
Special Article - Tracheostomy Decannulation
Phys Med Rehabil Int. 2015;2(6): 1053.
Tracheostomy Decannulation
Nicolini Antonello¹*, Piroddi Ines Maria Grazia¹ and Banfi Paolo²
1Respiratory Rehabilitation Unit, ASL4 Chiavarese, Italy
2Don Gnocchi Foundation, IRCSS, Milan, Italy
*Corresponding author: Nicolini Antonello, Respiratory Rehabilitation Unit, ASL4 Chiavarese, via Terzi 43 -16039 Sestri Levante
Received: June 04, 2015; Accepted: July 16, 2015; Published: July 20, 2015
Abstract
Tracheostomy is a surgical opening in the anterior wall of the trachea to facilitate ventilation. The indications for placement of a tracheostomy tube include failure to wean from mechanical ventilation, impaired neurologic status, inability to handle excessive secretions, and the need to bypass an upperairway obstruction. Tracheostomised patients represent today approx. 20% of patients mechanically ventilated by this route. The increased prevalence of tracheostomy is associated with earlier admissions to respiratory step-down wards, but the indications and timing of decannulation are still being discussed. To our knowledge, there are no guidelines on decannulation of tracheostomised patients. This review aims to point out a systematic approach to this procedure.
Keywords: Tracheostomy; Decannulation; Failure; Multidisciplinary team decision; Appropriate approach
Abbreviations
ICU: Intensive Care Unit; HMV: Home Mechanical Ventilation; ST: Surgical Tracheostomy; PDT: Percutaneous Dilatation Tracheostomy; PMV: Prolonged Mechanical Ventilation; MV: Mechanical Ventilation; PaCO2: Arterial Carbon Dioxide: PCF: Peak Cough Flow; VC: Vital Capacity; AD: Accidental Decannulation; MAC: Mechanical Assisted Cough; SpO2: Pulse Oxymetry
Introduction
Tracheostomy is a surgical opening in the anterior wall of the trachea to facilitate ventilation, removal of secretions or airway protection; the opening is usually maintained by use of a Tracheostomy tube [1]. The procedure may be performed either surgically or by a percutaneous method [2] and it is one of the most frequent procedures done in an intensive care unit (ICU) [3].
The indications for placement of a tracheostomy tube include failure to wean from mechanical ventilation, impaired neurologic status, inability to handle excessive secretions, and the need to bypass an upper-airway obstruction [4]. Also the placement of a tracheostomy tube facilitates the transfer of patient from the intensive care unit to a weaning facility such as a step-down unit or a long-term care hospital [5].
Tracheostomised patients represent today approx. 20% of patients mechanically ventilated by this route [6]. The increased prevalence of tracheostomy is associated with earlier admissions to respiratory step-down wards, but the indications and timing of decannulation are still being discussed [7,8].
In these patients, there are multiple variables that may impact clinical outcome and complications. Moreover, in patients with invasive HMV via tracheostomy, long-term mortality and readmission rate are high [9].
The presence of a Tracheostomy may reduce the work of breathing, the dead space and the respiratory resistance and therefore may facilitate the weaning from a ventilator.
No statistical differences were found between patients who received a surgical (ST) or percutaneous dilatational Tracheostomy (PDT) [2], but in critically ill adult patients, PDT techniques can be performed faster and reduce stoma inflammation and infection but are associated with increased technical difficulties when compared to ST [7,9].
The frequency of Tracheostomy in the management of patients receiving mechanical ventilation contrasts with the lack of evidence as to when a tracheostomy tube should be removed. It appears that the majority of critically ill tracheostomized patients who survive to ICU discharge could eventually be successfully decannulated [10].
For percutaneously tracheostomized patients with prolonged weaning and persisting respiratory failure, the adequate time point for safe decannulation and switch to noninvasive ventilation is an important clinical issue.
A systematic review compared patients with a tracheostomy tube in situ discharged from an ICU to a general ward who received care from a dedicated multidisciplinary team or standard care and showed reductions in time to decannulation, length of stay and adverse events [11].
Chronic comorbidities and the lack of evidence-based weaning and decannulation guidelines make it difficult to predict weaning outcomes of individual patients. Clinically stable patients undergoing prolonged mechanical ventilation usually begin the weaning process by spending increasing amounts of time on a spontaneous breathing trial via humidified Tracheostomy mask [11]. Therapistdriven weaning protocols, such as those involving spontaneous breathing trials or decreasing levels of pressure support, have been implemented in the post-acute-care setting and have been shown to shorten the time taken to wean patients from prolonged mechanical ventilation [12,13]. The procedure is usually straightforward, but adequate assessment and preparation as outlined below is required to maximize success. The steps by weaning are a progressively reduction of the support from mechanical ventilation for prepare the patient at the time of the decannulation and reduction the size of the tracheostomy tubes.
Determinants of Tracheostomy Decannulation
Removing a Tracheostomy is a fundamental step in the rehabilitation of a patient recovering from an ICU stay, not only because of the negative repercussions may that Tracheostomy have on social life activities but also because of the risk of complications: infections, bleeding, stenosis, and fistulas [2].
The prolonged presence of a tracheostomy may be detrimental to functional recovery in terms of delayed rehabilitation and higher morbidity but can also be associated with longer hospitalization, higher staffing and consumable costs [14].
There are several benefits to tracheostomy-tube removal. The tracheostomy tube is a foreign body that may cause bronchorrhea or excessive cough and impairs normal tracheal elevation during swallowing. Diverting breathing away from the upper airway and through the tracheostomy lumen has substantial deleterious effects. The physiologic benefit of pursed-lips breathing is eliminated. The vocal cords are by-passed, and there is no "laryngeal blast" to facilitate effective cough. Partial closure of the vocal cords maintains a subglottic pressure referred to as "physiologic PEEP" (positive end-expiratory pressure) [5]. Most importantly, patients are unable to speak when the tracheostomy tube bypasses the larynx. There are profound consequences of inability to speak. Care is further compromised when the patient is unable to express symptoms that would normally prompt further investigation or intervention. Clinical assessment is compromised when mental status cannot be appropriately assessed because of the lack of verbal communication [7].
For patients with long-term tracheostomy, it is common practice to take an intermediate step prior to completely removing the tracheostomy tube, using is the speaking valve [15].
Clinical judgement by experienced clinicians is an appropriate approach for making decannulation decisions, and favours timely decannulation [3].
The decision process prompting decannulation for management of an acute upper-airway obstruction is very different than assessment for removal of a tracheostomy tube that was placed for long-term management of complex airway abnormalities or for prolonged mechanical ventilation (PMV) [15].
Decannulation of patients with prolonged tracheostomy is not as straightforward as tube removal following a resolved acute upperairway obstruction.
Patients recently weaned from PMV have prolonged critical illness, multiple medical comorbidities, and a marginal respiratory status [15].
Five criteria most often cited in the reviewed literature are: stability of respiratory conditions, effective cough, slowly progressive underlying disease, effective swallowing and no or mild hypercapnia in stable patients. The other criteria are scored differently and this can be explained by the fact that there are no exact guidelines for closing tracheostomy [16].
Heffner [17] proposed the following checklist to determine whether the patient might be decannulated: 1) Is MV no longer required? 2) Are airway secretions controlled? 3) Is aspiration nonexistent or minimal and well tolerated? 4) Does the patient have an effective cough?
An important point is the absence in Heffner’s checklist of judgement about severity of disease (PaCO2, prognosis and clinical stability). Studies are needed to evaluate accepted criteria for safely closing tracheostomy.
It is common opinion that tracheostomy decannulation should be a multidisciplinary team decision, made either in the ICU or in wards following patient discharge from the ICU. The four most important determinants are: clinicians rated level of consciousness, ability to tolerate tracheostomy tube capping, cough effectiveness, and secretions [3].
Patient’s comorbidities, etiology of respiratory failure, swallowing function, respiratory rate, adequate nutritional state, absence of delirium or psychiatric disorders, patent upper-airway and oxygenation were judged to be of moderate importance [18].
Although the ability to tolerate tracheostomy capping was judged to be an important determinant of tracheostomy decannulation for patients with neuromuscular diseases, peak cough flow (PCF) can be significantly increased by providing maximal insufflations; also, flows can be further increased by appropriately timing an abdominal thrust to glottic opening (manually assisted coughing). All patients for whom greater than 160 L/min of PCF could be achieved were successfully extubated or decannulated, whereas no patients with PCFs under 160 L/min were successfully extubated or decannulated [19].
Tracheostomy Tube Changes and Decannulation
Decannulation of long-term tracheostomy patients can be divided into 2 basic categories: planned decannulation and accidental decannulation.
Accidental decannulation (AD) is an unplanned removal of the tracheostomy tube. Such unplanned decannulations can be uneventful or produce a life-threatening situation. There are few published data on the frequency at which this occurs in the home, but common practice is to provide all long-term tracheostomy patients back-up tubes, including tubes that are 1-2 sizes smaller, to be used in the event the primary tube cannot be quickly re-inserted. Many emergency decannulations are unreported, as patients and caregivers are often successful at replacing the primary tube [20].
Planned decannulation is a goal for many tracheostomy patients when the medical need for the tracheostomy no longer exists. A common decannulation process involves the sequential downsizing of the tube, often in conjunction with plugging periods leading to eventual decannulation. This process can take several days or weeks and is often dependent on the patient’s stability and tolerance of the downsizing and plugging procedures. Another planned decannulation is referred to as the one-step method. This method is more comprehensive and includes endoscopic evaluation of the airway and, if clinically indicated, the subsequent removal of the tube. The one-step procedure is considered more intensive and is often performed in the acute-care setting, followed by 24-48 hours of decannulation monitoring. (20)
All patients undergoing weaning from mechanical ventilation should be carefully monitored using continuous pulse oximetry and cardiac telemetry [5].
Various weaning methods to decannulation include: downsizing the tracheostomy tube (which sometimes includes capping/corking), insertion of fenestrated tubes, use of a tracheal button, cuff deflation for sustained periods of time and rapid removal of the tube as the patient’s condition improves [3].
Once a patient demonstrates the ability to tolerate a tracheostomy mask, it is important to establish that the upper airway (i.e., glottis, vocal cords, and subglottic space) is patent. The upper airway can be checked noninvasively by fully deflating the cuff on the tracheostomy tube and placing a gloved finger over the tracheostomy tube opening to deflect air through the upper airway and vocal cords, allowing phonation. Alternatively, tracheostomy tube manometry may be used to obtain objective measurements of airway pressures during the use of a speaking valve or cap. This technique helps identify patients who can tolerate occlusion of the tracheostomy tube and also those who may benefit from having a tracheostomy tube with a smaller external diameter [21].
If the patient is unable to phonate, has stridor or labored breathing, or manifests any respiratory distress, a through endoscopic examination of the airway, including the vocal cords and subglottic space, is recommended [21]. If the airway patency is compromised by stenosis, granulation tissue, or abnormal vocal cord movement, otolaryngology should be consulted for further evaluation and treatment. The initial tracheostomy tube placed can be up to 8 mm inner diameter to facilitate fiber optic bronchoscope. If no pathology is found on endoscopy, the tube may be downsized and changed to a tight-to-shaft (fully deflated) cuff to enhance air flow around the occluded tube [5].
There is little evidence on when to change a long-term tracheostomy tube. Routinely tracheostomy tube changes include: prevention of granulation tissue formation around the tracheostomy tube, prevention of the tube blocking from excessive secretions, and to facilitate weaning or speech by changing the size or type of tracheostomy tube. The Shiley Corporation recommends changing their polyvinyl chloride (PVC) tracheostomy tubes every 29 days. The Portex Blue Line package insert recommends 30 days as the maximum recommended period of use. The Portex Bivona tube package insert recommends it be used for up to 29 days. Furthermore, many manufacturers recommend that a tube with an inner cannula should not remain in situ for more than 30 days. However, to our knowledge, there are no guidelines on the frequency of changing a tracheostomy tube in an adult patient.
Decannulation in Neuromuscular Disease Patients
The assisted PCF but not age, ventilation free breathing time (VFBT), duration or extent of ventilator need, or vital capacity (VC ) significantly predict the ability to safely extubate or decannulate patients with neuromuscular conditions irrespective of the extent of ventilatory insufficiency [19].
Patients with neuromuscular disease may have reduced PCF because of inspiratory and expiratory muscle weakness, impaired bulbar function, and reduced VC. Criteria for decannulation include the ability to attain a PCF, whether assisted or not, greater than 160 L/ min with the tracheostomy capped [20,21].
In regard to neuromuscular disease patients there are no extubation studies on continuously NIV dependent. Goncalves et al. [22] defines extubation criteria for unweanable ventilator dependent patients as no ventilator-free breathing tolerance with 7 cm pressure support in ambient air on the basis of neuromuscular disease or critical care myopathy, VC less than 20% of normal, PaCO2 40 mm Hg or less, at peak inspiratory pressures less than 35 cm H2O on full setting assist/control mode at a rate of 10-13/minute, frequent and aggressive mechanically assisted cough (MAC) to expel secretions and maintain or return SpO2 over 95%, fully alert and cooperative, receiving no sedative medications, chest radiograph abnormalities cleared or clearing and absent flogosis signs, air leakage via upper airway sufficient for vocalization upon cuff deflation.
Besides hypo-ventilation, ineffective PCF has been associated with extubation failure.
Boitano suggests that the neuromuscular respiratorymanagement program that includes periodic evaluation of cough strength and timely initiation of effective cough-augmentation therapy can improve both the quality and duration of life for patients with respiratory insufficiency. Cough strength depends on several contributing factors, which can be independently evaluated with various pulmonary function tests. In his study forced vital capacity spirometry has been used to determine PCF as a measure of cough effectiveness and a significant decrease in normal vs. bulbar paralysis subjects is found [19].
Post-Decannulation Step
After decannulation continuous telemetry and oximetry monitoring patients for at least 24 hours to monitor for unexpected airway compromise is needed.
A patient may exhibit reduced voice quality due to air-flow diversion through the healing stoma on exhalation. Vocalization may be enhanced by gently placing 2 fingers over the gauze-covered stoma during speech to minimize leak and maximize air flow to the vocal cords. Vocalization will usually return to normal once the stoma has closed completely. The tracheostomy stoma heals by secondary intention within 5-7 days in the majority of patients. However, tracheostomy-stoma-closure rates are variable and closure may occur in a single day or may take weeks. A persistent tracheo-cutaneous fistula may remain in some patients and may require surgical closure [5].
Decannulation Failure
During the post-mechanical ventilation period, patients are predisposed to respiratory muscle fatigue, abnormal ventilatory drive, and another episode of respiratory failure. Individuals with a long-term tracheostomy are at risk for upper-airway obstruction due to complications of tracheostomy.
Additionally, there may be upper-airway abnormalities that were initially unappreciated or unrecognized at the time of decannulation. Patients may subsequently experience life-threatening airway compromise requiring emergency reinsertion of the tracheostomy tube [15].
In the literature, failed decannulation has been variously defined as the need to reinsert an artificial airway within 24h [3], 48 h [23], and 48-72 h [17] or within 3 days [19]. Others report a 1-week time frame or the requirement for a second tracheostomy during hospital admission.
However, there is currently no accepted definition for decannulation failure [18].
Bach and Saporito [19] defined successful decannulation as extubation or decannulation and site closure with no consequent respiratory symptoms or blood gas deterioration for at least 2 weeks.
Ceriana et al. evaluated the feasibility of tracheostomy, and defined its failure as the need to reopen the Tracheostomy because of an acute episode or progressive worsening of arterial blood gases not corrected by the application of noninvasive mechanical ventilation [2].
Data suggest that most clinicians would consider reinsertion of an artificial airway within 48 to 96 hours following planned tracheostomy removal to constitute a decannulation failure. Furthermore, clinicians appeared to consider a decannulation failure rate of 2% to 5% to be acceptable [18].
All anatomical and nearly all stridor problems appear mostly within the first 4h after decannulation. Thus, clinicians must assess airway patency, work of breathing and oxygenation attentively in the first 4h to detect, and respond to, potential respiratory problems. Over the first 24 h, sputum retention is the primary cause of decannulation failure, suggesting clinical vigilance for an inability to independently expectorate secretions is paramount, and that chest physiotherapy must be maintained to prevent associated adverse events. These findings can be used to provide guidance to inexperienced nurses managing patients who have been recently decannulated [3].
Patients with significant neurological injury who make very slow or little progress in their neurological recovery and have prolonged ICU lengths of stay are often prone to deconditioning, whereas for patients who are neurologically intact, independent expectoration of pulmonary secretions and early mobilization tends to result in successful decannulation [3].
The duration of unsupported breathing, oxygenation and age can be predictors of decannulation failure [24].
In patients with prolonged mechanical ventilation, weaning failure is associated with poor survival [10]. Moreover, in patients with invasive HMV via tracheostomy, long-term mortality and readmission rate are high [9]. Therefore, it appears important to liberate patients from a tracheostoma whenever possible and to keep the time with a tracheostoma as short as possible. In the decision of decannulation the duration of spontaneous breathing and oxygenation as well as a patient’s age should be considered [24].
Conclusion
In conclusion decannulation is usually well tolerated by the patient, but a previous clinical systematic and endoscopy approach to patient evaluation in needed. Following decannulation, patients require close monitoring to identify signs of airway compromise.
In a recent review has also been supposed a hypothetical score for a practical use we will name objective quantitative parameters ‘major criteria’, and semi-quantitative or subjective parameters ‘minor criteria’ to help clinicians in choosing decannulation timing. If all main criteria are satisfied, regardless of minor criteria, decannulation with high probability of positive outcome can be assumed, but that requires discussion and a prospective validation study [25].
Different protocols have been proposed for tracheostomy decannulation [2,7,8,25] but there are essentially no controlled studies. In our experience modified decisional flow ( Figure 1) proposed by Ceriana et al [2] should be useful in identifying which patients are ready to be weanned. Further larger prospective studies are needed to validate these clinical approaches.
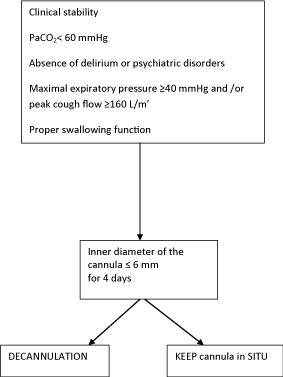
Figure 1: Decisional flow chart to decide tracheostomy removal.
References
- Yu M. Tracheostomy patients on the ward: multiple benefits from a multidisciplinary team? Crit Care. 2010; 14: 109.
- Ceriana P, Carlucci A, Navalesi P, Rampulla C, Delmastro M, Piaggi G, et al. Weaning from tracheotomy in long-term mechanically ventilated patients: feasibility of a decisional flowchart and clinical outcome. Intensive Care Med. 2003; 29: 845-848.
- Choate K, Barbetti J, Currey J. Tracheostomy decannulation failure rate following critical illness: a prospective descriptive study. Aust Crit Care. 2009; 22: 8-15.
- Frutos-Vivar F, Esteban A, Apezteguía C, Anzueto A, Nightingale P, González M, et al. Outcome of mechanically ventilated patients who require a tracheostomy. Crit Care Med. 2005; 33: 290-298.
- O'Connor HH, White AC. Tracheostomy decannulation. Respir Care. 2010; 55: 1076-1081.
- Esteban A, Anzueto A, Alía I, Gordo F, Apezteguía C, Pálizas F, et al. How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med. 2000; 161: 1450-1458.
- Christopher KL. Tracheostomy decannulation. Respir Care. 2005; 50: 538-541.
- Heili Frades SB, Peces Barba Romero G, Villar M, Pelicano S, Checa venegas MJ, Gutierrez Fonseca R, et al. Ventilacion mechanic Mechanical ventilation weaning protocol and decannulation of the intermediate Respiratory Care Unit of the Fundacion Jimenez dìDiaz. Rev Patol Resp. 2011; 14: 83-91.
- Marchese S, Lo Coco D, Lo Coco A. Outcome and attitudes toward home tracheostomy ventilation of consecutive patients: a 10-year experience. Respir Med. 2008; 102: 430-436.
- Engoren M, Arslanian-Engoren C, Fenn-Buderer N. Hospital and long-term outcome after tracheostomy for respiratory failure. Chest. 2004; 125: 220-227.
- Garrubba M, Turner T, Grieveson C. Multidisciplinary care for tracheostomy patients: a systematic review. Crit Care. 2009; 13: R177.
- Scheinhorn DJ, Chao DC, Stearn-Hassenpflug M, Wallace WA. Outcomes in post-ICU mechanical ventilation: a therapist-implemented weaning protocol. Chest. 2001; 119: 236-242.
- Vitacca M, Vianello A, Colombo D, Clini E, Porta R, Bianchi L, et al. Comparison of two methods for weaning patients with chronic obstructive pulmonary disease requiring mechanical ventilation for more than 15 days. Am J Respir Crit Care Med. 2001; 164: 225- 230.
- Leung R, MacGregor L, Campbell D, Berkowitz RG. Decannulation and survival following tracheostomy in an intensive care unit. Ann Otol Rhinol Laryngol. 2003; 112: 853-858.
- Christopher KL. Tracheostomy decannulation. Respir Care. 2005; 50: 538-541.
- Marchese S, Corrado A, Scala R, Corrao S, Ambrosino N. Intensive Care Study Group, Italian Association of Hospital Pulmonologists (AIPO). Tracheostomy in patients with long-term mechanical ventilation: a survey. Respir Med. 2010; 104: 749-753.
- Heffner JE. The technique of weaning from tracheostomy. Criteria for weaning; practical measures to prevent failure. J Crit Illn. 1995; 10: 729-733.
- Stelfox HT, Crimi C, Berra L, Noto A, Schmidt U, Bigatello LM, et al. Determinants of tracheostomy decannulation: an international survey. Crit Care. 2008; 12: R26.
- Bach JR, Saporito LR. Criteria for extubation and tracheostomy tube removal for patients with ventilatory failure. A different approach to weaning. Chest. 1996; 110: 1566-1571.
- Lewarski JS. Long-term care of the patient with a tracheostomy. Respir Care. 2005; 50: 534-537.
- Christopher KL. Tracheostomy decannulation. Respir Care. 2005; 50: 538-541.
- Bach JR, Gonçalves MR, Hamdani I, Winck JC. Extubation of patients with neuromuscular weakness: a new management paradigm. Chest. 2010; 137: 1033-1039.
- Thompson-Ward E, Boots R, Frisby J, Bassett L, Timm M. Evaluating suitability for tracheostomy decannulation: a critical evaluation of two management protocols. J Med Speech-Lang Pathol. 1999; 7: 273-281.
- Budweiser S, Baur T, Jörres RA, Kollert F, Pfeifer M, Heinemann F. Predictors of successful decannulation using a tracheostomy retainer in patients with prolonged weaning and persisting respiratory failure. Respiration. 2012; 84: 469-476.
- Santus P, Gramegna A, Radovanovic D, Raccanelli R, Valenti V, Rabbiosi D, et al. A systematic review on tracheostomy decannulation: a proposal of a quantitative semiquantitative clinical score. BMC Pulm Med. 2014; 14: 201.