
Special Article - Stroke Rehabilitation
Phys Med Rehabil Int. 2015; 2(7): 1057.
A Review of the Physical Demands of Stair Negotiation in Healthy Aging and Following Stroke
Ridgway HM, Bisson EJ and Brouwer B*
Motor Performance Laboratory, School of Rehabilitation Therapy, Queen’s University, Ontario, Canada
*Corresponding author: Brouwer B, School of Rehabilitation Therapy, Queen’s University, Kingston, Ontario, Canada
Received: July 31, 2015; Accepted: September 02,2015; Published: September 04, 2015
Abstract
Stair negotiation is an important determinant of community ambulation and functional independence. Compared to level ground walking, the ability to manage stairs requires greater joint range of motion, muscle strength and cardiovascular fitness which can pose challenges for older adults secondary to age-related decline in physical capacity. For older adults who have experienced a stroke, the superimposition of the resultant physical impairments can further compromise mobility and may limit the capacity for community ambulation risking social isolation. This paper summarizes the research describing the lower limb joint mobility, the muscle moment requirements, and the oxygen demands of stair negotiation relative to level walking to characterize the physical ‘costs’ of mobility essential for community ambulation. Specifically, alterations in movement patterns due to age-related changes in physical capacity are discussed in the context of better understanding the impact of stroke-related impairments on the ability to compensate in order to negotiate stairs. This information is relevant to physical rehabilitation specialists to assist in determining individuals’ capacity for community ambulation and to provide guidance in developing targeted strategies to enhance mobility in people aging with stroke.
Keywords: Aging; Biomechanics; Function; Gait; Mobility; Rehabilitation
Abbreviations
ROM: Range Of Motion; COM: Centre Of Mass; SOS: Step-Over- Step; SBS: Step-By-Step
Introduction
Stroke is the leading cause of adult neurological disability, disproportionately affecting the aging population. In 2005, it was estimated that 16 million people worldwide suffered from a first-ever stroke, and 62 million live with the effects of stroke [1]. In terms of disability, this translates into an annual loss of 43.7 million disabilityadjusted life-years reflecting the extraordinary disease burden [1]. Nearly all survivors of mild stroke, and 85% of survivors of moderate to severe stroke return to living in the community [2], although a much smaller percentage are functionally independent [3].
The extent of physical deficits including muscle weakness, spasticity, sensory loss, and gait or balance impairments generally relates to the location and the severity of the stroke [4-6]. The impact is often significant in terms of mobility restriction, loss of independence, social isolation, and reduced community participation [3,7-11] and may be exacerbated by comorbidities and aging.
Seventy-five percent of individuals discharged post-stroke prioritize being active in the community; however, one third of community ambulators pre-stroke were unable to walk unsupervised in their communities when discharged following stroke [12]. Mobility limitations, lower levels of physical activity post-stroke, and an inability to negotiate stairs can restrict independence outside the home [7,9]. Indeed many stroke survivors (60-70%) are able to walk by the time of discharge from hospital [12-14], though this is tempered by accounts that only 7-22% can walk independently outside of their homes [15]. Among long term stroke survivors (4 years post-stroke), 71% report incomplete recovery and 49% require assistance with daily activities [16]. While 76% are able to walk independently indoors, a much lower percentage (63%) can do so outdoors [16]. It stands to reason that the primary focus of rehabilitation is the restoration of independent ambulation; walking being the principle goal [17,18].
Arguably, independent living requires physical mobility beyond walking. Stair negotiation is an important determinant of discharge destination and independence, surpassing walking speed as the single best predictor of community ambulation [2,19]. Despite this, little is known about the physical demands of stair negotiation in rehabilitation populations such as stroke and studies involving healthy adults are few. In contrast, the movement patterns, strength requirements and energy demands of walking have been studied extensively in stroke [20-25]. A similar depth of understanding of the physical ‘cost’ of stair negotiation and the cost relative to walking is essential to gauge mobility function, establish physical rehabilitation goals, re-train safe stair ambulation and appreciate the merit of alternate movement strategies adopted by those with mobility limitation in order to manage stairs.
This review examines the physical demands associated with mobility, particularly stair negotiation in healthy older adults and in older adults with hemiparesis due to stroke. The specific objectives are: a) to describe the lower limb joint mobility, the active force output required from the major lower limb muscle groups and the energy demands of stair negotiation, b) to discuss age-related alterations in movement patterns as a means of compensating for changes in physical capacity, and c) to explore the impact of chronic stroke on the biomechanical and energy demands of stair negotiation. Information pertaining to level walking is presented to provide contextual reference serving to highlight the elevated demands of stair negotiation.
Methods
The authors searched the literature (PubMed and Google Scholar) for studies involving human subjects using the following key words: stair ascent, stair climbing, stair descent, and stair negotiation as well as outcome descriptors including kinematics, kinetics, metabolic demand, aerobic demand and oxygen uptake. Journal articles published from 1990 to 2014 that provided primary data (observational and experimental) or reviews of research studies relevant to the objectives were reviewed. In addition, we drew on findings from our laboratory to supplement available information.
Physical demands of stair negotiation
Stair negotiation requires both concentric and eccentric muscle activation to lift (or lower) the body vertically and translate it horizontally. Stair ascent primarily involves positive (concentric) work as the stance limb accepts body weight, then pulls the body up to provide full support on the next step, and finally maintains progression while the swing limb clears the intermediate step to make contact with the next step (forward continuance). During stair descent, the stance limb accepts body weight and controls the lowering of the body’s centre of mass (eccentric muscle work) as the swing limb is pulled forward to contact the lower step. A combination of adequate joint mobility, strength and aerobic capacity to meet the energy demand is required to accomplish the tasks of ascent and descent which involve physical requirements that typically exceed those associated with level walking.
Joint mobility
Compared to natural speed walking, stair negotiation requires greater sagittal plane range of motion (ROM) at all lower limb joints [26-29], see Table 1. The magnitude of the differences depends on the particular joint in question and the direction of movement (i.e. ascent or descent). The ankle ROM is greatest during descent, whereas mobility at the knee and hip, primarily in flexion, is greatest during ascent. At the hip joint, the ROM is about 40% greater than that observed during level walking or stair descent [27,30]. The greater hip flexion ensures step clearance and avoidance of tripping. At the knee, the angular displacement is comparable for ascent and descent reflecting an increase in ROM of more than 50% (or ~30o) over level walking in order to scale the rise of the step.
Subject characteristics
Range of Motion
Sagittal plane (degrees)
Peak Moment
(Nm/kg)
Ankle
Knee
Hip
PF
KE
HE
Level Walking
Reiner et al [27]
n=10 young men, ages 24-34 years; cadence:108 steps/min*
26
52
41
1.57
0.50
0.80
Winter[36]
n=19 young men & women, cadence:102 steps/min
33
64
30
1.62
0.61
0.60
Kerrigan et al [29]
n=31 young men & women, ages 18-36 years; speed/cadence: 1.37m/sec/119 steps/min
29
58
46
0.77
0.41
0.46
n=31 older men & women, ages 65-84 years; speed/cadence: 1.19m/sec/119 steps/min
23
55
40
0.75
0.27
0.38
Nadeau et al [30]
n=11 men & women, ages 41-70 years (n=9<64 years); speed/cadence: 1.16m/sec/105 steps/min
29
68
46
1.39
0.46
0.68
Stair Ascent
Reiner et al [27]
See above. Cadence: 86 steps/min*
32
95
70
1.26
1.14
0.54
Nadeau et al [30]
See above. Cadence: 94 steps/min
39
103
65
1.17
1.00
0.50
Protopapadaki et al [32]
n=33 men & women, ages 18-39 years; mean=28 years; cadence: 83 steps/min*
42
94
65
1.45
0.51
0.76
Novak & Brouwer [33]
n=23 young men & women, ages 20-30 years; Cadence: 102 steps/min
-
-
-
1.31
1.02
0.56
n=32 older men & women ages 55-83 years; Cadence: 95 steps/min
-
-
-
1.19
0.99
0.55
Reeves et al [34]
n=13 older men & women, mean age: 75 years; cadence: 92 steps/min
32
78
56
1.23
0.90
-
Reeves et al [60]
n=17 young men & women, mean age: 25 years; cadence:98 steps/min
33
83
-
1.48
1.19
-
n=15 older men & women, mean age:75 years; cadence: 92 steps/min
32
81
-
1.24
0.89
-
Stair Descent
Reiner et al [27]
See above. Cadence: 100 steps/min*
40
74
24
1.12
1.35
0.60
Protopapadaki et al [32]
See above. Cadence: 91 steps/min*
61
91
40
1.38
0.46
0.52
Novak & Brouwer [33]
See above. Cadence: 111 steps/min (young)
-
-
-
1.07
1.11
0.23
See above. Cadence 104 steps/min (older)
-
-
-
1.02
1.19
0.23
Reeves et al [34]
See above. Cadence: 94 steps/min
56
77
-
1.07
0.80
-
Reeves et al [64]
n=17 young men & women, mean age: 25 years; cadence: not reported
54
79
-
1.32
0.91
-
n=15 older men & women, mean age:75 years; cadence: not reported
56
78
-
1.03
0.83
-
PF: Plantar Flexor; KE: Knee Extensor, HE: Hip Extensor, - = not reported. * = estimated based on reported cycle time.
Table 1: Average joint angular displacement and internal extensor moments associated with natural speed level walking, and self- paced stair ascent and descent in healthy young adults and older adults (shaded). PF = Plantarflexors; KE = Knee Extensors; HE = Hip Extensors
The sagittal motion at the ankle joint is important for step clearance and foot placement during ascent requiring about 5°-10° greater range than achieved during level walking [27,30]. The ROM observed during descent is markedly higher; however, this is attributed to greater dorsiflexion in late stance as the body’s centre of mass (COM) shifts forward as weight is transferred to the lead limb resulting in substantial passive ankle dorsiflexion of the trail limb.
It follows that because greater joint mobility is required to ascend and descend stairs, restrictions in joint ROM can adversely affect stair negotiation despite having negligible or considerably lesser impact on walking. Recognizing the different physical requirements of specific mobility tasks and an individual’s capacity to meet them is important in determining how one might function in their home or community where stairs may be unavoidable.
Muscle output
To negotiate stairs, muscles must be able to generate the forces required to produce the necessary joint movement and to control the excursion of the COM relative to the base of support in order to translate the body vertically and horizontally. Figure 1 illustrates the sagittal lower limb internal joint moment profiles associated with walking, stair ascent and descent for visual comparison. Similar to walking, the plantar flexors generate an extensor moment during the stance phase of stair negotiation to maintain upright support. Output reaches a peak during late stance (Figure 1a) to propel the limb into the swing phase [27,31,32]. During ascent and descent, the plantar flexors have the added roles of stabilizing the support limb while weight is accepted and raising the COM (or controlling its lowering in descent) [27,31,33]. Though the magnitudes of the peak plantar flexor moments during stair negotiation are comparable to those reported for walking (Table 1), the overall work accomplished through concentric activity during ascent is greater as reflected by the larger area under the moment curve (Figure 1a). Effectively this elevates the total muscle effort associated with the task thus elevating the physical challenge.
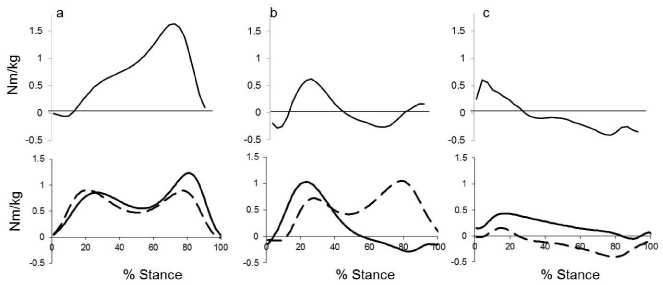
Figure 1: Net joint moment profiles for the ankle (a), knee (b) and hip (c) during the stance phase of level walking (top), stair negotiation (bottom; ascent: solid line,
descent: dashed line) in healthy young adults. Internal extensor moments are positive [unpublished data].
The knee extensors are important contributors to the work of stair negotiation with peak moment magnitudes approximately double those reported for walking [26,27,33,34]. In conjunction with the plantar flexors, the knee extensors contribute substantially to the total support moment during weight acceptance and initial forward continuance in ascent and controlled lowering of the COM during descent [35], see Figure 1b. In descent, the extensors work eccentrically to decelerate the forward and downward movement of the COM and to prevent collapse [31,33], a crucial role in ensuring stability and safety. Unlike level walking, the ability to manage stairs is critically dependent upon the activity and strength of the knee extensors as these muscles produce considerable joint angular displacement which is turn translates the COM.
During walking, the hip extensors mainly contribute to translating the body’s COM forward in the direction of progression. During stair negotiation, in ascent more so than descent, the early hip extensor moment of the lead limb contributes to upright support during weight acceptance and early transition to single limb support initiating the pull up phase [26,27,31,32]. Throughout the remainder of stance, the hip muscles aid primarily in stabilizing the mass of the trunk and upper body over the base of support [33,36], though their contribution can be quite variable from stride-to-stride within an individual as well as across individuals [31,33]. The generally lower hip extensor moment magnitudes relative to level walking reflect the difference in function, that is, stability rather than forward progression (Figure 1c).
Frontal plane moments generated at the hip have different functions in stair climbing and walking although their magnitudes are comparable [30]. In level walking, the abductor moment reflects eccentric activity as the hip is an adducted position, whereas the concentric activity in stair ascent serves to abduct the hip and raise the pelvis on the swing limb side to facilitate step clearance [30,33]. This action is important to accomplishing the task safely and underscores the significance of maintaining adequate hip abductor strength. In both level ground and stair walking, these muscles provide mediolateral stabilization during stance to control pelvic obliquity thereby limiting motion extraneous to the plane of progression.
Energy demands
The overall higher muscle output required for stair negotiation compared to level walking is necessarily linked to elevated energy demands. From a mechanical standpoint, the ability to exploit the energy of motion is greater during walking due largely to intersegmental and interlimb energy transfers, which assist propulsion and compensate active muscle force production to enhance efficiency [24,37,38]. The mechanical energy expenditure associated with the work performed by the ankle and knee muscles during stair ascent and descent are reportedly 1.5 to ten times higher than values documented for walking [39]. Not surprisingly, the metabolic energy demands are also much higher for stair negotiation, particularly during ascent in association with the production of positive work [40,41].
In healthy individuals, the metabolic or aerobic cost of natural speed stair climbing (~70-95 steps/min) can exceed three times that of natural speed walking [40-42]. Stair descent is about 50% less aerobically demanding (~17 ml/kg/min) [41] attributable to the predominantly negative work performed with gravity’s assistance, but nonetheless, it far exceeds the oxygen consumption associated with natural speed level walking (~11-12 ml/kg/min) [43,44].
Young adults are usually able to readily accommodate the energy demands associated with stair climbing; however, the same may not be true for older adults. Age-related decline in maximum aerobic capacity in men and women is in the order of 8-10% per decade commencing in the third decade [45,46]. As such, the higher physical demands of stair negotiation may pose significant challenges in later years. Indeed many older adults describe stair negotiation as one of the most difficult tasks attributed to aging [47].
Age-related changes in physical function and the impact on stair negotiation
Musculoskeletal changes: Characteristics of the aging musculoskeletal system have been well documented [48-54]. Agerelated declines in strength are directly impacted by, and correlated with, loss of skeletal muscle mass. Such loss is detectable as early as the third decade and declines at a rate of 12-14% per decade beyond 50 years of age; explained in part by the loss of motor units [48,55]. Post-mortem morphological studies estimate an average reduction of about 25% in lower motoneuron cell counts in the lumbosacral cord of previously healthy adults between the second and tenth decade [56]. Losses of this nature are strongly linked to decreases in physical ability including mobility function, with the greatest limitations associated with the most physically challenging tasks [57].
Other age-related musculoskeletal changes include increased joint stiffness secondary to losses in distensibility and elasticity of ligaments and muscles as well as degradation of articular cartilage [52]. These alterations can result in restrictions of joint mobility due to elevated passive resistance, and when paired with declines in active muscle force production, can limit joint range of motion. If excessive, and the physical task demands warrant near maximal joint range, then movement patterns will be modified to compensate which could impact efficiency and safety. Degradation of joint health (e.g. arthritic changes) and associated pain may further limit joint mobility [58,59], the impact of which tends to be most pronounced at the knee due to high loads during stair negotiation [28,30,31,60].
The progression of age-related changes in the musculoskeletal system corresponds to greater effort required to perform a given physical task as compared to younger counterparts. In relative terms, older adults operate closer to their maximum capacity. Most activities of daily living, including walking, require low to moderate physical effort for which the strength requirements fall well within individuals’ force generating capacity [21,61-63]; however, increasing the physical demands of the task by increasing speed or changing the nature of the activity can expose capacity limitations. For example, it is estimated that healthy adults (mean age: 46 years) use from 23% to 53% of their maximum lower limb flexor/extensor muscle strength capacity during level walking at slow speeds of ~0.75 m/s [62]. Increasing to natural speed walking (~1.25 m/s), elevates these estimates to 43% - 63%. Depending on musculoskeletal health, such speed may not be attainable or sustainable for any length of time. With respect to the higher demand of stair ascent, older adults reportedly utilize up to 90% and 75% of their maximum strength at the ankle and knee joints, respectively [60]. The corresponding values for stair descent are 75% and 42% [64], also well above the demands of walking. These values are an average of 22% (ascent) and 12% (descent) higher than those observed in young adults [60,64]. The higher relative ‘cost’ of mobility in older adults is mainly attributed to the reduction in maximum muscle output or strength. An early adaptation is a downward shift (slowing) in natural speed or natural cadence to lower the physical demands (see Table 1). Additionally, older adults naturally redistribute the extensor requirements among lower limb joints to keep the demands within an optimal and safe range [33,60,64].
Reducing walking cadence or speed is associated with lower muscle moments and is a well-established early adaptation to agerelated declines in strength [65]. The same strategy applies to stair negotiation [33, 60]. Further, individuals vary the relative magnitudes of the ankle, knee and hip moment outputs whilst maintaining the profile of the total support moment (net extensor moment that keeps the body upright during stair ascent and descent) [33]. This phenomenon is described in level walking [66] and provides a means of transferring or substituting the workload from one joint to another.
Older adults negotiating stairs generate lower sagittal peak joint moments, particularly for the plantar flexors and knee extensors which produce most of the work of stair ascent (Figure 2, left panel). During stair descent, lower plantar flexor moments are paired with higher hip extensor moments in early stance thought to enhance stability during weight-acceptance without affecting the overall extensor support (Figure 2, right panel). The higher knee extensor output as the body’s COM is lowered in descent promotes stability by increasing the total support moment alleviating concerns of falling [33]. Prolonging the task duration secondary to reduced cadence; however, impacts the metabolic energy demands.
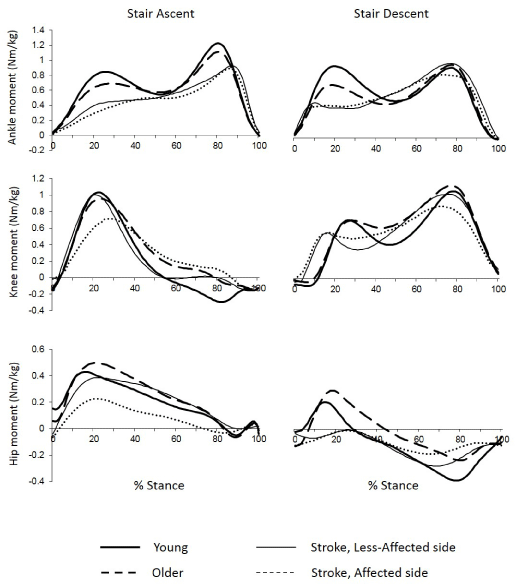
Figure 2: Mean net joint moment profiles generated during stair ascent and descent at the ankle (top), knee (middle) and hip (bottom) for young (n=23; mean age
of 23 years) and older adults (n=32; mean age of 67 years) and the less-affected and affected limbs of older adults with stroke (n=13; mean age of 65 years) [see
33 and 101]. Mean cadence for stair ascent (steps/min): 102 (young), 95 (older), and 69 (stroke); descent (steps/min): 110 (young), 104 (older), and 63 (stroke);
No aids or handrails were used. Internal extensor moments are positive.
Changes in energy requirements: Individuals young and old adopt natural speeds of locomotion that are optimally energy efficient and beyond which, in either direction, energy requirements increase i.e. the well-established U-shaped relationship between speed and energy cost [67]. In healthy older adults the curve is shifted upwards reflecting a 15-25% higher energy cost compared to young adults at any given speed [68-71]. The cause includes greater gait instability, elevated mechanical work associated with alterations in joint kinematics and kinetics, and agonist-antagonist co-activation to increase joint stability. While there remains considerable debate about the relative contributions of these factors to the higher cost [68,69,72,73], the net result of increased energy demands is clear.
The mechanical energy expended during stair negotiation without any transfer to adjoining limb segments is 1.5 to 10 times higher than reported for level walking during which pendular motion enhances energy interchange and transfer thus promoting greater mechanical efficiency [39]. Older adults are less able to compensate the work done by active muscles through inter-segmental energy transfer than their younger counterparts resulting in a loss in efficiency, particularly during stair descent [39]. The greater instability during stair walking and particularly with descent [74] may be counteracted by increasing active stiffness via flexor and extensor co-activation. Co-activation is higher in older adults than young adults during walking [69] and it is reasonable to speculate that it might be exaggerated in response to the greater instability associated with stair walking. It follows that greater muscle output would then be required to generate the needed net joint moment for mobility thus elevating the energy demand [75,76].
In terms of metabolic energy expenditure, there is a paucity of information comparing the energy cost of stair negotiation in young and older adults. Teh and Aziz [41] reported average oxygen consumption of 33.5 ml/kg/min and 17.0 ml/kg/min for adults ascending (mean age: 44 years) and descending (mean age: 37 years) 180 steps at a cadence of 95 and 106 steps/minute, respectively. Extrapolating the oxygen consumed after 45 seconds of ascent or descent from the oxygen uptake vs time curve for a typical subject, the corresponding values would be 20.5 ml/kg/min and 12.5 ml/kg/min, respectively. In contrast, elderly adults (mean age: 79 years) walking up and down a total of 18 steps (9 up and 9 down) at a cadence of 24 steps/min (~ 45 seconds of activity) consumed a mean of 17 ml/kg/ min of oxygen at about one quarter the speed [72]. Though an indirect comparison, the near equivalence in the rate of O2 consumption suggests that the capacity for stair negotiation in older adults may be severely compromised. Given the age-related decline in maximum aerobic capacity of 8-10% per decade after 25 years [45,46], limitations in aerobic capacity may underlie the reduced stair cadence.
The effects of age-related decline in physical function impact mobility and manifests most prominently when performing tasks that are more physically demanding. If physical impairments are superimposed on aging, it follows that the impact on mobility would likely be exaggerated and the ability to compensate may be limited.
Stair negotiation in people with stroke: Over 75% of strokes occur in people over the age of 65 years [77]. Associated mobility restrictions and sensorimotor impairments would therefore be superimposed on natural age-related declines in physical function. The majority of community-dwelling stroke survivors report restrictions in physical capacity and mobility that impact their reintegration into the community [10]. Over half (57%) of home-dwelling stroke survivors of one year require some assistance with managing stairs and 4% require full assistance [78]. A prospective study of 205 adults assessed one and three years post-stroke found that 21% showed mobility deterioration (Rivermead Mobility Index) over time [79]. Due to the high demands of stair negotiation, the rate of decline in the ability to manage stairs safely may be particularly steep thus impacting community ambulation. Recent studies are beginning to explore this aspect of mobility in individuals with stroke, which will improve our understanding of their unique physical demands.
Impact of physical limitations
Following hemispheric stroke, increased joint stiffness [80-82] and muscle weakness affecting distal muscles to a greater extent than proximal muscles, particularly on the contralateral side [83-85], contribute to limited joint mobility and often manifest in asymmetrical gait patterns. Natural walking speed is slower [21,86], stance times tend to be shorter on the paretic side [23,25,87-90], angular joint motion [20,24] and flexor/extensor moments are both of smaller magnitude than observed in similarly aged healthy adults [20,21,91]. Compensatory activity of the hip flexors to pull the limb forward and upward in late stance/early swing secondary to restricted ankle mobility and low propulsive plantar flexor power is associated with a greater mechanical cost of walking [92]. The slower gait speed also limits the transformation between potential and kinetic energies and is associated with an almost doubling of mechanical work compared to healthy adults [93]. The significantly higher metabolic energy requirements observed in stroke mirror the elevated mechanical energy [94,95].
Milot et al [62] examined muscle output in hyphenated level walking, reporting that peak plantar flexor and hip flexor and extensor moments generated in walking relative to their maximum strength was 19.5%, 55.2% and 40.8% higher, respectively, than detected for healthy adults walking at a similar cadence of about 85 steps/min. When the healthy group walked at ~120 steps/min, the corresponding relative strength costs more closely approximated those associated with the slower (self-selected) cadence observed in stroke. This finding suggests that the slower hyphenated gait maintains the level of effort in a range better suited to their strength capacity. Evidently though, this comes at a higher mechanical and metabolic energy cost (see above), which may prove limit performance of the more challenging task of stair negotiation, especially in the presence of cardiovascular compromise.
A recent study examining the kinematics and kinetics of stair ascent and descent in stroke noted that the sagittal plane joint angular displacement profiles were qualitatively similar to healthy control subjects though the moment profiles reflected lower peak values and compensatory patterns [35]. As illustrated in Figure 2 (left panel), the extensor moments are lower on the affected side compared to both the less affected side and healthy older adults (controls) during stair ascent [35]. The reduction relative to controls is partly explained by the slower cadence; a consequence of paresis and reduced power output. The similarity in moment magnitudes between the lessaffected side in stroke and controls is therefore notable, and reflects an augmentation of extensor support primarily during periods of transition as weight is transferred from one limb to the other. This serves to compensate instability and weakness associated with the affected limb, but also accentuates inter-limb asymmetry (Figure 2). The pattern was similar for stair descent (Figure 2, right panel).
In early stance during descent, people with stroke rely on the knee extensors to maintain upright support and stability compensating for lower plantar flexor and hip extensor output. Unlike controls, individuals with stroke use the hip musculature to a greater extent to control the position of the upper body over the base of support during weight acceptance and controlled lowering of the COM. The low magnitude flexor moments help control the trunk to limit translation of the COM thus enhancing stability.
These findings are useful in understanding stroke-related differences in task performance; however, they are less helpful in appreciating the actual demands of performing the task. Expressing the peak moment magnitudes relative to maximum strength reveals that people with stroke use a significantly greater percentage of maximum strength; from 15% to 50% higher utilization than ageand sex-matched healthy older adults when ascending stairs [35]. Similarly, during descent, the percentage of maximum strength used is 20% to nearly 40% higher than observed in controls despite the slower cadence. The elevated strength cost of stair negotiation in stroke bodes poorly for maintaining mobility independence with continued aging. Additionally, it raises concern about the metabolic energy cost to support the high muscle activation and prolonged output associated with accomplishing the task at a slower speed.
Approximately 75% of people with stroke have cardiovascular disease [96], a condition associated with low aerobic capacity [97,98]. During walking, people with hemiparesis use 66%-76% of their maximum aerobic capacity compared to healthy adults who use only 27% [5,99]. It is reasonable to infer that the higher physical demands of stair negotiation may pose considerable metabolic challenge in stroke. In individuals unable to meet the demands, alternative strategies to complete the task of stair negotiation may be adopted.
Strategies to manage the demands of stair negotiation in stroke
The abnormally high strength and aerobic demands of stair negotiation in stroke is likely a factor underlying its rating as the most difficult activity to perform following stroke rehabilitation, often leading to avoidance of stairs [100]. Alternatively, the asymmetric nature of stroke-related physical deficits may lead to the adoption of alternate stepping strategies [74].
The reciprocal nature of the normal step-over-step (SOS) pattern of stair ascent and descent imposes similar demands on muscle output and joint mobility of each lower limb as demonstrated in healthy adults [33]. In stroke, interlimb asymmetry reflects the redistribution of workload across muscle groups as a compensatory strategy for paresis (see Figure 2); however the external work to be accomplished is not side-dependent. Consequently, when impairment severity limits the capacity to adequately compensate, reciprocal stair negotiation may not be possible. Adoption of a step-by-step (SBS) pattern (placement of both feet on each step before progressing) yields asymmetrical demands in that the physical requirements are greatest for the lead limb in ascent and the trail limb in descent [101]; which may be better suited to some individuals with hyphenated.
In stroke, adoption of the SBS pattern enables the majority of mechanical work to be accomplished by the stronger muscles on the less affected side, an adaptation to unilateral paresis and limitations in joint range of motion [74]. The reduction in cadence by nearly 50% since each foot makes contact with each step is associated with an overall decrease in the magnitude of lower limb extensor moments required (Figure 3), thus preserving muscle strength. Data from our laboratory [102] reveal that the peak knee and hip extensor moments generated by the SBS trail limb in stroke (paretic side) are 28% to 66% lower than those associated with the SOS pattern or with the SBS lead limb during ascent (see Figure 3). The trail limb plantar flexors generate higher moments than the lead limb in order to generate upward propulsion to facilitate step clearance, though the peak magnitude is almost 35% lower than that required using a SOS pattern. A similar pattern was observed during descent enabling individuals with stroke to off-load the paretic limb in favour of having the less-affected limb perform the majority of the work. On the basis of strength requirements, there are considerable savings to be garnered by adopting a SBS pattern enabling some stroke survivors to manage stairs who otherwise could not. The impact of stepping pattern on the energy demands however, may be less advantageous associated with each foot having to contact each step.
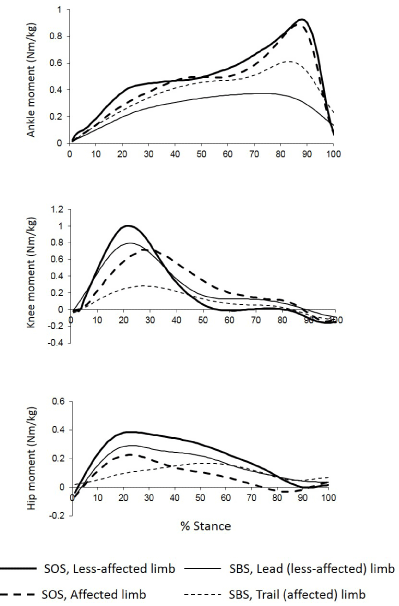
Figure 3: Mean net joint moment profiles in older adults with hemi paretic
stroke (n=13; mean age of 65 years) ascending stairs using two different
strategies: a step-over-step (SOS; thick solid and dashed lines; mean
cadence: 69 steps/min) and step-by-step (SBS; thin solid and dashed lines;
mean cadence: 32 steps/min). No aids or handrails were used. Internal
extensor moments are positive [see ref 102].
In healthy adults, the SBS pattern of ascent and descent increased the metabolic energy costs by 8% to 19% over the SOS [103]. The longer task duration secondary to reduced cadence associated with the SBS strategy contributed to the elevated cost. Our own data indicated that the rate of oxygen consumption was about 35% higher when people with hemispheric stroke climbed stairs using an SBS pattern compared to SOS, whereas a 27% increase was shown in healthy adults. These findings indicate that while modifying the stepping pattern can adequately compensate for strength deficits, the associated elevated aerobic demand may warrant consideration for those with cardiovascular risk. This information is important for rehabilitation specialists as it underscores the need to consider multiple physical factors when assessing mobility. Modifying the way in which mobility tasks are performed can compensate for weakness or physical limitations in one physical system, but may increase the challenge in another. As such, interventions directed toward optimizing function across multiple physical domains may be most effective at enhancing mobility and preserving independence in community settings.
Conclusion
There is ample evidence that stair negotiation, compared to level walking, is more physically demanding in terms of joint mobility, muscle strength and metabolic energy requirements; all physical performance measures that decline with increasing age. When physical limitations attributed to diseases such as stroke are superimposed on age-related losses in physical function, the ability to engage in higher demand tasks like stair ambulation can be restricted. This paper describes the physical challenges associated with stair ascent and descent and the compensation strategies adopted by healthy older adults and those aging with stroke as a means of modulating the physical requirements of the task such that they fall within the limits of their ability. What is evident is that modulations in movement patterns secondary to weakness can reduce strength requirements, but increase energy costs. For physical rehabilitation specialists such knowledge is important and contributes to the determination of appropriate intervention strategies. Research to further our understanding of how multiple physiological systems interact and may be impacted by changes in one contributing factor is essential to advance our knowledge of how best to optimize mobility in healthy older adults and in those aging with stroke.
Acknowledgments
This study was supported by the Heart and Stroke Foundation of Ontario (Grant NA 7369).
References
- Mukherjee D, Patil CG. Epidemiology and the global burden of stroke. World Neurosurg. 2011; 76: S85-90.
- Stineman MG, Granger CV. Outcome, efficiency, and time-trend pattern analyses for stroke rehabilitation. Am J Phys Med Rehabil. 1998; 77: 193-201.
- Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Anderson CS. Long-term disability after first-ever stroke and related prognostic factors in the Perth Community Stroke Study, 1989-1990. Stroke. 2002; 33: 1034-1040.
- Gordon NF, Gulanick M, Costa F, Fletcher G, Franklin BA, Roth EJ, et al. American Heart Association Council on Clinical Cardiology . Physical activity and exercise recommendations for stroke survivors: an American Heart Association scientific statement from the Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention; the Council on Cardiovascular Nursing; the Council on Nutrition, Physical Activity, and Metabolism; and the Stroke Council. Circulation. 2004; 109: 2031-2041.
- Michael KM, Allen JK, Macko RF. Reduced ambulatory activity after stroke: the role of balance, gait, and cardiovascular fitness. Arch Phys Med Rehabil. 2005; 86: 1552-1556.
- Kluding P, Gajewski B. Lower-extremity strength differences predict activity limitations in people with chronic stroke. Phys Ther. 2009; 89: 73-81.
- Pound P, Gompertz P, Ebrahim S. A patient-centred study of the consequences of stroke. Clin Rehabil. 1998; 12: 338-347.
- Duncan PW, Goldstein LB, Horner RD, Landsman PB, Samsa GP, Matchar DB. Similar motor recovery of upper and lower extremities after stroke. Stroke. 1994; 25: 1181-1188.
- Hamel KA, Cavanagh PR. Stair performance in people aged 75 and older. J Am Geriatr Soc. 2004; 52: 563-567.
- Mayo NE, Wood-Dauphinee S, Côté R, Durcan L, Carlton J. Activity, participation, and quality of life 6 months poststroke. Arch Phys Med Rehabil. 2002; 83: 1035-1042.
- Jørgensen HS, Nakayama H, Raaschou HO, Vive-Larsen J, Støier M, Olsen TS. Outcome and time course of recovery in stroke. Part I: Outcome. The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995; 76: 399-405.
- Lord SE, McPherson K, McNaughton HK, Rochester L, Weatherall M. Community ambulation after stroke: how important and obtainable is it and what measures appear predictive? Arch Phys Med Rehabil. 2004; 85: 234-239.
- Thorngren M, Westling B, Norrving B. Outcome after stroke in patients discharged to independent living. Stroke. 1990; 21: 236-240.
- Wade DT, Wood VA, Heller A, Maggs J, Langton Hewer R. Walking after stroke. Measurement and recovery over the first 3 months. Scand J Rehabil Med. 1987; 19: 25-30.
- Hill K, Ellis P, Bernhardt J, Maggs P, Hull S. Balance and mobility outcomes for stroke patients: a comprehensive audit. Aust J Physiother. 1997; 43: 173-180.
- Gadidi V, Katz-Leurer M, Carmeli E, Bornstein NM. Long-term outcome poststroke: predictors of activity limitation and participation restriction. Arch Phys Med Rehabil. 2011; 92: 1802-1808.
- Jette DU, Latham NK, Smout RJ, Gassaway J, Slavin MD, Horn SD. Physical therapy interventions for patients with stroke in inpatient rehabilitation facilities. Phys Ther. 2005; 85: 238-248.
- Belda-Lois JM, Mena-del Horno S, Bermejo-Bosch I, Moreno JC, Pons JL, Farina D, et al. Rehabilitation of gait after stroke: a review towards a top-down approach. J Neuroeng Rehabil. 2011; 8: 66.
- Alzahrani MA, Dean CM, Ada L. Ability to negotiate stairs predicts free-living physical activity in community-dwelling people with stroke: an observational study. Aust J Physiother. 2009; 55: 277-281.
- Olney SJ, Griffin MP, McBride ID. Temporal, kinematic, and kinetic variables related to gait speed in subjects with hemiplegia: a regression approach. Phys Ther. 1994; 74: 872-885.
- Nadeau S, Gravel D, Arsenault AB, Bourbonnais D. Plantarflexor weakness as a limiting factor of gait speed in stroke subjects and the compensating role of hip flexors. Clin Biomech (Bristol, Avon). 1999; 14: 125-135.
- Kim CM, Eng JJ. Magnitude and pattern of 3D kinematic and kinetic gait profiles in persons with stroke: relationship to walking speed. Gait Posture. 2004; 20: 140-146.
- Parvataneni K, Olney SJ, Brouwer B. Changes in muscle group work associated with changes in gait speed of persons with stroke. Clin Biomech (Bristol, Avon). 2007; 22: 813-820.
- Olney SJ, Griffin MP, Monga TN, McBride ID. Work and power in gait of stroke patients. Arch Phys Med Rehabil. 1991; 72: 309-314.
- Brouwer B, Parvataneni K, Olney SJ. A comparison of gait biomechanics and metabolic requirements of overground and treadmill walking in people with stroke. Clin Biomech (Bristol, Avon). 2009; 24: 729-734.
- Costigan PA, Deluzio KJ, Wyss UP. Knee and hip kinetics during normal stair climbing. Gait Posture. 2002; 16: 31-37.
- Riener R, Rabuffetti M, Frigo C. Stair ascent and descent at different inclinations. Gait Posture. 2002; 15: 32-44.
- Andriacchi TP, Andersson GB, Fermier RW, Stern D, Galante JO. A study of lower-limb mechanics during stair-climbing. J Bone Joint Surg Am. 1980; 62: 749-757.
- Kerrigan DC, Todd MK, Della Croce U, Lipsitz LA, Collins JJ. Biomechanical gait alterations independent of speed in the healthy elderly: evidence for specific limiting impairments. Arch Phys Med Rehabil. 1998; 79: 317-322.
- Nadeau S, McFadyen BJ, Malouin F. Frontal and sagittal plane analyses of the stair climbing task in healthy adults aged over 40 years: what are the challenges compared to level walking? Clin Biomech (Bristol, Avon). 2003; 18: 950-959.
- Mc Fadyen BJ, Winter DA. An integrated biomechanical analysis of normal stair ascent and descent. J Biomech. 1988; 21: 733-744.
- Protopapadaki A, Drechsler W, Cramp MC, Coutts FJ, Scott OM. Hip, knee, ankle kinematics and kinetics during stair ascent and descent in healthy young individuals. Clin Biomech (Bristol, Avon). 2007; 22: 203-210.
- Novak AC, Brouwer B. Sagittal and frontal lower limb joint moments during stair ascent and descent in young and older adults. Gait Posture. 2011; 33: 54-60.
- Reeves ND, Spanjaard M, Mohagheghi AA, Baltzopoulos V, Maganaris CN. Influence of light handrail use on the biomechanics of stair negotiation in old age. Gait Posture. 2008; 28: 327-336.
- Novak AC, Brouwer B. Strength and aerobic requirements during stair ambulation in persons with chronic stroke and healthy adults. Arch Phys Med Rehabil. 2012; 93: 683-689.
- Winter D. Human balance and posture control during standing and walking. Gait Posture. 1995; 3: 193-214.
- Robertson DG, Winter DA. Mechanical energy generation, absorption and transfer amongst segments during walking. J Biomech. 1980; 13: 845-854.
- Zajac FE, Neptune RR, Kautz SA. Biomechanics and muscle coordination of human walking. Part I: introduction to concepts, power transfer, dynamics and simulations. Gait Posture. 2002; 16: 215-232.
- Novak AC, Li Q, Yang S, Brouwer B. Mechanical energy transfers across lower limb segments during stair ascent and descent in young and healthy older adults. Gait Posture. 2011; 34: 384-390.
- Bassett DR, Vachon JA, Kirkland AO, Howley ET, Duncan GE, Johnson KR. Energy cost of stair climbing and descending on the college alumnus questionnaire. Med Sci Sports Exerc. 1997; 29: 1250-1254.
- Teh KC, Aziz AR. Heart rate, oxygen uptake, and energy cost of ascending and descending the stairs. Med Sci Sports Exerc. 2002; 34: 695-699.
- Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011; 43: 1575-1581.
- Parvataneni K, Ploeg L, Olney SJ, Brouwer B. Kinematic, kinetic and metabolic parameters of treadmill versus overground walking in healthy older adults. Clin Biomech (Bristol, Avon). 2009; 24: 95-100.
- Platts MM, Rafferty D, Paul L. Metabolic cost of over ground gait in younger stroke patients and healthy controls. Med Sci Sports Exerc. 2006; 38: 1041-1046.
- Fitzgerald MD, Tanaka H, Tran ZV, Seals DR. Age-related declines in maximal aerobic capacity in regularly exercising vs. sedentary women: a meta-analysis. J Appl Physiol (1985). 1997; 83: 160-165.
- Edvardsen E, Scient C, Hansen BH, Holme IM, Dyrstad SM, Anderssen SA. Reference values for cardiorespiratory response and fitness on the treadmill in a 20- to 85-year-old population. Chest. 2013; 144: 241-248.
- Williamson JD, Fried LP. Characterization of older adults who attribute functional decrements to "old age". J Am Geriatr Soc. 1996; 44: 1429-1434.
- Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002; 25: 17-25.
- Roos MR, Rice CL, Vandervoort AA. Age-related changes in motor unit function. Muscle Nerve. 1997; 20: 679-690.
- Porter MM, Vandervoort AA, Lexell J. Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports. 1995; 5: 129-142.
- Booth FW, Weeden SH, Tseng BS. Effect of aging on human skeletal muscle and motor function. Med Sci Sports Exerc. 1994; 26: 556-560.
- Bailey AJ. Molecular mechanisms of ageing in connective tissues. Mech Ageing Dev. 2001; 122: 735-755.
- Doherty TJ. The influence of aging and sex on skeletal muscle mass and strength. Curr Opin Clin Nutr Metab Care. 2001; 4: 503-508.
- Kragstrup TW, Kjaer M, Mackey AL. Structural, biochemical, cellular, and functional changes in skeletal muscle extracellular matrix with aging. Scand J Med Sci Sports. 2011; 21: 749-757.
- Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol (1985). 2003; 95: 1717-1727.
- Tomlinson BE, Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci. 1977; 34: 213-219.
- Brouwer B, Olney S. Aging Skeletal Muscle and the Impact of Resistance Exercise. Physiother Canada. 2004; 56: 80-87.
- Mills K, Hunt MA, Ferber R. Biomechanical deviations during level walking associated with knee osteoarthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2013; 65: 1643-1665.
- Astephen JL, Deluzio KJ, Caldwell GE, Dunbar MJ. Biomechanical changes at the hip, knee, and ankle joints during gait are associated with knee osteoarthritis severity. J Orthop Res. 2008; 26: 332-341.
- Reeves ND, Spanjaard M, Mohagheghi AA, Baltzopoulos V, Maganaris CN. Older adults employ alternative strategies to operate within their maximum capabilities when ascending stairs. J Electromyogr Kinesiol. 2009; 19: e57-68.
- Bohannon RW. Muscle strength and muscle training after stroke. J Rehabil Med. 2007; 39: 14-20.
- Milot MH, Nadeau S, Gravel D, Requião LF. Bilateral level of effort of the plantar flexors, hip flexors, and extensors during gait in hemiparetic and healthy individuals. Stroke. 2006; 37: 2070-2075.
- Requião LF, Nadeau S, Milot MH, Gravel D, Bourbonnais D, Gagnon D. Quantification of level of effort at the plantarflexors and hip extensors and flexor muscles in healthy subjects walking at different cadences. J Electromyogr Kinesiol. 2005; 15: 393-405.
- Reeves ND, Spanjaard M, Mohagheghi AA, Baltzopoulos V, Maganaris CN. The demands of stair descent relative to maximum capacities in elderly and young adults. J Electromyogr Kinesiol. 2008; 18: 218-227.
- Prince F, Corriveau H, Hébert R, Winter DA. Gait in the elderly. Gait Posture. 1997; 5: 128-135.
- Winter DA. Kinematic and kinetic patterns in human gait: Variability and compensating effects. Hum Mov Sci. 1984; 3: 51-76.
- Abernathy B, Kippers V, Hanrahan SJ, Pandy MG, McManus AM, Mackinnon L. Biophysical Foundations of Human Movement 3rd edition. Human Kinetics, Champaign, Illinois. 61825-5076. 2013: p. 136
- Malatesta D, Simar D, Dauvilliers Y, Candau R, Borrani F, Prefaut C, et al. Energy cost of walking and gait instability in healthy 65- and 80-yr-olds. J Appl Physiol (1985). 2003; 95: 2248-2256.
- Mian OS, Thom JM, Ardigò LP, Narici MV, Minetti AE. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta Physiol (Oxf). 2006; 186: 127-139.
- Ortega JD, Farley CT. Minimizing center of mass vertical movement increases metabolic cost in walking. J Appl Physiol (1985). 2005; 99: 2099-2107.
- Martin PE, Rothstein DE, Larish DD. Effects of age and physical activity status on the speed-aerobic demand relationship of walking. J Appl Physiol (1985). 1992; 73: 200-206.
- Knaggs JD, Larkin KA, Manini TM. Metabolic cost of daily activities and effect of mobility impairment in older adults. J Am Geriatr Soc. 2011; 59: 2118-2123.
- McGibbon CA. Toward a better understanding of gait changes with age and disablement: neuromuscular adaptation. Exerc Sport Sci Rev. 2003; 31: 102-108.
- Startzell JK, Owens DA, Mulfinger LM, Cavanagh PR. Stair negotiation in older people: a review. J Am Geriatr Soc. 2000; 48: 567-580.
- Hortobágyi T, Mizelle C, Beam S, DeVita P. Old adults perform activities of daily living near their maximal capabilities. J Gerontol A Biol Sci Med Sci. 2003; 58: M453-460.
- Peterson DS, Martin PE. Effects of age and walking speed on coactivation and cost of walking in healthy adults. Gait Posture. 2010; 31: 355-359.
- Mayo NE, Neville D, Kirkland S, Ostbye T, Mustard CA, Reeder B, et al. Hospitalization and case-fatality rates for stroke in Canada from 1982 through 1991. The Canadian Collaborative Study Group of Stroke Hospitalizations. Stroke. 1996; 27: 1215-1220.
- Hartman-Maeir A, Soroker N, Ring H, Avni N, Katz N. Activities, participation and satisfaction one-year post stroke. Disabil Rehabil. 2007; 29: 559-566.
- van de Port IG, Kwakkel G, van Wijk I, Lindeman E. Susceptibility to deterioration of mobility long-term after stroke: a prospective cohort study. Stroke. 2006; 37: 167-171.
- Levin MF, Hui-Chan C. Ankle spasticity is inversely correlated with antagonist voluntary contraction in hemiparetic subjects. Electromyogr Clin Neurophysiol. 1994; 34: 415-425.
- Rydahl SJ, Brouwer BJ. Ankle stiffness and tissue compliance in stroke survivors: a validation of Myotonometer measurements. Arch Phys Med Rehabil. 2004; 85: 1631-1637.
- Den Otter AR, Geurts AC, Mulder T, Duysens J. Abnormalities in the temporal patterning of lower extremity muscle activity in hemiparetic gait. Gait Posture. 2007; 25: 342-352.
- Teixeira-Salmela LF, Olney SJ, Nadeau S, Brouwer B. Muscle strengthening and physical conditioning to reduce impairment and disability in chronic stroke survivors. Arch Phys Med Rehabil. 1999; 80: 1211-1218.
- Kim CM, Eng JJ. The relationship of lower-extremity muscle torque to locomotor performance in people with stroke. Phys Ther. 2003; 83: 49-57.
- Eng JJ, Kim CM, Macintyre DL. Reliability of lower extremity strength measures in persons with chronic stroke. Arch Phys Med Rehabil. 2002; 83: 322-328.
- Turnbull GI, Charteris J, Wall JC. A comparison of the range of walking speeds between normal and hemiplegic subjects. Scand J Rehabil Med. 1995; 27: 175-182.
- Hsu AL, Tang PF, Jan MH. Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild to moderate stroke. Arch Phys Med Rehabil. 2003; 84: 1185-1193.
- Wall JC, Turnbull GI. Gait asymmetries in residual hemiplegia. Arch Phys Med Rehabil. 1986; 67: 550-553.
- Patterson KK, Parafianowicz I, Danells CJ, Closson V, Verrier MC, Staines WR, et al. Gait asymmetry in community-ambulating stroke survivors. Arch Phys Med Rehabil. 2008; 89: 304-310.
- Griffin MP, Olney SJ, McBride ID. Role of symmetry in gait performance of stroke subjects with hemiplegia. Gait Posture. 1995; 3: 132-142.
- Lamontagne A, Malouin F, Richards CL, Dumas F. Mechanisms of disturbed motor control in ankle weakness during gait after stroke. Gait Posture. 2002; 15: 244-255.
- Olney SJ, Richards C. Hemiparetic gait following stroke. Part I: Characteristics. Gait Posture. 1996; 4: 136-148.
- Detrembleur C, Dierick F, Stoquart G, Chantraine F, Lejeune T. Energy cost, mechanical work, and efficiency of hemiparetic walking. Gait Posture. 2003; 18: 47-55.
- Zamparo P, Francescato MP, De Luca G, Lovati L, di Prampero PE. The energy cost of level walking in patients with hemiplegia. Scand J Med Sci Sports. 1995; 5: 348-352.
- HIRSCHBERG GG, RALSTON HJ. ENERGY COST OF STAIR-CLIMBING IN NORMAL AND HEMIPLEGIC SUBJECTS. Am J Phys Med. 1965; 44: 165-168.
- Roth EJ. Heart disease in patients with stroke: incidence, impact, and implications for rehabilitation. Part 1: Classification and prevalence. Arch Phys Med Rehabil. 1993; 74: 752-760.
- MacKay-Lyons MJ, Howlett J. Exercise capacity and cardiovascular adaptations to aerobic training early after stroke. Top Stroke Rehabil. 2005; 12: 31-44.
- Pang MY, Eng JJ, McKay HA, Dawson AS. Reduced hip bone mineral density is related to physical fitness and leg lean mass in ambulatory individuals with chronic stroke. Osteoporos Int. 2005; 16: 1769-1779.
- Ivey FM, Macko RF, Ryan AS, Hafer-Macko CE. Cardiovascular health and fitness after stroke. Top Stroke Rehabil. 2005; 12: 1-16.
- Tsuji T, Sonoda S, Domen K, Saitoh E, Liu M, Chino N. ADL structure for stroke patients in Japan based on the functional independence measure. Am J Phys Med Rehabil. 1995; 74: 432-438.
- Reid SM, Lynn SK, Musselman RP, Costigan PA. Knee biomechanics of alternate stair ambulation patterns. Med Sci Sports Exerc. 2007; 39: 2005-2011.
- Ridgway H. Strength Requirements and Energy Efficiency of Different Stair-Stepping Strategies in Persons with Chronic Stroke and Healthy Adults [thesis]. Pro Quest, UMI Dissertations Publishing, 2013.
- Shiomi T. Effects of different patterns of stairclimbing on physiological cost and motor efficiency. J Hum Ergol (Tokyo). 1994; 23: 111-120.