
Research Article
Phys Med Rehabil Int. 2015;2(5): 1048.
Ultrasound Identification of Maximal Subdeltoid Bursa Distention Alters the Ultrasound-Guided Injection Approach for Shoulder Pain
Ramey LN1,2, Knowlton SE1,2, Amorese-O’Connell L³ and Kohler MJ2,4*
1Department of Physical Medicine and Rehabilitation,Spaulding Rehabilitation Hospital, USA
2Harvard Medical School, USA
3Division of Rheumatology, Brown University, USA
4Division of Rheumatology, Allergy and Immunology, Massachusetts General Hospital, USA
*Corresponding author: Kohler MJ, Division of Rheumatology, Allergy and Immunology, Massachusetts General Hospital, USA
Received: April 09, 2015; Accepted: June 01, 2015; Published: June 03, 2015
Abstract
Objective: Landmark-guided injections (LMGIs) of corticosteroid (CS) into the subdeltoid bursa (SDB) are typically provided from a posterolateral approach, aiming at the subacromial aspect of the bursa with varying accuracy and response. Ultrasound (US) can visualize bursal abnormalities and is known to improve injection accuracy. Our objective was to evaluate how US affects the injection approach used for treatment of subacromial impingement.
Methods: We reviewed clinical and US characteristics of 67 patients with impingement syndrome who received 96 SDB US-guided injections (USGIs) in one rheumatology US clinic. Images were obtained and interpreted by 1 musculoskeletal ultrasound (MSUS) trained rheumatologist and reviewed by 2 MSUS trained physiatrists.
Results: Patients were mostly female (62.7%) with a mean age of 63.5±14.7 years and BMI of 28.8±7.02. Twenty-six (38.8%) received prior LMGIs with more than half (61.5%) reporting little to no benefit. USGI approach was determined by localizing the area of maximal SBD thickening and distension on US. USGIs were targeted subacromially in only 40.6% of cases; 59.4% were targeted anterior to the acromion: 37.5% anterior over the subscapularis tendon, 19.8% anterolateral over the supraspinatus tendon, and 2.0% anterior over the biceps tendon. Nearly all patients reported significant improvement immediately post-procedure.
Conclusion: Point-of-care US identified SDB abnormality to occur more frequently anterior to the acromion, rather than below it. This may contribute to LMGI failure, as LMGIs are typically directed at the posterior border of the bursa beneath the acromion. This method has the potential to improve clinical outcomes. Future prospective studies are needed.
Keywords: Subacromial impingement; Subdeltoid bursitis; Ultrasound; Ultrasound-guided injection; Corticosteroid injection
Abbreviations
SDB: Sub Deltoid Bursa; CS: Corticosteroid; LMGI: Landmarkguided Injection; US: Ultrasound; USGI: Ultrasound-guided Injection; MSUS: Musculoskeletal Ultrasound; AMSSM: American Medical Society of Sports Medicine; BMI: Body Mass Index
Introduction
Shoulder pain is a common musculoskeletal complaint seen in sports, orthopedic, rheumatology, and primary care clinics. Subacromial impingement syndrome, defined as subdeltoid bursitis and/or rotator cuff tendinitis, is a common cause of shoulder pain [1-3]. Symptoms are thought to occur because of impingement of the subdeltoid bursa (SDB) and neighboring tendons beneath their rigid overlying structures, including the acromion process, the coracoid process and the coracoacromial ligament, together referred to as the coracoacromial arch.
SDB corticosteroid (CS) injections are widely used to treat shoulder pain due to subacromial impingement unresponsive to more conservative treatments, including analgesics, nonsteroidal anti-inflammatories and physical therapy [4-9]. Injections are traditionally given by palpating known bony landmarks for guidance. These landmark-guided injections (LMGIs) can be given using various approaches. However, based on anecdotal data and technical instructions published in multiple national guidelines, they are traditionally given from a posterolateral approach with medication directed subacromially [7,10]. The American Family Physician guidelines describe a posterolateral approach with needle insertion inferior to the posterolateral edge of the acromion directing the needle toward the opposite nipple [10]. A review by Gruson et al similarly describes the posterolateral approach as being identified 2 cm distal and 1 cm medial to the posterolateral tip of the acromion with the needle angled approximately 45° cephalad [7]. This approach is potentially favored because the bursa is easily accessible from this angle and it provides reproducible, palpable bony landmarks for guidance. Mathews and Glousman described a cadaveric study comparing the accuracy of posterior and anterolateral approaches and found no statistical difference between these techniques [11]. There are no studies, to our knowledge, that compare the outcomes of different LMGI approaches.
Current literature supports the use of SDB CS LMGIs for impingement syndrome [4,5,7,12]. While outcomes have been variable, a number of studies have shown benefit from these injections with respect to pain and range of motion [5,7,12]. Over the past 10 years, point-of-care ultrasound (US) use has been rapidly growing by musculoskeletal specialists for diagnostic and therapeutic purposes. Ultrasound-guided injections (USGIs) are known to be accurate [13,14] and the use of US-guidance for SDB injections has become a common occurrence in clinical practice. US can visualize bursal fluid, bursal thickening, tendinopathy, and tendon tears [15- 17]. It is effective in guiding a needle into the SDB [18]. Research comparing the outcomes of LMGIs versus USGIs of CS into the SDB is limited in quantity and quality, but has shown a trend toward better outcomes following USGIs [14,18-22]. One theory postulates that poor outcomes following LMGIs are due to inaccuracy without direct visualization. US has been shown to improve the accuracy of bursal and intra-articular injections [14]. Only a few studies have directly compared the accuracy of LMGIs and USGIs of the SDB. In a randomized comparison, Naredo et al found that the majority of the LMGIs were inaccurately placed, whereas more than 90% of the USGIs were accurately delivered, with corresponding clinical improvement [20]. The American Medical Society of Sports Medicine (AMSSM) reviewed the data on LMGIs versus USGIs of the SDB and found that while LMGIs had an accuracy of a 81%, USGIs had an accuracy of 100% [14,20,23]. The AMSSM position statement reports that there is strong evidence that USGIs are more accurate than LMGIs of the SDB [14].
In addition to improving accuracy, we believe other factors may contribute to the better outcomes seen following USGIs of the SDB. The use of US may alter the injection approach used in SDB CS injections. US can help by identifying the area of most significant bursal thickening and distension; this allows physicians to localize CS injections to the most affected area of the bursa, potentially improving patient outcomes in comparison to the standard posterolateral approach of LMGIs. Our objective was to evaluate how point-of-care US affects the injection approach used for treatment of subacromial impingement with bursal involvement. We hypothesize that, when injections are directed based on bursal abnormalities seen on US with clinical correlation, SDB CS injections will more commonly be given anterior to the acromion, rather than directly below it, as would be done with LMGIs.
Methods
A retrospective review of the medical records and US images was performed on adult patients who received an USGI of the SDB at one academic rheumatology musculoskeletal ultrasound (MSUS) clinic. Institutional review board approval was obtained prior to chart review and data collection. Records were reviewed from December 2011- July 2014.
A query was performed for patients with an ICD-9 code for shoulder pain who received an USGI using a research patient data repository [24]. These patients were considered for inclusion in this study (n=129). Patients who received an injection at any location other than the SDB were excluded (n=62). The most commonly excluded were injections to the glenohumeral joint and biceps tendon sheath. Patients with both subdeltoid bursitis and biceps tenosynovitis were included in this study if their primary pain generator was attributed to the SDB, thus receiving an USGI of the SDB. Many patients underwent either bilateral injections or repeat injections at a later date. The decision was made to treat each of these injections as an individual case because they were frequently given in the contralateral arm and/or utilizing a different probe position.
Medical records were retrospectively reviewed for patient demographics, including age, body mass index (BMI), coexisting inflammatory conditions, and history of chronic widespread pain. The mean ± standard deviation or frequency of each characteristic was calculated. As is standard practice, patients were clinically evaluated based on history and a standard shoulder examination evaluating for impingement, biceps tenosynovitis and joint involvement prior to US examination. If documented, both the area of primary pain location identified during the history and the area of most significant reproducible tenderness to palpation on examination were noted. These areas were described as occurring primarily at the anterior, anterolateral, lateral, or posterior aspect of the shoulder.
The US evaluation and USGIs were performed or supervised by an expert MSUS-trained rheumatologist (MK). All injections were given using an in-plane needle approach with direct needle visualization throughout the procedure. It is standard practice in this clinic to direct the injection to the area of most significant abnormality (e.g. bursal thickening and fluid distension) identified on US if clinical correlation exists. If there was a discrepancy in these two parameters, bursal fluid distension was selected to take priority.
Images were reviewed by two MSUS-trained physiatry residents (LR, SK) for the following parameters: area of most significant bursal thickening, area of most significant bursal fluid collection, location of tendinopathy (biceps, subscapularis or supraspinatus), and location in which CS injection was given. All images were then reviewed and confirmed by the same MSUS-trained rheumatologist (MK). If there was a discrepancy between the two physiatry reviewers, the rheumatologist made the final interpretation. Intra-rater reliability was 100% for all reviewers on a test sample of 10 images. Inter-rater reliability between the physiatry residents (LR, SK) was 90%, while 100% inter-rater reliability was found between the rheumatologist (MK) and at least one of the physiatrists (LR or SK) for all cases.
To develop a standardized method to identify locations for the above parameters, four common shoulder probe positions were used: 1- anterior over the biceps tendon (Figure 1), 2- anterior over the subscapularis tendon (Figure 2), 3- anterolateral over supraspinatus tendon (Figure 3), and 4- lateral over the supraspinatus tendon (Figure 4). An alternate probe position was used for probe positions 3 and 4 when a patient was unable perform a modified crass position due to pain (Figure 5). A detailed description of each probe position can be found in Table 1.
Probe Position
Arm Position
Probe Position
Location of SDB
Injection Approach
Image
1. Anterior over Biceps
- Shoulder neutral
- Elbow flexed
- Forearm supinated
- Short axis to the long head of the biceps tendon
- Proximally within the bicipital groove
- Directly over the biceps tendon but not contiguous with it
- In-Plane
- Lateral-to-medial OR medial-to lateral
Figure 1
2. Anterior over Subscapularis
- Shoulder externally rotated
- Elbow flexed
- Forearm supinated
- Long axis to the subscapularis tendon
- Insertion site on the lesser tuberosity of the humerus
- Directly overlying the subscapularis tendon
- In-Plane
- Lateral-to-medial OR medial-to lateral
Figure 2
3. Anterolateral over Supraspinatus ("bird’s beak view")
- Modified Crass position, if tolerated
- Alternate position in Figure 5
- Long axis to the supraspinatus tendon
- Anterior to the acromion process
- Insertion site on the greater tuberosity
- Directly over the supraspinatus tendon
- In-Plane
- Lateral-to-medial OR medial-to lateral
Figure 3
4. Lateral over Supraspinatus
("subacromial view")
- Modified Crass position, if tolerated
- Alternate position in Figure 5
- Long axis to the supraspinatus tendon
- Directly beneath the acromion process
- Directly over the supraspinatus tendon and beneath the acromion
- In-Plane
- Lateral-to-medial OR medial-to lateral
Figure 4
Table 1: Description of probe positions.
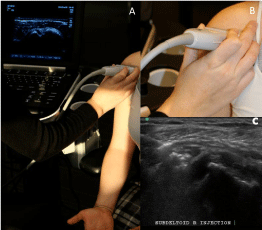
Figure 1: Probe position one: anterior over the biceps tendon. ADemonstration
of arm position, probe position and appearance on US in
probe position one. B- Orientation of the probe in position one. C- US image
of a lateral-to-medial in-plane subdeltoid bursa injection in probe position one.
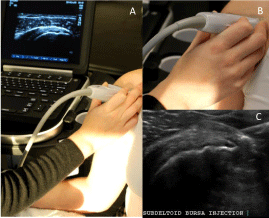
Figure 2: Probe position two: anterior over the subscapularis tendon. ADemonstration
of arm position, probe position and appearance on US in
probe position two. B- Orientation of the probe in position two. C- US image
of a lateral-to-medial in-plane subdeltoid bursa injection in probe position two.
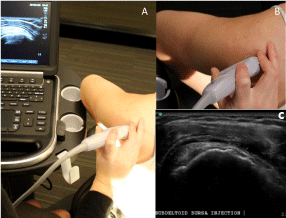
Figure 3: Probe positions three: anterolateral over the supraspinatus tendon
("bird’s beak view"). A- Demonstration of arm position, probe position and
appearance on ultrasound in probe position three. B- Orientation of the probe
in position three. C- US image of a lateral-to-medial in-plane subdeltoid bursa
injection in probe position three.
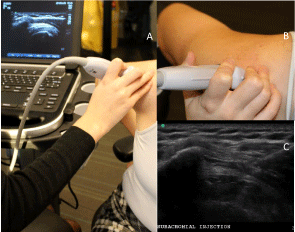
Figure 4: Probe position four: lateral over the supraspinatus tendon
("subacromial view"). A- Demonstration of arm position, probe position and
appearance on ultrasound in probe position four. B- Orientation of the probe
in position four. C- US image of a lateral-to-medial in-plane subdeltoid bursa
injection in probe position four.
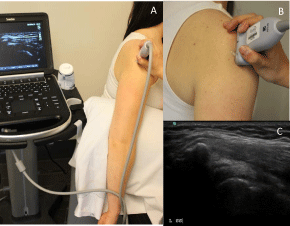
Figure 5: Alternate arm (A) and probe (B) position for position 4 if patient
unable to tolerate modified crass position with resulting US image (C). Similar
modifications were made if patient unable to tolerate modified crass arm
position for probe position 3.
The location of pain or tenderness noted during the history and examination, as described above, was identified as being congruent or non-congruent with the abnormalities seen on US. Symptoms were deemed congruent if: the patient had anterior pain or tenderness and most prominent bursal abnormalities occurred in probe positions 1 or 2; or the patient had anterolateral pain or tenderness and most prominent bursal abnormalities occurred in probe position 3; or the patient had lateral pain or tenderness and most prominent bursal abnormalities occurred in probe position 4 (Table 1). Any other combination of symptoms and bursal abnormalities on US were considered non-congruent. The goal was to identify if patientreported symptoms could reliably be used to identify the area of most significant bursal abnormalities without the need for US. It is important to note that an injection is only performed at this clinic if there is clinical correlation defined as: (1) the patient’s history and exam is consistent with impingement syndrome and (2) there is tenderness to palpation over the region of bursal abnormality during US evaluation that reproduces their symptoms. Immediate post-injection symptomatic improvement was reviewed using the following 5-point scale: 1-provided no relief, 2- provided minimal relief, 3- provided partial relief, 4- provided significant relief, and 5- providing complete relief. Recording immediate post-injection pain is standard practice for this clinic and was done for all cases. In addition, the results of prior LMGIs of the SDB, if documented, were recorded using the same post-injection pain rating scale. No follow-up data regarding the long-term efficacy of these injections was available for review.
Results and Discussion
A total of 96 USGIs of the SDB were performed on 67 patients. All patients were age 18 and older and had US images available for review. Patients were mostly female (62.7%) with a mean age of 63.5±14.7 years and average BMI of 28.8±7.02. Most patients (70.1%) had no coexisting inflammatory condition at the time of injection. Only 7 of the 67 patients (10.4%) had a history of chronic, widespread pain. Twenty-six patients (38.8%) had received a prior LMGI of the SDB and more than half of these patients (61.5%) reported little to no benefit. There was no significant difference in the mean age or BMI in those that responded to LMGIs and those that did not. There was a higher rate of coexisting inflammatory conditions in the responders compared to the non-responders and the entire group, and a higher rate of chronic widespread pain in the non-responders compared to the responders and the entire group. Details regarding patient demographics can be found in Table 2 broken down by LMGIresponders and non-responders, as well as by probe position.
No. of Cases
No. of Patients
BMI (Mean ± Std Dev)
No. of Patients with Coexisting Inflammatory Condition (%)
No. of Patients with Chronic Widespread Pain (%)
All
96
67*
28.8±7.02
20 (29.9%)
7 (10.4%)
Position 1
2
1
32.4±0
0 (0%)
0 (0%)
Position 2
37
28
27.7±8.48
10 (35.7%)
1 (3.6%)
Position 3
19
15
29.4±6.14
5 (33.3%
1 (6.7%)
Position 4
38
30
29.7±5.48
8 (26.7%)
6 (20%)
LMGI Responders
10
10
27.3±3.3
4 (40%)
1 (10%)
LMGI Nonresponders
16
16
29.7±4.8
5 (27.8%)
3 (16.7%)
*While only 67 patients were included in this study, the sum of the number of patients in each position totals 74, as repeat injections were commonly given in a different probe position than the origin injection, so this patient was included in both groups.
Table 2: Patient Demographics.
Probe position 4 (lateral over the supraspinatus) was considered to be equivalent to the traditional posterolateral LMGI approach, given the shared subacromial target. USGIs were given using this approach in only 40.6% of cases. However, 59.4% of USGIs were targeted anterior to the acromion as follows: 37.5% in probe position 2 (anterior over the subscapularis tendon); 19.8% in probe position 3 (anterolateral over the supraspinatus tendon); and 2.0% in probe position 1 (anterior over the biceps tendon).
Review of the US images confirmed that bursal distension and thickening occurred anterior to the acromion process in the majority (59.4%) of cases. Of the 96 cases, there were only 3 cases (3.1%) with discrepancy between the area of most prominent bursal thickening and the area of most prominent bursal fluid collection. This finding supports that while bursal thickening and fluid distension can occur independently, when they occur simultaneously it is nearly always in the same portion of the bursa.
The ability to correlate the location of pain or reproducible tenderness was somewhat limited, as it was not localized in 28 of the 96 cases (29.2%), suggesting difficulty in identifying focal symptoms on clinical exam or lack of documentation. In cases where focal pain or tenderness was documented, these symptoms correlated with the abnormalities seen on US in only 36.8% of cases, and did not correlate in the remaining 63.2% of cases. As noted previously, whenever bursal abnormalities were noted on US, clinical tenderness in this area was confirmed prior to injection.
Although not the main focus of this study, nearly all patients (97.9%) reported partial to complete relief immediately after USGI with the following breakdown: 2.1% with minimal improvement, 24.0% with partial improvement, 44.8% with significant improvement, and 29.1% with complete relief. Only 2 patients had minimal relief immediately post-procedure: one who had failed prior LMGI and one who had received no prior injections. Details can be found in Table 3. Of the 16 patients who had little to no response to prior LMGIs, 15 had partial to complete relief immediately following USGI. Of the 10 patients that responded to LMGIs, all 10 had partial to complete relief immediately following USGI. The response profile of the 14 USGIs provided to patients with chronic, widespread pain was not significantly different than that of the general population (total response rate of 100% with the following breakdown: 35.7% with partial relief, 35.7% with significant relief, and 28.6% with complete relief).
LMGIs of CS provide variable benefit for impingement syndrome, but their use is supported by current literature and considered standard clinical care. A 2003 Cochrane Database Systematic Review found that SDB CS LMGIs have a small benefit over placebo with respect to pain and active abduction range for rotator cuff disease [5]. A 2005 meta-analysis showed SDB CS LMGIs to be effective for symptomatic improvement for rotator cuff tendonitis for up to a 9-month period [12]. Koester’s 2007 review found little reproducible evidence to support the efficacy of SDB LMGI in rotator cuff disease [4], while Gruson et al concluded that accurate, sterile SDB CS injections lead to good short-term outcomes [7]. The disparity in the literature is likely influenced by study design variability, including duration of symptoms, the number of injections administered, the amount and type of medication given, and the adjunctive use of NSAIDs and physical therapy. While outcomes are variable, current literature shows the benefit of CS LMGIs into the SDB for impingement syndrome.
Research comparing the outcomes of LMGIs versus USGIs of CS into the SDB is limited in quantity and quality but available studies have shown USGIs to produce significantly greater improvements in shoulder abduction range [19], patient-rated pain [20] and patientrated shoulder functioning [18,20]. A 2011 systematic review noted that USGIs had significantly greater improvement in shoulder pain and function at 6 weeks post-injection, but noted the paucity of randomized controlled trials (RCTs) on this topic [21]. A 2012 Cochrane Review found insufficient evidence to establish an advantage of USGIs of the SDB or glenohumoral joint, but used LMG shoulder and intramuscular gluteal injections as the comparison groups, which may contribute to this discrepancy [22]. A 2013 randomized trial found that USGIs result in better outcomes in regards to passive shoulder abduction and shoulder function in comparison to LMGIs in patients with chronic subdeltoid bursitis [18]. The American Medical Society of Sports Medicine (AMSSM) recently published a position statement on MSUS stating there is moderate evidence that USGIs are more efficacious and preliminary evidence that they are more cost effective than LMGIs, with specific data supporting its use at the SBD [14]. Many have attributed the improved outcomes of USGIs to improved accuracy; however, no studies have evaluated how US alters the injection approach used for CS SDB injections, which may also contribute to the improved outcomes.
This study shows that the majority of bursal fluid distention and thickening identified on US occurs anterior to the acromion. As described previously, LMGIs are traditionally given from a posterolateral approach with medication directed at the posterior edge of the bursa beneath the acromion process. However, we found that by using US to identify abnormalities, more than 50% of SBD CS injections are directed to a more anterior portion of the bursa. This data demonstrates that identification of bursal abnormalities by US with clinical correlation can alter the US-guided approach. This finding has direct clinical implications and may contribute to the better outcomes seen using US-guidance.
The bursa is not confined to the subacromial region but is composed of subacromial and subdeltoid portions [7]. It is located beneath the deep surface of the deltoid muscle and above the superficial surface of the subscapularis muscle, supraspinatus muscle and the tendon of the long head of the biceps. In cadaveric shoulders, the bursa lined the anterior half of the anteroposterior distance of the acromion [25], supporting a more anterior location than was previously thought. The anterior portion of the bursa can extend as far forward as the coracoid process and is thought to play a major role in impingement. It is significantly widened in patients with anteromedial impingement symptoms [26] and may serve as a more appropriate target for injections in some individuals [27]. While the bursa is a fluid-filled space that should allow for homogenous distribution of the steroid throughout the bursa, soft tissue thickening may inhibit equal distribution of the medication and prevent the medicine from gaining access to the most affected, inflamed, symptomatic portion of the bursa.
While all patients who received USGI for impingement syndrome had physical exam maneuvers positive for impingement and reproducible symptoms during the US evaluation, we did not find an obvious correlation between the location of patient-reported symptoms or tenderness on initial examination with the location of symptom reproduction and bursal abnormalities seen on US. This suggests that the patients in this study had difficulty localizing bursal pain without US and that using the area of patient-reported pain or tenderness may not be as reliable as using US-guidance to identify SDB abnormalities, which can then be used as a target for CS injection.
It should be noted that many other factors are known to contribute to patient outcomes besides injection technique. For example, psychosocial comorbidities such as depression and chronic, widespread pain are known predictors of poor clinical response following SDB injections. When looking at the patient demographics stratified by response to prior LMGI (see Table 3), there was an increased frequency of patients with chronic, widespread pain in the group that did not improve following LMGI. However, this group had a 100% response rate to USGI, suggesting this may be a good option for this patient population. Further studies with a larger sample size would be needed to analyze this trend.
Response to Prior LMGI
Immediate Response to USGI
Total Given
Improvement*
No Improvement**
Improvement*
No Improvement**
All
26 (38.8%)
10 (38.5%)
16 (61.5%)
92 (95.8%)
2 (2.1%)
Position 1
0 (0%)
0 (0%)
0 (0%)
2 (100%)
0 (0%)
Position 2
7 (25%)
3 (42.9%)
4 (57.1%)
37 (97.3%)
0 (0%)
Position 3
5 (33.3%)
2 (40.0%)
3 (60.0%)
19 (100%)
0 (0%)
Position 4
14 (46.7%)
5 (16.7%)
9 (30.0%)
36 (94.7%)
2 (5.3%)
No Prior Injection
47
-
-
46 (97.9%)
1 (2.1%)
LMGI Responders
10
10 (100%)
0 (0%)
10 (100%)
0 (0%)
LMGI Non-Responders
16
0 (0%)
16 (100%)
15 (93.8%)
1 (6.2%)
*A patient was considered to have pain improvement if they rated their pain as somewhat, significantly, or completely improved (scores 3-5 as described in the methods section).
**A patient was considered to have no pain improvement if they rated their pain as little to no improvement post-injection (score 1-2 as described in the methods section).
Table 3: Patient response to injections.
Limitations of this study include the use of retrospective review for data analysis and the lack of validated clinical outcome measures. We opted to focus on technique used rather than clinical outcomes in this initial exploratory study. While we did record immediate post-procedure symptomatic improvement, we did not have any other follow-up in regards to patient-rated pain, shoulder function or shoulder range of motion, and we acknowledge that this reflects the anesthetic effects rather than steroid effects. Further prospective, longitudinal studies to compare patient-rated and objective clinical outcomes, both short-term and long-term, of USGIs given laterally over the supraspinatus tendon (as is typically done with LMGIs) versus focally to the US-identified area of most significant bursal abnormality would be of great interest. Additionally, using contrast material injectate to visualize medication distribution pattern within the bursa, particularly in patients with chronic symptoms and evidence of bursal thickening, may provide further insight. Contrast injections have been used to study the accuracy of subdeltoid bursal injections but the extent of distribution of the injected material within the bursa has not been described. This study only reviewed the data from one provider in an academic point-of-care US clinic; given the operator-dependent nature of US and the variety of techniques utilized at different facilities, larger, multicenter studies with multiple clinicians are needed. Finally, we acknowledge that the data regarding the benefit of subdeltoid injections, both landmark and US-guided, is limited, as is comparison data between the two techniques, and deserves additional investigation with high-quality randomized controlled studies.
Conclusion
US identified maximal distention and inflammation of the SDB to occur anterior to the acromion, rather than subacromially, in 59.4% of cases, which suggests that no single LMGI approach will reliably target the most inflamed or distended portion of the bursa. This finding may contribute to the variable clinical outcomes seen following LMGIs and the trend toward improved outcomes seen following USGIs.
References
- Neer CS 2nd. Impingement lesions. Clin Orthop Relat Res. 1983; 70-77.
- Ostör AJ, Richards CA, Prevost AT, Speed CA, Hazleman BL. Diagnosis and relation to general health of shoulder disorders presenting to primary care. Rheumatology (Oxford). 2005; 44: 800-805.
- Ottenheijm R, van't Klooster I, Starmans L, Vanderdood K, de Bie R, Cals J, et al. Ultrasound-Diagnosed Disorders in Shoulder Patients in Daily General Practice: a Retrospective Observational Study. BMC Family Practice. 2014; 15: 2-12.
- Koester MC, Dunn WR, Kuhn JE, Spindler KP. The efficacy of subacromial corticosteroid injection in the treatment of rotator cuff disease: A systematic review. J Am Acad Orthop Surg. 2007; 15: 3-11.
- Buchbinder R, Green S, Youd JM. Corticosteroid injections for shoulder pain. Cochrane Database Syst Rev. 2003; CD004016.
- Green S, Buchbinder R, Hetrick S. Physiotherapy interventions for shoulder pain. Cochrane Database Syst Rev. 2003; : CD004258.
- Gruson KI, Ruchelsman DE, Zuckerman JD. Subacromial corticosteroid injections. J Shoulder Elbow Surg. 2008; 17: 118S-130S.
- Kuhn JE. Exercise in the Treatment of Rotator Cuff Impingement: a Systematic Review and a Synthesized Evidence-Based Rehabilitation Protocol. J Shoulder Elbow Surg. 2009; 18: 138-160.
- Morrison DS, Frogameni AD, Woodworth P. Non-operative treatment of subacromial impingement syndrome. J Bone Joint Surg Am. 1997; 79: 732-737.
- Tallia AF, Cardone DA. Diagnostic and therapeutic injection of the shoulder region. Am Fam Physician. 2003; 67: 1271-1278.
- Mathews PV, Glousman RE. Accuracy of subacromial injection: anterolateral versus posterior approach. J Shoulder Elbow Surg. 2005; 14: 145-148.
- Arroll B, Goodyear-Smith F. Corticosteroid injections for painful shoulder: a meta-analysis. Br J Gen Pract. 2005; 55: 224-228.
- Gilliland CA, Salazar LD, Borchers JR. Ultrasound versus anatomic guidance for intra-articular and periarticular injection: a systematic review. Phys Sportsmed. 2011; 39: 121-131.
- Finnoff JT, Hall MM, Concoff AL, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. PMR. 2015; 7: 151-168.
- Tsai YH, Huang TJ, Hsu WH, Huang KC, Li YY, Peng KT, et al. Detection of subacromial bursa thickening by sonography in shoulder impingement syndrome. Chang Gung Med J. 2007; 30: 135-141.
- Malvestiti O, Mariani C, Scorsolini A, Ratti F, Ferraris G, Columbaro G. [Subacromial impingement syndrome and rotator cuff tear. Ultrasonography of 140 cases]. Radiol Med. 1997; 94: 37-42.
- Papatheodorou A, Ellinas P, Takis F, Tsanis A, Maris I, Batakis N. US of the shoulder: rotator cuff and non-rotator cuff disorders. Radiographics. 2006; 26: e23.
- Hsieh LF, Hsu WC, Lin YJ, Wu SH, Chang KC, Chang HL. Is ultrasound-guided injection more effective in chronic subacromial bursitis? Med Sci Sports Exerc. 2013; 45: 2205-2213.
- Chen MJ, Lew HL, Hsu TC, Tsai WC, Lin WC, Tang SF, et al. Ultrasound-guided shoulder injections in the treatment of subacromial bursitis. Am J Phys Med Rehabil. 2006; 85: 31-35.
- Naredo E, Cabero F, Beneyto P, Cruz A, Mondejar B, Uson J, et al. A Randomized Comparative Study of Short Term Response to Blind Injection versus Sonographic-Guided Injection of Local Corticosteroids in Patients with Painful Shoulder. J Rheumatol. 2004; 31: 308-314.
- Soh E, Li W, Ong KO, Chen W, Bautista D. Image-guided versus blind corticosteroid injections in adults with shoulder pain: a systematic review. BMC Musculoskelet Disord. 2011; 12: 137.
- Bloom JE, Rischin A, Johnston RV, Buchbinder R. Image-guided versus blind glucocorticoid injection for shoulder pain. Cochrane Database Syst Rev. 2012; 8: CD009147.
- Henkus HE, Cobben LP, Coerkamp EG, Nelissen RG, van Arkel ER. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy. 2006; 22: 277-282.
- Nalichowski R, Keogh D, Chueh HC, Murphy SN. Calculating the benefits of a Research Patient Data Repository. AMIA Annu Symp Proc. 2006; .
- Beals TC, Harryman DT 2nd, Lazarus MD. Useful boundaries of the subacromial bursa. Arthroscopy. 1998; 14: 465-470.
- Stallenberg B, Destate N, Feipel V, Gevenois PA. Involvement of the anterior portion of the subacromial-subdeltoid bursa in the painful shoulder. AJR Am J Roentgenol. 2006; 187: 894-900.
- Drakes S, Thomas S, Kim S, Guerrero L, Lee SW. Ultrasonography of subcoracoid bursal impingement syndrome. PM R. 2015; 7: 329-333.