, Behavior, and Immunity. 2020; 87: 59-73.
- Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nature Microbiology. 2020; 5: 562-569.
- Wan Y. An analysis based on decade-long structural studies of SARS 3. JVI. J Virol. 2020; 94: 1-9.
- Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020; 181: 281-292.
- Berry JD, Jones S, Drebot MA, Andonov A, Sabara M, Yuan XY, et al. Development and characterisation of neutralising monoclonal antibody to the SARS-coronavirus. Journal of Virological Methods. 2004; 120: 87-96.
- Chacón-Torres JC, Reinoso C, Navas-León DG, Briceño S, González G. Optimized and scalable synthesis of magnetic nanoparticles for RNA extraction in response to developing countries' needs in the detection and control of SARS-CoV-2. Scientific Reports. 2020; 10.
- Gorshkov K, Susumu K, Chen J, Xu M, Pradhan M, Zhu W, et al. Quantum Dot-Conjugated SARS-CoV-2 Spike Pseudo-Virions Enable Tracking of Angiotensin Converting Enzyme 2 Binding and Endocytosis. ACS Nano. 2020; 14: 12234-12247.
- Samson R, Navale GR, Dharne MS. Biosensors: frontiers in rapid detection of COVID-19. 3 Biotech. 2020; 10.
- Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY). 2020; 12: 6049-6057.
- Winichakoon P, Chaiwarith R, Liwsrisakun C, Salee P, Goonna A, Limsukon A, et al. Negative Nasopharyngeal and Oropharyngeal Swabs Do Not Rule Out COVID-19. Journal of Clinical Microbiology. 2020; 58.
- Pan Y, Li X, Yang G, Fan J, Tang Y, Zhao J, et al. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients. The Journal of Infection. 2020; 81: e28-e32.
- Lin D, Liu L, Zhang M, Hu Y, Yang Q, Guo J, et al. Evaluations of the serological test in the diagnosis of 2019 novel coronavirus (SARS-CoV-2) infections during the COVID-19 outbreak. European Journal of Clinical Microbiology & Infectious Diseases. 2020; 39: 2271-2277.
- Lipsitch M, Swerdlow DL, Finelli L. Defining the Epidemiology of Covid-19 - Studies Needed. The New England journal of medicine. 2020; 382: 1194- 1196.
- Okba NMA, Müller MA, Li W, Wang C, GeurtsvanKessel CH, Corman VM, et al. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients. Emerging Infectious Diseases. 2020; 26: 1478-1488.
- Shen, Z., et al., Genomic diversity of severe acute respiratory syndrome– coronavirus 2 in patients with coronavirus disease 2019. Clinical infectious diseases. 2020; 71: 713-720.
- Moitra P, Alafeef M, Dighe K, Frieman MB, Pan D. Selective Naked-Eye Detection of SARS-CoV-2 Mediated by N Gene Targeted Antisense Oligonucleotide Capped Plasmonic Nanoparticles. ACS Nano. 2020; 14: 7617-7627.
- Yan S, et al. New strategy for COVID-19: an evolutionary role for RGD motif in SARS-CoV-2 and potential inhibitors for virus infection. Frontiers in Pharmacology. 2020; 11: 912.
- Smith YR, et al. Anodic functionalization of titania nanotube arrays for the electrochemical detection of tuberculosis biomarker vapors. 2015; 163: B83.
- Hematian A, Sadeghifard N, Mohebi R, Taherikalani M, Nasrolahi A, Amraei M, et al. Traditional and Modern Cell Culture in Virus Diagnosis. Osong Public Health and Research Perspectives. 2016; 7: 77-82.
- Azmi A, Azman AA, Ibrahim S, Yunus MA Md. Techniques in Advancing the Capabilities of Various Nitrate Detection Methods: A Review. International Journal on Smart Sensing & Intelligent Systems. 2017; 10: 223-261.
- Vadlamani BS, Uppal T, Verma SC, Misra M. Functionalized TiO2 nanotubebased electrochemical biosensor for rapid detection of SARS-CoV-2. Sensors. MDPI. 2020; 20: 5871.
- Kumar N, Shetti NP, Jagannath S, Aminabhavi TM. Electrochemical sensors for the detection of SARS-CoV-2 virus. Chemical Engineering Journal. 2022; 430: 132966.
- Farzin L, Shamsipur M, Samandari L, Sheibani S. HIV biosensors for early diagnosis of infection: The intertwine of nanotechnology with sensing strategies. Talanta. 2020; 206: 120201.
- Seo G, Lee G, Kim MJ, Baek S, Choi M, Ku KB, et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano. 2020; 14: 5135-5142.
- Ahmadivand A, Gerislioglu B, Ramezani Z, Kaushik A, Manickam P, Ghoreishi SA. Functionalized terahertz plasmonic metasensors: Femtomolar-level detection of SARS-CoV-2 spike proteins. Biosensors & Bioelectronics. 2021; 177: 112971.
- Azzi L, Carcano G, Gianfagna F, Grossi P, Gasperina DD, Genoni A, et al. Saliva is a reliable tool to detect SARS-CoV-2. The Journal of Infection. 2020; 81: e45-e50.
- Kevadiya BD, Machhi J, Herskovitz J, Oleynikov MD, Blomberg WR, Bajwa N, et al. Diagnostics for SARS-CoV-2 infections. Nature Materials. 2021; 20: 593-605.
- Fruncillo S, Su X, Liu H, Wong LS. Lithographic Processes for the Scalable Fabrication of Micro- and Nanostructures for Biochips and Biosensors. ACS Sensors. 2021; 6: 2002-2024.
- Shan B, Broza YY, Li W, Wang Y, Wu S, Liu Z, et al. Multiplexed Nanomaterial-Based Sensor Array for Detection of COVID-19 in Exhaled Breath. ACS Nano. 2020.
- Cheng N, Chen D, Lou B, Fu J, Wang H. A biosensing method for the direct serological detection of liver diseases by integrating a SERS-based sensor and a CNN classifier. Biosensors & bioelectronics. 2021; 186: 113246.
- Abduljalil JM. Laboratory diagnosis of SARS-CoV-2: available approaches and limitations. New Microbes and New Infections. 2020; 36: 100713.
- Kevadiya BD, Machhi J, Herskovitz J, Oleynikov MD, Blomberg WR, Bajwa N, et al. Diagnostics for SARS-CoV-2 infections. Nature Materials. 2021; 20: 593-605.
- Shrotri M, Schalkwyk MCIV, Post N, Eddy D, Huntley C, Leeman D, et al. T cell response to SARS-CoV-2 infection in humans: A systematic review. PLoS ONE. 2021; 16: e0245532.
- Zwirglmaier K, Weyh M, Krüger C, Ehmann R, Müller K, Wölfel R, et al. Rapid detection of SARS-CoV-2 by pulse-controlled amplification (PCA). Journal of Virological Methods. 2021; 290: 114083.
- Infantino, M., et al., Serological assays for SARS-CoV-2 infectious disease: benefits, limitations and perspectives. Isr Med Assoc J. 2020; 22: 203-210.
- Dheda K, Ruhwald M, Theron G, Peter JC, Yam W. Point-of-care diagnosis of tuberculosis: Past, present and future. Respirology. 2013; 18: 217-232.
- Shen M, Zhou Y, Ye J, AL-maskri AAA, Kang Y, Zeng S, et al. Recent advances and perspectives of nucleic acid detection for coronavirus. Journal of Pharmaceutical Analysis. 2020; 10: 97-101.
- Qin L, Yang Y, Cao Q, Cheng Z, Wang X, Sun Q, et al. A predictive model and scoring system combining clinical and CT characteristics for the diagnosis of COVID-19. European Radiology. 2020; 30: 6797-6807.
- Xu Y, Cheng M, Chen X, Zhu J. Current approaches in laboratory testing for SARS-CoV-2. International Journal of Infectious Diseases. 2020; 100: 7-9.
- D'Cruz RJ, AW Currier, VB Sampson. Laboratory testing methods for novel severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). Frontiers in cell and developmental biology. 2020; 8: 468.
- Qi J, Lai X, Wang J, Tang H, Ren H, Yang Y, et al. Multi-shelled hollow micro-/nanostructures. Chemical Society reviews. 2015; 44: 6749-6773.
- Long L, Liu X, Chen L, Li D, Jia J. A hollow CuOx/NiOy nanocomposite for amperometric and non-enzymatic sensing of glucose and hydrogen peroxide. Microchimica Acta. 2019; 186: 1-11.
- He G, Tian L, Cai Y, Wu S, Su Y, Yan H, et al. Sensitive Nonenzymatic Electrochemical Glucose Detection Based on Hollow Porous NiO. Nanoscale Research Letters. 2018; 13.
- Abunahla H, Mohammad B, Alazzam A, Jaoude MA, Al-Qutayri M, Hadi SA, et al. MOMSense: Metal-Oxide-Metal Elementary Glucose Sensor. Scientific Reports. 2019; 9.
- Zhang J, Sun Y, Li X, Xu J. Fabrication of porous NiMn2O4 nanosheet arrays on nickel foam as an advanced sensor material for non-enzymatic glucose detection. Scientific Reports. 2019; 9.
- Qian J, Wang Y, Pan J, Chen Z, Wang C, et al. Non-enzymatic glucose sensor based on ZnO–CeO2 whiskers. Materials Chemistry and Physics. 2020; 239: 122051.
- Gandhi M, Yokoe DS, Havlir DV. Asymptomatic Transmission, the Achilles’ Heel of Current Strategies to Control Covid-19. The New England Journal of Medicine. 2020; 382: 2158-2160.
- Muti M, Sharma S, Erdem A, Papakonstantinou P. Electrochemical Monitoring of Nucleic Acid Hybridization by Single-Use Graphene Oxide- Based Sensor. Electroanalysis. 2011; 23: 272-279.
- Bi S, Zhao T, Luo B. A graphene oxide platform for the assay of biomolecules based on chemiluminescence resonance energy transfer. Chemical communications. 2012; 48: 106-108.
- Liu F, Xiang G, Zhang L, Jiang D, Liu L, et al. A novel label free long non-coding RNA electrochemical biosensor based on green L-cysteine electrodeposition and Au–Rh hollow nanospheres as tags. RSC advances. 2015; 5: 51990-51999.
- Liu X, Cheng Z, Fan H, Ai S, Han R, et al. Electrochemical detection of avian influenza virus H5N1 gene sequence using a DNA aptamer immobilized onto a hybrid nanomaterial-modified electrode. Electrochimica Acta. 2011; 56: 6266-6270.
- Low SS, Tan MTT, Loh H, Khiew PS, Chiu WS. Facile hydrothermal growth graphene/ZnO nanocomposite for development of enhanced biosensor. Analytica chimica acta. 2016; 903: 131-141.
- Loczechin A, Séron K, Barras A, Giovanelli E, Belouzard S, Chen Y, et al. Functional Carbon Quantum Dots as Medical Countermeasures to Human Coronavirus. ACS Applied Materials & Interfaces. 2019; 11: 42964-42974.
- Mao X, Liu S, Yang C, Liu F, Wang K, Chen G. Colorimetric detection of hepatitis B virus (HBV) DNA based on DNA-templated copper nanoclusters. Analytica chimica acta. 2016; 909: 101-108.
- Chen X, Xie H, Seow ZY, Gao Z. An ultrasensitive DNA biosensor based on enzyme-catalyzed deposition of cupric hexacyanoferrate nanoparticles. Biosensors & bioelectronics. 2010; 25: 1420-1426.
- Tsang N, Chang K, Lin S, Hao S, Tseng C, Kuo T, et al. Detection of Epstein-Barr Virus–Derived Latent Membrane Protein-1 Gene in Various Head and Neck Cancers: Is It Specific for Nasopharyngeal Carcinoma?. The Laryngoscope. 2003; 113: 1050-1054.
- Riccò R, Meneghello A, Enrichi F. Signal enhancement in DNA microarray using dye doped silica nanoparticles: application to human papilloma virus (HPV) detection. Biosensors & bioelectronics. 2011; 26: 2761-2765.
- Tsang M, Ye W, Wang G, Li J, Yang M, Hao J. Ultrasensitive Detection of Ebola Virus Oligonucleotide Based on Upconversion Nanoprobe/ Nanoporous Membrane System. ACS nano. 2016; 10: 598-605.
- Chen C, Lai Z, Wang G, Wu C. Polymerase chain reaction-free detection of hepatitis B virus DNA using a nanostructured impedance biosensor. Biosensors & bioelectronics. 2016; 77: 603-608.
- Hyeon T, Y Piao, YI Park. Method of preparing iron oxide nanoparticles coated with hydrophilic material, and magnetic resonance imaging contrast agent using the same. 2016, Google Patents.
- Mashhadizadeh MH, RP Talemi. A highly sensitive and selective hepatitis B DNA biosensor using gold nanoparticle electrodeposition on an Au electrode and mercaptobenzaldehyde. Analytical Methods. 2014; 6: 8956-8964.
- Ma C, Xie G, Zhang, W, Liang M, Liu B, et al. Label-free sandwich type of immunosensor for hepatitis C virus core antigen based on the use of gold nanoparticles on a nanostructured metal oxide surface. Microchimica Acta. 2012; 178: 331-340.
- Sabela M, Balme S, Bechelany M, Janot JM, Bisetty K. A review of gold and silver nanoparticle-based colorimetric sensing assays. Advanced Engineering Materials. 2017; 19: 1700270.
- Yu K, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nature Biomedical Engineering. 2018; 2: 719-731.
- Castillo-Henríquez L, Brenes-Acuña M, Castro-Rojas A, Cordero-Salmerón R, Lopretti-Correa M, Vega-Baudrit JR. Biosensors for the Detection of Bacterial and Viral Clinical Pathogens. Sensors (Basel, Switzerland). 2020; 20: 6926.
- Rosén T, Hsiao BS, Söderberg LD. Elucidating the Opportunities and Challenges for Nanocellulose Spinning. Advanced Materials. 2020; 33: 2001238.
- Yockell-Lelièvre H, Bukar N, Toulouse JL, Pelletier JN, Masson J. Nakedeye nanobiosensor for therapeutic drug monitoring of methotrexate. The Analyst. 2016; 141: 697-703.
- Jianrong C, Yuqing M, Nongyue H, Xiaohua W, Sijiao L. Nanotechnology and biosensors. Biotechnology advances. 2004; 22: 505-518.
- Socorro-Leránoz AB, Santano D, Villar I Del, Matias IRl. Trends in the design of wavelength-based optical fibre biosensors (2008–2018). Biosensors and Bioelectronics: X. 2019; 1: 100015.
- Mishra, R.K. and R. Rajakumari, Chapter 1 - Nanobiosensors for Biomedical Application: Present and Future Prospects, in Characterization and Biology of Nanomaterials for Drug Delivery, S.S. Mohapatra, et al., Editors. 2019, Elsevier. 1-23.
- Fu Z, Lu Y, Lai JJ. Recent Advances in Biosensors for Nucleic Acid and Exosome Detection. Chonnam Medical Journal. 2019; 55: 86.
- Veselinovic J, AlMashtoub S, Nagella S, Seker E. Interplay of Effective Surface Area, Mass Transport, and Electrochemical Features in Nanoporous Nucleic Acid Sensors. Analytical chemistry. 2020.
- Park CS, Lee C, Kwon OS. Conducting Polymer Based Nanobiosensors. Polymers. 2016; 8: 249.
- Chamorro-Garcia A, Merkoçi A. Nanobiosensors in diagnostics. Nanobiomedicine. 2016; 3: 184954351666357.
- Harvey JD, Baker HA, Ortiz MV, Kentsis A, Heller DA. HIV Detection via a Carbon Nanotube RNA Sensor. ACS sensors. 2019; 4: 1236-1244.
- Lin Z, Wu G, Zhao L, Lai K. Carbon nanomaterial-based biosensors: a review of design and applications. 2019; 13: 4-14.
- Pirzada M, Altintas Z. Nanomaterials for Healthcare Biosensing Applications. Sensors (Basel, Switzerland). 2019; 19: 5311.
- Shetti NP, Bukkitgar SD, Reddy KR, Reddy CV, Aminabhavi TM. ZnO-based nanostructured electrodes for electrochemical sensors and biosensors in biomedical applications. Biosensors & bioelectronics. 2019; 141: 111417.
- Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. The Lancet. Microbe. 2021; 2: e13-e22.
- Shetti NP, Malode SJ, Nayak DS, Aminabhavi TM, Reddy KR. Nanostructured silver doped TiO2/CNTs hybrid as an efficient electrochemical sensor for detection of anti-inflammatory drug, cetirizine. Microchemical Journal. 2019; 150: 104124.
- Shetti NP, Nayak DS, Malode SJ, Kakarla RR, Shukla SS, Aminabhavi TM. Sensors based on ruthenium-doped TiO2 nanoparticles loaded into multiwalled carbon nanotubes for the detection of flufenamic acid and mefenamic acid. Analytica chimica acta. 2019;1051:58-72.
- Kumar S, Bukkitgar SD, Singh S, Pratibha, Singh V, et al. Electrochemical Sensors and Biosensors Based on Graphene Functionalized with Metal Oxide Nanostructures for Healthcare Applications. Chemistry Select. 2019; 4: 5322-5337.
- Yoon S, H-K Kim. Cost-effective stretchable Ag nanoparticles electrodes fabrication by screen printing for wearable strain sensors. Surface and Coatings Technology. 2020; 384: 125308.
- Guo W, K Shim, Y-T Kim. Ag layer deposited on Zn by physical vapor deposition with enhanced CO selectivity for electrochemical CO2 reduction. Applied Surface Science. 2020; 526: 146651.
- Shetti NP, Malode SJ, Bukkitgar SD, Bagihalli GB, Kulkarni RM, et al. Electro-oxidation and determination of nimesulide at nanosilica modified sensor. Materials Science for Energy Technologies. 2019; 2: 396-400.
- Teng Z, Li W, Tang Y, Elzatahry A, Lu G, Zhao D. Mesoporous Organosilica Hollow Nanoparticles: Synthesis and Applications. Advanced Materials. 2018; 31: 1707612.
- Tamayo-Velasco, Peñarrubia-Ponce MJ, álvarez FJ, Gonzalo-Benito H, Fuente IDL, Martín-Fernández M, et al. Evaluation of Cytokines as Robust Diagnostic Biomarkers for COVID-19 Detection. Journal of Personalized Medicine. 2021; 11: 681.
- Lee T, Park SY, Jang H, Kim G, Lee Y, Park C, et al. Fabrication of electrochemical biosensor consisted of multi-functional DNA structure/ porous au nanoparticle for avian influenza virus (H5N1) in chicken serum. Materials science & engineering. C, Materials for biological applications. 2019; 99: 511-519.
- Shariati M, Ghorbani M, Sasanpour P, Karimizefreh A. An ultrasensitive label free human papilloma virus DNA biosensor using gold nanotubes based on nanoporous polycarbonate in electrical alignment. Analytica chimica acta. 2019; 1048: 31-41.
- Wang J. Electrochemical biosensing based on noble metal nanoparticles. Microchimica Acta. 2012; 177: 245-270.
- Zhang Y, Li B, Wei X, Gu Q, Chen M, Zhang J, et al. Amplified electrochemical antibiotic aptasensing based on electrochemically deposited AuNPs coordinated with PEI-functionalized Fe-based metal-organic framework. Microchimica Acta. 2021; 188: 1-11.
- Masud MK, Umer M, Hossain MSA, Yamauchi Y, Nguyen N, Shiddiky MJA. Nanoarchitecture Frameworks for Electrochemical miRNA Detection. Trends in biochemical sciences. 2019; 44: 433-452.
- Rezaei B, Ghani M, Shoushtari AM, Rabiee M. Electrochemical biosensors based on nanofibres for cardiac biomarker detection: A comprehensive review. Biosensors & bioelectronics. 2016; 78: 513-523.
- Mahari S, Roberts A, Shahdeo D, Gandhi S, et al. eCovSens-ultrasensitive novel in-house built printed circuit board based electrochemical device for rapid detection of nCovid-19 antigen, a spike protein domain 1 of SARSCoV- 2. BioRxiv, 2020.
- Bhattacharyya D, Smith YR, Mohanty SK, Misra M, et al. Titania nanotube array sensor for electrochemical detection of four predominate tuberculosis volatile biomarkers. Journal of The Electrochemical Society. 2016; 163: B206.
- Smith YR, Bhattacharyya D, Mohanty SK, Misra M, et al. Anodic functionalization of titania nanotube arrays for the electrochemical detection of tuberculosis biomarker vapors. Journal of the Electrochemical Society. 2015; 163: B83.
- Kumar S, Bukkitgar SD, Singh S, Pratibha, Singh V, et al. Electrochemical sensors and biosensors based on graphene functionalized with metal oxide nanostructures for healthcare applications. Chemistry Select. 2019; 4: 5322- 5337.
- Bukkitgar SD, Shetti NP. Fabrication of a TiO2 and clay nanoparticle composite electrode as a sensor. Analytical Methods. 2017; 9: 4387-4393.
- Bukkitgar SD, NP Shetti, RM Kulkarni. Construction of nanoparticles composite sensor for atorvastatin and its determination in pharmaceutical and urine samples. Sensors and Actuators B: Chemical. 2018; 255: 1462- 1470.
- Shikandar D, Shetti NP, Kulkarni RM, Kulkarni SD, et al. Silver-doped titania modified carbon electrode for electrochemical studies of furantril. ECS Journal of Solid State Science and Technology. 2018; 7: Q3215.
- Mishra A, Basu S, Shetti NP, Reddy KR, Aminabhavi TM, et al. Photocatalysis of graphene and carbon nitride-based functional carbon quantum dots, in Nanoscale Materials in Water Purification. 2019; 759-781.
- Mishra A, Shetti NP, Basu S, Reddy KR, Aminabhavi TM. Carbon Clothbased Hybrid Materials as Flexible Electrochemical Supercapacitors. ChemElectroChem. 2019; 6: 5771-5786.
- Singh L, Kruger HG, Maguire GE, Govender T, Parboosing R. The role of nanotechnology in the treatment of viral infections. Therapeutic Advances in Infectious Disease. 2017; 4: 105-131.
- Cho I, Kim DH, Park S. Electrochemical biosensors: perspective on functional nanomaterials for on-site analysis. Biomaterials Research. 2020; 24.
- Zhao F, Bai Y, Cao L, Han G, Fang C, Wei S, et al. New electrochemical DNA sensor based on nanoflowers of Cu3(PO4)2-BSA-GO for hepatitis B virus DNA detection. Journal of Electroanalytical Chemistry. 2020; 867: 114184.
- Khristunova E, Barek J, Kratochvil B, Korotkova E, Dorozhko E, Vyskocil V. Electrochemical immunoassay for the detection of antibodies to tick-borne encephalitis virus by using various types of bioconjugates based on silver nanoparticles. Bioelectrochemistry. 2020; 135: 107576.
- Kwon J, Lee Y, Lee T, Ahn J. Aptamer-based field-effect transistor for detection of avian influenza virus in chicken serum. Analytical chemistry. 2020.
- Kaushik A, Yndart A, Kumar S, Jayant RD, Vashist A, Brown AN, et al. A sensitive electrochemical immunosensor for label-free detection of Zikavirus protein. Scientific Reports. 2018; 8.
- Bandodkar AJ, Imani S, Nuñez-Flores R, Kumar R, Wang C, Mohan AMV, et al. Re-usable electrochemical glucose sensors integrated into a smartphone platform. Biosensors & bioelectronics. 2018; 101: 181-187.
- Walgama C, Nguyen MP, Boatner LM, Richards I, Crooks RM. Hybrid paper and 3D-printed microfluidic device for electrochemical detection of Ag nanoparticle labels. Lab on a chip. 2020; 20: 1648-1657.
- McArdle H, Spain E, Keyes TE, Stallings RL, Fournet MB, et al. Triangular silver nanoplates: Properties and ultrasensitive detection of miRNA. Electrochemistry Communications. 2017; 79: 23-27.
- Sheridan C. Fast, portable tests come online to curb coronavirus pandemic. Nature Biotechnology. 2020; 38: 515-518.
- Li X, Scida K, Crooks RM. Detection of hepatitis B virus DNA with a paper electrochemical sensor. Analytical chemistry. 2015; 87: 9009-9015.
- Zhang X, Pascal T, Graff A, Palivan CG, Meier W, et al. Mimicking the cell membrane with block copolymer membranes. Journal of polymer science part A: polymer chemistry. 2012; 50: 2293-2318.
- Eguilaz MRD, Cumba LR, Forster RJ. Electrochemical detection of viruses and antibodies: A mini review. Electrochemistry Communications. 2020; 116: 106762.
- Dai Y, Liu CC. Recent Advances on Electrochemical Biosensing Strategies toward Universal Point of Care Systems. Angewandte Chemie. 2019; 58: 12355-12368.
- Chen JH, Yip CC, Poon RW, Chan K, Cheng VC, Hung IF, et al. Evaluating the use of posterior oropharyngeal saliva in a point-of-care assay for the detection of SARS-CoV-2. Emerging Microbes & Infections. 2020; 9: 1356- 1359.
- Choi JR. Development of Point-of-Care Biosensors for COVID-19. Frontiers in Chemistry. 2020; 8.
- Lee J, Park S, Choi J. Electrical Property of Graphene and Its Application to Electrochemical Biosensing. Nanomaterials. 2019; 9: 297.
- Chan C, Shi J, Fan Y, Yang M. A microfluidic flow-through chip integrated with reduced graphene oxide transistor for influenza virus gene detection. Sensors and Actuators B: Chemical. 2017; 251: 927-933.
- Ono T, Oe T, Kanai Y, Ikuta, T, Ohno Y, et al. Glycan-functionalized graphene-FETs toward selective detection of human-infectious avian influenza virus. Japanese Journal of Applied Physics. 2017; 56: 030302.
- Seo G, Lee G, Kim MJ, Baek S, Choi M, Ku KB, et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano. 2020; 14: 5135-5142.
- Iravani S. Nano- and biosensors for the detection of SARS-CoV-2: challenges and opportunities. Materials Advances. 2020; 1: 3092-3103.
- Liu J, Chen X, Wang Q, Xiao M, Zhong D, Sun W, et al. Ultrasensitive Monolayer MoS2 Field-Effect Transistor Based DNA Sensors for Screening of Down Syndrome. Nano letters. 2019; 19: 1437-1444.
- Janissen R, Sahoo PK, Santos CA, Silva AMD, Zuben AAGV, Souto DEP, et al. InP Nanowire Biosensor with Tailored Biofunctionalization: Ultrasensitive and Highly Selective Disease Biomarker Detection. Nano letters. 2017; 17: 5938-5949.
- Layqah LA, Eissa S. An electrochemical immunosensor for the corona virus associated with the Middle East respiratory syndrome using an array of gold nanoparticle-modified carbon electrodes. Mikrochimica Acta. 2019; 186.
- Wen F, He T, Liu H, Chen HY, Zhang T, et al. Advances in chemical sensing technology for enabling the next-generation self-sustainable integrated wearable system in the IoT era. Nano Energy. 2020: 105155.
- Ryvolová M, Macka M, Ryvolova M, Preisler J, Macka M. Portable capillarybased (non-chip) capillary electrophoresis. TrAC Trends in Analytical Chemistry. 2010; 29: 339-353.
- Ganganboina AB, Chowdhury AD, Khoris IM, Doong R, Li T, Hara T, et al. Hollow magnetic-fluorescent nanoparticles for dual-modality virus detection. Biosensors & bioelectronics. 2020; 170: 112680.
- Chuong TT, Pallaoro A, Chaves CA, Li Z, Lee J, Eisenstein M, et al. Dualreporter SERS-based biomolecular assay with reduced false-positive signals. Proceedings of the National Academy of Sciences. 2017; 114: 9056-9061.
- Park J, Jeong Y, Kim J, Gu J, Wang J, Park I. Biopsy needle integrated with multi-modal physical/chemical sensor array. Biosensors & bioelectronics. 2019; 148: 111822.
- Wu Z, Zeng T, Guo W, Bai Y, Pang D, Zhang Z. Digital Single Virus Immunoassay for Ultrasensitive Multiplex Avian Influenza Virus Detection Based on Fluorescent Magnetic Multifunctional Nanospheres. ACS applied materials & interfaces. 2019; 11: 5762-5770.
- Shrivastava S, Trung TQ, Lee N. Recent progress, challenges, and prospects of fully integrated mobile and wearable point-of-care testing systems for selftesting. Chemical Society reviews. 2020; 49: 1812-1866.
- Guerrero-Martínez A, Pérez-Juste J, Liz-Marzán LM. Recent Progress on Silica Coating of Nanoparticles and Related Nanomaterials. Advanced Materials. 2010; 22: 1182-1195.
- Chowdhury AD, Ganganboina AB, Tsai Y, Chiu H, Doong R. Multifunctional GQDs-Concanavalin A@Fe3O4 nanocomposites for cancer cells detection and targeted drug delivery. Analytica chimica acta. 2018; 1027: 109-120.
- Song L, Wang Z, Liu J, Wang T, Jiang Q, Ding B. Tumor-Targeted DNA Bipyramid for in Vivo Dual-Modality Imaging. ACS applied bio materials. 2020; 3: 2854-2860.
- Wang R, Lin J, Lassiter K, Srinivasan B, Lin L, Lu H, et al. Evaluation study of a portable impedance biosensor for detection of avian influenza virus. Journal of virological methods. 2011; 178: 52-58.
- Lake RJ, Yang Z, Zhang J, Lu Y. DNAzymes as Activity-Based Sensors for Metal Ions: Recent Applications, Demonstrated Advantages, Current Challenges, and Future Directions. Accounts of chemical research. 2019; 52: 3275-3286.
- Medina CB, Mehrotra P, Arandjelovic S, Perry JSA, Guo Y, Morioka S, et al. Metabolites released from apoptotic cells act as novel tissue messengers. Nature. 2020; 580: 130-135.
- Takemura K, Satoh J, Boonyakida J, Park S, Chowdhury AD, Park EY. Electrochemical detection of white spot syndrome virus with a silicone rubber disposable electrode composed of graphene quantum dots and gold nanoparticle-embedded polyaniline nanowires. Journal of Nanobiotechnology. 2020; 18.
- Nasrin F, Chowdhury AD, Takemura K, Kozaki I, Honda H, Adegoke O, et al. Fluorometric virus detection platform using quantum dots-gold nanocomposites optimizing the linker length variation. Analytica chimica acta. 2020; 1109: 148-157.
- Chowdhury AD, Takemura K, Li T, Suzuki T, Park EY. Electrical pulseinduced electrochemical biosensor for hepatitis E virus detection. Nature Communications. 2019; 10.
- Zhou H, Zhang J, Li B, Liu J, Xu J, Chen H. Dual-Mode SERS and Electrochemical Detection of miRNA Based on Popcorn-like Gold Nanofilms and Toehold-Mediated Strand Displacement Amplification Reaction. Analytical chemistry. 2021.
- Sathiyamoorthy K, VK Mohankumar, VM Murukeshan. Real time monitoring of fluorescent particles in micro-channels by high resolution dual modality probe imaging. Optics and Photonics Journal. 2011; 1: 197-203.
- Ali MA, Tabassum S, Wang Q, Wang Y, Kumar R, Dong L. Integrated dual-modality microfluidic sensor for biomarker detection using lithographic plasmonic crystal. Lab on a chip. 2018; 18: 803-817.
- Zhuang J, Yin J, Lv S, Wang B, Mu Y. Advanced “lab-on-a-chip” to detect viruses – Current challenges and future perspectives. Biosensors & Bioelectronics. 2020; 163: 112291.
- Qiu G, Gai Z, Tao Y, Schmitt J, Kullak-Ublick GA, Wang J. Dual-Functional Plasmonic Photothermal Biosensors for Highly Accurate Severe Acute Respiratory Syndrome Coronavirus 2 Detection. ACS Nano. 2020; 14: 5268- 5277.
- Damborský P, Švitel J, Katrlík J. Optical biosensors. Essays in Biochemistry. 2016; 60: 91-100.
- Takemura K. Surface Plasmon Resonance (SPR)- and Localized SPR (LSPR)-Based Virus Sensing Systems: Optical Vibration of Nano- and Micro-Metallic Materials for the Development of Next-Generation Virus Detection Technology. Biosensors. 2021; 11: 250.
- Nag P, K Sadani, S Mukherji. Optical fiber sensors for rapid screening of COVID-19. Transactions of the Indian National Academy of Engineering. 2020; 5: 233-236.
- Socorro-Leránoz AB, Santano D, Villar ID, Matias IR. Trends in the design of wavelength-based optical fibre biosensors (2008–2018). Biosensors and Bioelectronics: X. 2019; 1: 100015.
- Roh C, Jo SK. Quantitative and sensitive detection of SARS coronavirus nucleocapsid protein using quantum dots-conjugated RNA aptamer on chip. Journal of Chemical Technology and Biotechnology. 2011; 86: 1475-1479.
- Ye H, Liu Y, Zhan L, Liu Y, Qin Z. Signal amplification and quantification on lateral flow assays by laser excitation of plasmonic nanomaterials. Theranostics. 2020; 10: 4359-4373.
- Lung, J., et al., The potential chemical structure of anti-SARS-CoV-2 RNAdependent RNA polymerase. Journal of Medical Virology. 2020; 92: 693- 697.
- Yu R, Chen L, Lan R, Shen R, Li P. Computational screening of antagonists against the SARS-CoV-2 (COVID-19) coronavirus by molecular docking. International Journal of Antimicrobial Agents. 2020; 56: 106012.
- Jauffred L, Samadi A, Klingberg H, Bendix PM, Oddershede LB. Plasmonic Heating of Nanostructures. Chemical reviews. 2019; 119: 8087-8130.
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020; 25.
- Khalid K, Tan X, Zaid HFM, Tao Y, Chew CL, Chu D, et al. Advanced in developmental organic and inorganic nanomaterial: a review. Bioengineered. 2020; 11: 328-355.
- Bruinink A, Wang J, Wick P. Effect of particle agglomeration in nanotoxicology. Archives of Toxicology. 2015; 89: 659-675.
- Cao J, Sun T, Grattan KT. Gold nanorod-based localized surface plasmon resonance biosensors: A review. Sensors and actuators B: Chemical. 2014; 195: 332-351.
- Zhang L, Mazouzi Y, Salmain M, Liedberg B, Boujday S. Antibody-Gold Nanoparticle Bioconjugates for Biosensors: Synthesis, Characterization and Selected Applications. Biosensors & bioelectronics. 2020; 165: 112370.
- Qiu G, Yue Y, Tang J, Zhao Y, Wang J. Total Bioaerosols Detection by a Succinimidyl-Ester-Functionalized Plasmonic Biosensor to Reveal Different Characteristics at Three Locations in Switzerland. Environmental science & technology. 2020.
- Turunc E, Binzet R, Gumus I, Binzet G, Arslan H. Green synthesis of silver and palladium nanoparticles using Lithodora hispidula (Sm.) Griseb. (Boraginaceae) and application to the electrocatalytic reduction of hydrogen peroxide. Materials Chemistry and Physics. 2017; 202: 310-319.
- Baghayeri M, Alinezhad H, Tarahormi M, Fayazi M, Ghanei-Motlagh M, et al. A non-enzymatic hydrogen peroxide sensor based on dendrimer functionalized magnetic graphene oxide decorated with palladium nanoparticles. Applied Surface Science. 2019; 478: 87-93.
- Petryayeva E, Krull UJ. Localized surface plasmon resonance: nanostructures, bioassays and biosensing--a review. Analytica chimica acta. 2011; 706: 8-24.
- Funari R, Chu K, Shen AQ. Detection of antibodies against SARS-CoV-2 spike protein by gold nanospikes in an opto-microfluidic chip. Biosensors & Bioelectronics. 2020; 169: 112578.
- Xia Y, Chen Y, Tang Y, Cheng G, Yu X, He H, et al. A Smartphone-based Point-of-care Microfluidic Platform Fabricated with ZnO Nanorod Template for Colorimetric Virus Detection. ACS sensors. 2019; 4: 3298-3307.
- Verma N, Badhe Y, Gupta R, Maparu A, Rai B. Interactions of Peptide Coated Gold Nanoparticles with Spike Protein of the SARS-CoV-2: A Basis for Design of a Simple and Rapid Detection Tool. 2020.
- Hussein HA, Hassan RYA, Chino M, Febbraio F. Point-of-Care Diagnostics of COVID-19: From Current Work to Future Perspectives. Sensors (Basel, Switzerland). 2020; 20: 4289.
- Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. Viral load of SARS-CoV-2 in clinical samples. The Lancet. Infectious Diseases. 2020;20(4):411-412.
- Chen Z, Zhang Z, Zhai X, Li Y, Lin L, Zhao H, et al. Rapid and Sensitive Detection of anti-SARS-CoV-2 IgG, Using Lanthanide-Doped Nanoparticles- Based Lateral Flow Immunoassay. Analytical Chemistry. 2020.
- Li Z, Yi Y, Luo X, Xiong N, Liu Y, Li S, et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. Journal of Medical Virology. 2020; 92: 1518-1524.
- Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020; 581: 221-224.
- Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Medicine. 2020; 46: 586-590.
- Barnes CO, Jette CA, Abernathy ME, Dam KA, Esswein SR, Gristick HB, et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020; 588: 682-687.
- Thakur AK, Sathyamurthy R, Ramalingam V, Lynch I, Sharshir SW, Ma Z, et al. A case study of SARS-CoV-2 transmission behavior in a severely air-polluted city (Delhi, India) and the potential usage of graphene based materials for filtering air-pollutants and controlling/monitoring the COVID-19 pandemic. Environmental science. Processes & impacts. 2021.
- Celina MC, Martinez E, Omana MA, Sanchez A, Wiemann D, Tezak M, et al. Extended use of face masks during the COVID-19 pandemic - Thermal conditioning and spray-on surface disinfection. Polymer Degradation and Stability. 2020; 179: 109251.
- Rai NK, Ashok A, Akondi BR. Consequences of chemical impact of disinfectants: safe preventive measures against COVID-19. Critical Reviews in Toxicology. 2020; 50: 513-520.
- Egorova KS, Gordeev EG, Ananikov VP. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chemical reviews. 2017; 117: 7132-7189.
- Klyachko NL, Manickam DS, Brynskikh AM, Uglanova SV, Li S, Higginbotham SM, et al. Cross-linked antioxidant nanozymes for improved delivery to CNS. Nanomedicine : nanotechnology, biology, and medicine. 2012; 8: 119-129.
- Yi X, Manickam DS, Brynskikh A, Kabanov AV. Agile delivery of protein therapeutics to CNS. Journal of controlled release : official journal of the Controlled Release Society. 2014; 190: 637-663.