
Special Article - Stroke Rehabilitation
Phys Med Rehabil Int. 2015; 2(7): 1058.
Reducing Cognitive Deficits after Stroke through Computerized Progressive Attentional Training (CPAT): A Pilot Study
Sampanis DS¹, Mevorach C¹, Shalev L², Mohammed S¹ and Humphreys GW³*
¹School of Psychology, University of Birmingham, England
²School of Education and School of Neuroscience, Tel Aviv University, Israel
³Department of Experimental Psychology, University of Oxford, England
*Corresponding author: Glyn W Humphreys, Department of Experimental Psychology, University of Oxford, England
Received: March 10, 2015; Accepted: September 09, 2015; Published: September 11, 2015
Abstract
Background and Purpose: Cognitive deficits following stroke are common and are associated with poor rehabilitation outcome. Computerized Progressive Attentional Training (CPAT) has been tested and found effective in children with Attention Deficit/Hyperactivity Disorder (ADHD) and there is evidence also for similar training effects on healthy older adults (Anguera et al., 2013). This pilot trial explored the potential effectiveness of CPAT for improving cognition in stroke survivors with cognitive deficits within 2 months of their stroke.
Methods: Eight sub acute stroke participants underwent the CPAT protocol for 10 sessions during a period of two weeks and were compared with controls (who did not receive training) on both attention tasks (eight healthy controls) and general cognitive assessments (eight other sub acute patients). Attention was assessed before and after training using four lab-based attention tasks while cognitive impairment was assessed using the Birmingham Cognitive Screen (BCoS).
Results: The CPAT intervention resulted in improvements on both attention functions (specifically sustained attention) and non-attention functions (e.g., language, memory, number skills and praxis). These improvements could not be simply attributed to the passage of time or repetition of the test (as evident from healthy and the neuropsychological control group performance).
Conclusion: While the small sample size and the pilot nature of the study should be taken into account, the results indicate that CPAT is potentially an effective and valuable instrument that can be applied to help ameliorate attentional deficits following stroke.
Keywords: Attention; Stroke; Rehabilitation; Neuropsychology
Introduction
Stroke is recognized worldwide as one of the major causes of disability [1]. Cognitive deficits following stroke are common. Recently, Humphreys et al. [2] reported that 70% of stroke patients tested on the Birmingham Cognitive Screen (BCoS) showed impaired cognitive functions. These impairments are also important predictors of outcome, and may lead to long term disability with significant impact on daily activities and independence of stroke survivors [3]. Post stroke attention deficits may even have a dramatic impact on functional recovery and are responsible for poor attendance during rehabilitation [4]. Cognitive and attentional problems in stroke often have impact on behaviour and can lead to chronic depression [5].
Given the high prevalence of stroke and its impact on cognitive and attentional aspects of daily living and functioning, considerable effort has been allocated to the development of various interventions that could ameliorate cognitive deficits following stroke [6]. Previous studies into the effects of attentional training have indicated positive outcome not only on attention but also on other domains of cognition (speed of processing, attention/vigilance, working memory, verbal learning and memory, visual learning and memory, and reasoning and problem solving - 7) and on everyday functional skills [8-10].
Stimulated by research showing that playing action video games can improve perceptual and attentional performance in young normal participants [11,12], a substantial number of attempts have now been made to use computer-based training to improve cognition in individuals showing some aspects of cognitive decline. This has included research on normal healthy ageing populations [13], patients with mild cognitive impairment [14], Alzheimer’s patients [15], and individuals with multiple sclerosis [16], acquired brain injury [17] and stroke survivors [18]. The results are mixed. In many of the studies training has produced benefits on the trained cognitive functions (e.g., improvements in working memory after working memory training; 13, 17]), but very often there have been failures to generalise improvements to non-trained functions [13,19]. In a recent Cochrane review of cognitive training of patients following stroke or other non-progressive forms of acquired brain damage, Chung et al. [20] concluded that there was insufficient high quality evidence for training having a benefit. Few studies used training tasks specifically designed to address critical cognitive processes, and few were designed with appropriate controls to measure effects of repeat testing and time. The authors highlight the need for high quality research which provides a fine-grained test of whether targeted cognitive training can improve cognition in neurological populations, whether training generalizes, and whether training effects supersede improvements produced by recovery through time and engagement in other ongoing activities.
One approach to enhance generalizability of computerised training is to follow early intervention studies [9] and to target cognitive processes that in themselves may sub-serve other cognitive processes. Aspects of attention are known to be important for a variety of cognitive processes and have been shown to produce generalised improvement [9, 21]. For instance, if there is poor sustained attention (the ability to keep attention ‘on task’ throughout a long period of time) or impairment in exerting executive control over processing then patients may show increased visual neglect and increased problems in language [22, 23]. It follows that computerised attention training of such processes (sustained attention, executive control) may yield generalised effects for stroke patients. Recently, Shalev et al. [21] used a Computerised Progressive Attention Training (CPAT) in a group of children with ADHD. The CPAT included aspects of sustained, executive and selective attention training. These authors reported improvements in the experimental group that transferred to a variety of non-trained tests such as maths, word copying as well as behavioural symptoms.
In stroke, problems in executive functions (task switching and inhibiting irrelevant stimuli and responses) and in sustained attention are common in occurrence [2] and potentially critical to a number of other cognitive domains. Thus the targeting of executive functions and sustained attention may be beneficial to induce generalized improvements after training in stroke patients too. To test this we employed the CPAT program developed by Shalev et al. [21] in a group of sub-acute stroke patients. We had patients train with three CPAT tasks, each of which was separately challenging. The tasks covered sustained attention, selective attention and executive control. Importantly, all of the tasks had progressive levels of difficulty which could be tuned to the abilities of individual patients, all used engaging ‘game-like’ displays (see Figure 1), and all generated easyto- understand graphical feedback to help motivate patients (see 21 for the use of CPAT in individuals with ADHD). In order to evaluate the outcome of computerised attention training and its potential generalization to other cognitive domains we measured performance before and after training (and in control groups) in a set of attention tasks as well as the patients’ cognitive abilities using the BCoS test battery (which examines a number of different aspects of cognition).
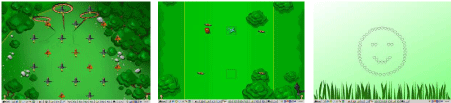
Figure 1: Example displays from the CPAT tasks of selective attention, executive functions and sustained attention. Left: Selective Attention. In this task the
participant has to decide whether the display includes a target (which is an orange quid itch on a broom with open arms). The example is taken from a relatively
high level of difficulty in which the visual load is high (many items presented on a noisy background) posing a high demand on selective attention. Middle:
Sustained attention. In this task the participant is required to detect the appearance of occasional targets. Again, the example depicts a relatively high level of
difficulty. Here the participant has to respond only when the target - a red car - appears in one of the two target locations (black outlined squares). Targets
appear on only 30% of the trials. Non-target trials may include the appearance of non-targets in the target square as well as targets appearing outside the
target squares. In this example the target (red car) can be seen outside the target squares thus this is a non-target trial. Right: Executive attention. In this task
the participants are asked to decide whether the global configuration of the hierarchical figure forms a smiley face (level 1). As the level of difficulty increases,
elements of working memory and task switching are inserted into the task together with a requirement for conflict resolution (e.g., when a smiley appears on the
non-target level).
Method
Participants
Three groups of participants were used. The experimental group included eight first stroke participants, mean age 56.3 years (SD 7.5), four with right-side lesions and four with left-side lesions, six males and two females, all recruited from the National Health Service (NHS) in United Kingdom. Time post stroke for inclusion in the experimental group (on the time of recruitment) was twenty one days post stroke (+/- 7days). A second group of healthy controls included eight age and gender matched healthy participants who were used as a control group for the attention tests (see below). Finally a third group of patient controls included eight stroke patients (mean age 54 (SD 6.0)) who were matched for lesion side and who were used to assess the effects of time and general participation in cognitive research on functional recovery. This patient control group took part in ongoing studies conducted during weekly sessions in the University of Birmingham for the same length of time as the attention training protocol that was applied to the experimental group. The patient control group served as control for the BCoS test battery. The study was approved by the Integrated Research Application System (IRAS) for research in NHS premises.
The training protocol - The Computerized Progressive Attention Training (CPAT) program (Shalev, Tsal, & Mevorach, 2007)
Within the CPAT program three training tasks were included: the Computerized Continuous Performance Task (CCPT; based on 24), which was designed to improve sustained attention; the Conjunctive Search task (CST; based on 25), which was designed to improve spatial selective attention and the Task Switching Stroop-like Task (TSST; based on 26), which was designed to improve executive attention and cognitive control. Snapshots of the training tasks are presented in Figure 1. The three training tasks with their different levels of difficulty have been previously reported [21].
Each patient in the experimental group performed 10 sessions of CPAT (5 sessions per week over a period of two weeks) with 8 - 12 blocks from the three different training tasks in each session. Numbers of blocks varied across participants primarily due to individual differences in severity of symptoms (this was similar in nature to the procedure in [21] in children with ADHD). Each block in the CST and the TSST contained 40 trials, while blocks in the CCPT were either 80 trials long (low levels of difficulty) or 160 trials long (high levels of difficulty). Participants advanced in levels of difficulty according to pre-specified criteria based on maintaining high levels of accuracy and improving (individually) in their reaction time (RT; see 21 for a more detailed description). The progression in levels of difficulty was fully controlled by the program based on the participants’ performance. The training tasks also included a tight schedule of feedback. Participants received auditory feedback (beep) every time an error was committed as well as immediate written positive feedback each time a correct response was performed, which was tied with RT performance (i.e., different messages appeared on the screen immediately following a correct response as a function of how quick the response was relative to the individual average RT in that level of difficulty). The immediate written feedback was not given during the CCPT task. The feedback was also translated into points which were presented on the screen at the end of each block. Each participant was supervised by an experimenter during the entire session.
Assessment tools
Two sets of assessment batteries for attention and for cognitive impairment were used in the experimental group before and after training. The healthy controls performed the attention test twice similarly to the experimental group and the patient controls performed the cognitive battery twice similarly to the experimental group. For attention testing a computerized battery of 4 attention tasks (not the trained tasks within CPAT; 27) was run on a PC with a graphic display which controlled stimulus presentation and data collection (Figure 2). Similar attention tasks were used to evaluate attention difficulties of ADHD children that underwent the CPAT protocol [21] and were therefore used here as well. All stimuli in these four tasks were presented against a dark background. Viewing distance was set at about 50cm so that 1cm represented about 1.15 deg of visual angle. Each task was preceded by practice trials during which auditory feedback was given for incorrect responses. Practice trials were repeated if the rate of errors during the practice exceeded 20%. No feedback was provided during the experimental blocks. Reaction times (RT) were recorded from the onset of the stimulus to the nearest msec. In each task, participants were required to respond as fast and as accurately as possible.
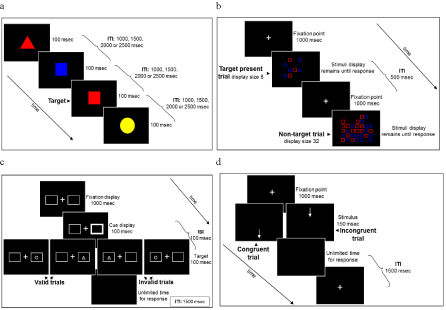
Figure 2: Schematic diagrams of the attention tests used for baseline measurements. a. Schematic diagram of the Sustained Attention task (CCPT). b. Schematic
diagram of the Selective Attention task (CST). c. Schematic diagram of the Orienting Attention task. d. Schematic diagram of the of Executive Attention task.
The four attention tasks that were used here were developed along with the four functions of attention model proposed by [27] in the context of ADHD and measure different aspects of attention. The Conjunctive Continuous Performance Task (CCPT) measures sustained attention - the ability to allocate attentional resources to a non-attractive task over time while maintaining a constant level of performance; The Conjunctive Search Task (CST) measures selective (spatial) attention - the ability to focus attention on a relevant target while ignoring adjacent distracters; The Spatial Cued-Identification Task (SCIT) measures orienting of attention - the ability to direct attention over the visual or auditory field according to sensory input, and to disengage and reorient efficiently; The Location-Direction Strooplike Task (L-DST) measures executive attention - the ability to resolve conflicts of information and/or responses. It is important to note that while these tasks shared some features with the training tasks within CPAT they all used different stimuli and therefore any change in performance in these tasks following training cannot be attributed to the mere exposure with similar stimuli. The four tasks are described in more details in the Appendix.
Assessment of cognitive impairment (BCoS, 2)
The BCoS test [2] was administered twice to all stroke participants in the experimental and patient control groups (duration approximately one hour). The BCoS instrument has been developed to enable comprehensive and efficient screening of post stroke cognitive function and maximises inclusion for stroke survivors by being ‘aphasia and neglect friendly’ (i.e. tests are designed not to be contaminated by aphasia or neglect) and time efficient (to minimise testing time). It assesses five primary domains of cognition: attention and executive function, language (spoken and written), memory (orientation in time and place, longer term verbal recall and recognition, and task recognition), number skills (reading, writing and calculations) and praxis and action (visuo-spatial construction, everyday multiple task construction, gesture production - recognition - imitation).
Scores on the BCoS battery were simplified by averaging performance across the sub-tests within each domain (attention and executive function, language, memory, number processing and praxis). This was done by calculating a z score for each test for each patient, based on the mean and standard deviation of performance for the normal control participants reported by [2] (BCoS z= (patients score - mean of controls) /standard deviation of controls). The z scores for the tests within each domain were then averaged to create a single z score per domain (see the Appendix for a list of the individual tests). Results from BCoS for the experimental group were compared with data derived from the eight patients in the patient control group who had attended the University of Birmingham regularly to take part in visual cognition experiments during the intervention period but who did not take part in the intervention. The experimental and patient control groups were matched for their baseline performance on the BCoS.
Assessment procedure
One week before and one week after the training procedure the two assessment batteries were administered to participants in the experimental group. The attention tests were administered in the experimental and the healthy control groups while the BCoS was administered in the experimental and patients control groups. The following scales were also obtained in the experimental group before and after training (or the time equivalent): Barthel Index [28], MoCA (Montreal Cognitive Assessment) [29], NIHSS (National Institute Health Stroke Scale) and HADS (Hospital Anxiety Depression Scale) [30]. The different assessment schedules are described in Table 1.
Participant groups and schedule
Sub-acute stroke survivors (experimental group)
Age matched control group of stroke survivors (patients control)
Age matched control group of healthy individuals (healthy controls)
1week prior to intervention: computer assessment and behavioural tests
10 days of intervention
1 week post intervention: computer assessment and behavioural tests
BCoS (behavioural) test twice to assess natural recovery
Computer assessment twice (3 weeks apart) to assess effects of test repetition.
Table 1: Participant groups and testing schedule.
The experimental patient group was compared with the healthy normal controls for their performance on attentional tasks pre- and post-intervention. The healthy control participants performed the attention tests twice, similarly to the experimental group following a three-week interval. Here we ask whether the patients not only showed improvement but also whether they improved to a normal level after the intervention. The patient controls (chronic stroke participants) were assessed on the BCoS battery twice similarly to the experimental group. These patients were invited in for regular cognitive assessments (but not training) in the period between the initial assessment and the follow-up, controlling for general engagement with therapists during the intervention period.
Results
Table 2 presents data on the Barthel Index, MoCA NIHSS and HADS assessments, pre- and post-intervention for the experimental group. These measurements applied only for the experimental patient group.
Outcome Measure
Mean Pre (std)
Mean Post (std)
Barthel Index
MOCA
NIHSS
HADS depression
HADS anxiety
14.8 (1.6)
21.6 (4.1)
23.6 (1.8)
4.0 (1.7)
5.6 (1.5)
15.6 (1.4)
24.7 (1.7)
24.5 (1.7)
3.9 (1.6)
5.2 (1.6)
Table 2: Pre and post intervention general outcome measures.
Paired t-tests on the outcome measures (pre and post) revealed significant improvements for the Barthel Index (t(7) = -2.393, p= 0.048), the MoCA (t(7) = -2.818, p= 0.026), and the NIHSS (t(7) = -2.497, p= 0.041). There was no significant change for the HADS depression and anxiety scales.
Attention tests
Attention was assessed by four attention tests in the experimental group and the healthy controls group before and after training (or the equivalent time for the controls).
Sustained attention: The conjunctive continuous performance test (CCPT): Within this task standard deviation of RTs (SD-RT) as well as omission rates represent measurement of sustained attention [27] with large SD-RT and high rates of omissions both indicating poor sustained attention. An ANOVA with time (pre vs. post) as a within subject factor and group (Experimental vs. Control) as a between subject factor was conducted on RT’s, the standard deviation of RT(SD-RT) and omission errors. The ANOVA on RT revealed no significant main effect of time (F(1, 14) = 2.390, p=0.144, η² = 0.146) nor interaction between time and group (F(1, 14) = 2.914, p=0.11, η² = 0.172). There was, however, a significant main effect of group (F(1, 14) = 7.869 p=0.013, η² = 0.36). The patients were significantly slower (789 ms) than the healthy controls (515 ms). A similar ANOVA on the SD-RTs revealed both significant main effects of time (F(1, 14) = 8.719, p=0.01, η² = 384; 161 and 111 ms for pre and post training, respectively) and group (F(1, 14) = 12.262 p=0.004, η² = 0467; 195 and 78 for experimental and healthy control groups, respectively). Moreover, a significant interaction of time and group was obtained (F(1, 14) = 17.291, p=0.001, η² = 0.553; See Figure 3a). A Wilcoxon test revealed that SD-RT was significantly reduced following training in the experimental group (T(8) = 0, p = 0.01; 255 and 135 ms for pre and post training, respectively). In contrast, in the healthy control group no significant effect of time was found (T(8) = 17, p>= 0.1; 68 and 88 ms for the first and second administration, respectively).
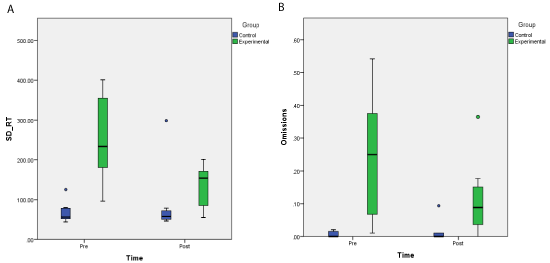
Figure 3: Boxplot charts depicting performance in the CCPT task before and after training. a. SD of RTs and b. percentages of omission errors for the experimental
patients group and healthy controls before and after training (for the patients) and on the first and second administration (for the controls).
A similar interaction of time and group was revealed for the omission errors (F(1, 14) = 6.683, p=0.022, η² = 0.323; see Figure 3b).A Wilcoxon test revealed a marginally significant reduction of omissions after training in the experimental group (T(8) = 4, 0.05<p < 0.1; 24% vs. 12% for pre and post training, respectively). However, no change in omission rates was found for the healthy control group over time (T(3) = 3, n.s.; 0.7% and 1.4% for the first and second administration, respectively).
Selective attention - Conjunction search: An ANOVA with time (pre vs. post) and set size (4, 8, 16 and 32) as within subjects factors and group (experimental vs. healthy control) as a between subjects factor was conducted on mean RTs. There were reliable main effects of group (F(1, 14) =21.51 p^lt 0.001, η² = 0.606), time (F(1, 14) = 20.512, p < 0.001, η² = 0.594) and set size (F(4, 56) = 36.174, p<0.001, η² = 0.721). Critically, there was also an interaction between time and group (F(1, 14) = 25.256, p < 0.001, η² = 0.643). For the experimental group RTs were significantly faster following training (T(8) = 0, p = 0.01; 1781 and 1179 ms for pre and post training, respectively). There was, however, no significant change in performance from the first to the second assessment for the healthy control group (T(8) = 8, p >0.1; 936 and 967, respectively). Thus, for the experimental group, there was a general improvement in RTs following training while no significant change was detected for the healthy controls.
A similar ANOVA conducted on the accuracy data revealed a significant main effect of set size ( F(3, 42) = 9.632, p 0.001, η² = 0.408) indicating a fall in accuracy in particular for the search displays including 32 items (0.98, 0.95, 0.96 and 0.87 for the 4, 8, 16 and 32 set size, respectively) across the two groups. No other effects were significant.
Orienting attention: A spatial cued-identification yask (SCIT): An ANOVA with cue validity (valid vs. invalid) and time (pre vs. post) as within subjects factors and group (experimental vs. healthy controls) as a between subjects factor was conducted on the RT data. There were significant main effects of group (F(1, 14) = 20.315, p<0.001, η² = 0.592; 1227 vs. 658 ms for the experimental and healthy control groups, respectively), time (F(1, 14) = 10.678, p =0.006, η² = 0.433; 1007 vs. 878 ms for pre and post training, respectively) and cue validity (F(1, 14) = 28.338, p <0.001, η² = 0.669; 898 and 987 ms for valid and invalid cues, respectively). There was also a reliable interaction between time and group (F(1, 14) = 14.558, p = 0.002, η² = 0.508). For the experimental group RTs improved after training (T(8) =1, p = 0.02; 1367 and 1086 ms for pre and post training, respectively). However, the mere repetition of the task in the healthy control group did not have an effect on the mean RTs (T(8) = 8, p >0.1; 648 and 669 ms for the first and second administration, respectively).
A similar ANOVA on accuracy revealed a significant interaction of group and validity (F(1, 14) = 5.174, p=0.039, η² = 0.27). The experimental and healthy control groups differed in accuracy for the valid trials (t(14) = 3.172, p = 0.007; 0.92 vs. 0.97 for the experimental and healthy control groups, respectively). However, there was no difference in accuracy between the groups for the invalid trials (t(14) = 0.428, p = 0.675; 0.95 vs. 0.94 for the experimental and healthy control groups, respectively). No other significant main effects or interactions were found.
Executive attention -Direction - Location stroop-like task: An ANOVA with congruency (congruent vs. incongruent), subtask (location vs. direction), and time (pre vs. post training) as within subjects factors and group (experimental vs. healthy controls) as a between subjects factor was conducted on RTs. There were reliable main effects of group (F(1, 14) = 15.692, p = 0.001, η² = 0.528; 1246 vs. 663 ms for the experimental and healthy control groups, respectively), task (F(1, 14) = 4.917, p = 0.044, η² = 0.26; 936 vs. 973 ms for the Location and Direction tasks, respectively), congruency (F(1, 14) = 40.916, p < 0.001, η² = 0.745; 925 vs. 984 ms for congruent vs. incongruent displays, respectively) and time (F(1, 14) = 9.992, p = 0.007, η² = 0.416; 1049 vs. 860 ms for pre and post training, respectively). More importantly, there was an interaction between time and group (F(1, 14) = 14.437, p = 0.002, η² = 0.508). For the experimental group there was once again a significant effect of time (T(8) = 0, p = 0.01) where performance improved after training (1454 and 1038 ms before and after training, respectively) while the healthy controls showed no difference in performance as a function of time (T(8) = 17, p > 0.1; 644 and 682 ms for the first and second assessment, respectively). No other significant interactions were returned.
A similar ANOVA on the accuracy data revealed significant main effects of group (F(1, 14) = 14.169, p= 0.002, η² = 0.503; 0.91 and 0.98 for the experimental and healthy controls, respectively), congruency (F(1, 14) = 13.201, p = 0.003, η² = 0.485; 0.96 vs. 0.93 for congruent and incongruent displays, respectively) and time (F(1, 14) = 12.456, p = 0.003, η² = 0.471; 0.92 vs. 0.97 for pre and post training, respectively). Furthermore, a significant interaction of time and group was also revealed (F(1, 14) = 10.401, p = 0.006, η² = 0.426). Similarly to the RT data, for the experimental group, performance improved significantly following training (T(8) = 0, p = 0.01; 0.86 and 0.96 for pre and post training, respectively). No change in performance was evident for the healthy control group (T(8)=3, p > 0.1; 0.98 and 0.98 for the first and second assessment, respectively).
Cognitive impairment - BCoS battery
The BCoS battery was used to evaluate transfer effects from the attention training to other cognitive domains and to compare the experimental group with a patients control group to ascertain that any observable change in the cognitive profile following training can indeed be attributed to the intervention program (and not to the mere repetition of assessments or the passage of time).
Normalised data from the five domains (attention, language, memory, number skills and praxis) of BCoS were analysed using a repeated measure MANOVA with time (pre vs.post) as a within subjects factor and group(experimental vs. patients controls) as a between subjects factor. The mean scores for the five domains were treated as different dependent variables. The data are presented in Figure 4. There was no significant main effect of group (F(5,10)=< 1), but the main effect of time was significant (F(5,10)= 12.535, p < 0.001, η² = 0.862). Critically, however, the analysis indicated there was a difference between the experimental and patient control groups in all domains over time ( F (5, 10) = 8.175, p = 0.003, η² = 0.803). Univariate tests also supported this result (F(1, 14) = 7.391, p = 0.017, η² = 0.346, for Attention; F(1, 14) = 16.281, p = 0.001, η² = 0.538, for Language; F(1, 14) = 8.264, p = 0.012, η² = 0.371, for Memory; F(1, 14) = 18.164, p = 0.001, η² = 0.565, for Number; F(1, 14) = 8.363, p = 0.012, η² = 0.374, for Praxis; all for the interaction of group and time in each univariate test). For the experimental group all domains showed significant improvement following training (T(8) = 0, p = 0.01 for Attention; T(8) = 0, p = 0.01 for Language; T(8) = 1, p = 0.02 for Memory; T(8) = 0, p < 0.01 for Number; T(8) = 0, p = 0.01 for Praxis). In contrast, for the patients control group no significant changes were observed in any domain (T(8) = 16, p > 0.1 for Attention; T(8) = 6.5, p > 0.1 for Language; T(8) = 11, p > 0.1 for Memory; T(8) = 11, p > 0.1 for Number; T(8) = 16, p > 0.1 for Praxis).Importantly, no significant differences across the groups were observed in the initial BCoS scores before training (all t’s(14)< 1).
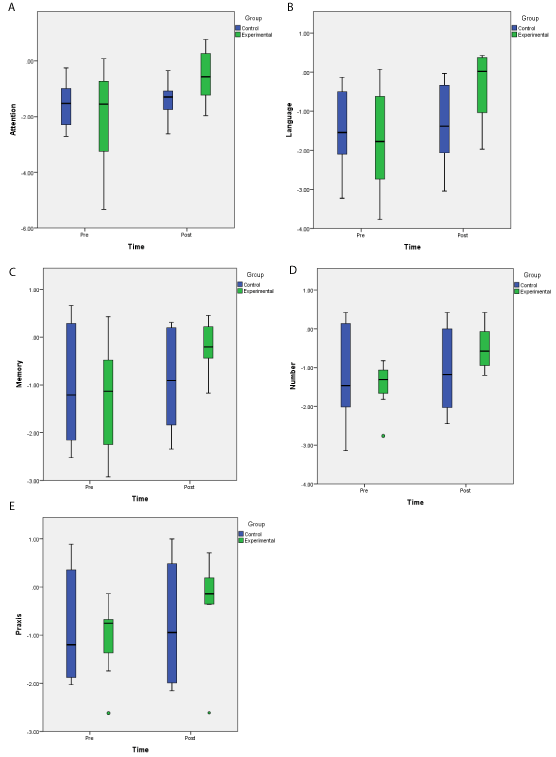
Figure 4: Box plot charts depicting standardized BCoS scores for the experimental and control patients before and after training (or the passage of time for the
controls). a. BCoS scores in the attention and executive function domain as a function of time. b. BCoS scores in the language domain as a function of time. c.
BCoS scores in the Memory domain as a function of time. d. BCoS scores in the Number Skills domain as a function of time. e. BCoS scores in the Praxis domain
as a function of time.
Discussion
We assessed the effects of attention training on cognitive impairment and attention function in stroke patients using the Computerised Progressive Attention Training (CPAT; 21). We report significant improvement in performance following a relatively small number of CPAT sessions (10). As has previously been shown in studies of computer-based ‘brain training’ [13] the patients showed improved performance on tasks that bear similarity to the trained ones. However, the attention tasks the patients performed for the assessment test were not identical to the trained ones so that mere exposure could not explain the improved performance. In fact, while three of the attention tasks had different levels of similarity with the training tasks (CCPT, conjunctive search and the locationdirection Stroop-like tasks) the peripheral cueing task did not have a corresponding training task. Nevertheless, performance improved in all four assessment tasks. The improved performance across the attention tasks was also not merely an effect of test repetition, since there were no significant improvements for the healthy control participants performing the same attention tasks twice.
Performance on the attention tests seems to have generally improved following attention training. That is, following training our patients exhibited in three out of four attention tasks overall faster RTs and in two out of the four attention tasks overall increased accuracy but no changes in specific attention related measures. For the conjunctive search task our patients exhibited an overall reduction of RTs following training. However, selective attention is typically associated with the efficiency of the search (the search slope) rather than with overall RTs in this task, where we did not find significant changes following training. Similarly, for the peripheral cueing task, patients’ overall RTs were significantly reduced following training. However, attention orienting is typically associated with the validity effect in this task (the difference in performance between valid and invalid cue trials) where again we found no change following training. A similar result was observed for the Location-Direction Stroop-like task. Again, our patients exhibited an overall reduction in RTs (as well as increased overall accuracy) in this task following training. However, executive control in this task is typically associated with the congruency effect (the difference between congruent and incongruent displays). Once again, we did not find a change in the magnitude of the congruency effect following training.
One obvious exception to the above pattern was observed in the CCPT that assesses sustained attention. Rather than overall improved RTs following training (which were not observed for this task) significant reductions following training were found for both the SD of RT and the omissions rates. These two measures represent participants’ specific ability to remain focused on task over a long period of time(to sustain their attention on task). Indeed, poor sustained attention is associated with larger standard deviations of responses (when participants cannot remain on task) as well as increased rates of target omissions (when attention is drifting elsewhere and participants miss the target). Thus, the change in performance in the CCPT represents a genuine improvement in the patients’ sustained attention following training.
One possible explanation for this discrepancy between the effects of training over the four different tasks (general vs. attention specific) may be associated with the patients’ attention profile prior to training. At least at the group level it appears that the patients’ sustained attention was considerably impaired at baseline (255ms vs. 68ms SD of RT for the patients and controls, respectively; 0.24 vs. 0.007 omissions rate for the patients and controls, respectively). In contrast, their selective attention (14ms vs. 21ms search slope for the patients and controls, respectively), orienting of attention(79ms vs. 100ms validity effect for the patients and controls, respectively) and executive control (66ms vs. 80ms congruency effect for the patients and controls, respectively) fell within the range of the healthy controls (though, overall performance was of course slower). Thus, it may well be the case that the group of patients in this study was primarily impaired in their sustained attention but less so in their other attention functions. The improvement in sustained attention that was documented in this study corroborates findings of recent studies which investigated memory and attention changes in adult stroke patients using computer assisted cognitive rehabilitation [31,32].
On top of measuring changes in attention, we were particularly interested in changes in the patients’ cognitive functioning following attention training. We, therefore, note the change we observed after training in the BCoS battery. We divided the BCoS into its 5 main domains and assessed if there was general improvement across each domain in the experimental patient group relative to a patient group who underwent other cognitive tests across the training time period. The two groups were matched for their pre-training BCoS performance to negate the possibility that any changes in the experimental group following training can be attributed to a general group difference at baseline. The analysis of the BCoS data revealed a remarkable improvement among the experimental patients in all 5 domains (4 of which were not the subject of specific training in the CPAT procedure: language, memory, number skills and praxis). Thus, following 10 sessions of attention training using the CPAT, the patients in the experimental group showed reduced cognitive impairment across domains. Moreover, the observed gains were not merely statistical; the experimental patient group improved so that they fell within 0.5 SD of the mean for healthy aged matched controls while the control patients were over 1 SD away on average on both pre and post assessments. This suggests that there was a real gain in aspects of cognitive processing which were not directly targeted under the CPAT regime. This is also supported by the improved scores the patients showed in the MoCA and the NIHSS - with the latter in particular pointing to a functional gain from the training.
The question remain as to what were the critical factors underlying these effects? While this is difficult to conclude from the present data as the training program included a few interleaved components we argue that improved sustained attention is the likely cause behind the generalised effects of the training. As discussed above, the participants in the experimental-patient group showed substantial improvement in sustained attention along with general improvements in all tasks.
One way to conceptualise these results is that the training tasks facilitated some domain-general processes which can be applied to a range of different input and output modalities. We would therefore argue that improved sustained attention is the likely domain-general process. Improvements in sustained attention can support the better maintenance of items in memory, the processing of sentences, simple calculations and so forth. Hence there can be generalization into more domain-specific processes in language, memory, number skills and praxis. To support this conjecture we assessed the link between the improved sustained attention (as measured in the change of both SD of RT and omission rates in the CCPT) and the degree of improvement in the 5 BCoS domains. While our sample is too small to assess this meaningfully we looked at the correlation between the changes in the sustained attention measurements and the BCoS before and after training. While not statistically significant, it is still noteworthy that the change in SD of RT following training yielded a relatively strong correlation with the overall change in the BCoS score (r = -0.587). This result should be taken with caution given the small sample size. Nevertheless, it points to the possible link between improved sustained attention (especially as measured by SD of RT) and the generalised effect across the BCoS domains.
The above conceptualisation is consistent with the unique generalisation that was obtained in a previous study where the CPAT was used with children with ADHD [21]. In that study, gains of the CPAT were translated to improved reading comprehension and speed of copying text as well as to a significant reduction of inattentive symptoms. Interestingly, although the present study involved patients who suffered from acquired attention deficits (as a result of stroke) and the Shalev and colleagues’ study included children who suffered from developmental attention deficits, in both studies substantial far transfer/generalisation effects to everyday life were obtained.
While some of the effects we report are striking they should be taken with caution particularly, given the small sample (n = 8). Thus, we concede that the full impact of the training can only be judged by a larger-scale randomised trial with a control group randomised into a procedure with active control (e.g., where therapist time with the patients is matched but the demands on the attentional processing of the patient is reduced). Despite these limitations though the present results hold promise that attention-training of stroke patients can lead to functional benefits in cognition and everyday life through the improvement of domain-general processes such as sustained attention.
Acknowledgements
This work was supported by the INDIREA EU Initial training Network, awarded to the last author.
References
- Zhang Y, Chapman AM, Plested M, Jackson D, Purroy F. The Incidence, Prevalence, and Mortality of Stroke in France, Germany, Italy, Spain, the UK, and the US: A Literature Review. Stroke Res Treat. 2012; 2012: 436125.
- Humphreys GW, Bickerton W, Samson D & Riddoch MJ. BCoS: Cognitive profiling after brain injury. London: Psychology Press. 2012.
- Cumming TB, Marshall RS, Lazar RM. Stroke, cognitive deficits, and rehabilitation: still an incomplete picture. Int J Stroke. 2013; 8: 38-45.
- Heruti RJ, Lusky A, Dankner R, Ring H, Dolgopiat M, Barell V, et al. Rehabilitation outcome of elderly patients after a first stroke: effect of cognitive status at admission on the functional outcome. Arch Phys Med Rehabil. 2002; 83: 742-749.
- Hackett ML, Anderson CS. Predictors of depression after stroke: a systematic review of observational studies. Stroke. 2005; 36: 2296-2301.
- Lincoln NB, Majid MJ, & Weyman N. Cognitive rehabilitation for attention deficits following stroke. Cochrane Database Syst Rev. 2000: 4.
- Nuechterlein KH, Robbins TW, & Einat H. Distinguishing separable domains of cognition in human and animal studies: what separations are optimal for targeting interventions? A summary of recommendations from breakout group 2 at the measurement and treatment research to improve cognition in schizophrenia new approaches conference. Schizophrenia Bulletin. 2005; 31: 870-874.
- Sohlberg MM, Mateer CA. Effectiveness of an attention-training program. J Clin Exp Neuropsychol. 1987; 9: 117-130.
- Ben-Yishay Y, Diller L, & Rattok J. A modular approach to optimizing orientation, psychomotor alertness and purposive behaviour in severe head trauma patients. Rehabilitation Monograph. 1978; 59: 63-67.
- Gray JM, Robertson I, Pentland B, Anderson S. Microcomputer-based attentional retraining after brain damage: a randomised group controlled trial. Neuropsychological Rehabilitation. 1992; 2: 97-115.
- Green CS, Pouget A, Bavelier D. Improved probabilistic inference as a general learning mechanism with action video games. Curr Biol. 2010; 20: 1573-1579.
- Green CS, & Bavelier D. Action video game modifies visual selective attention. Nature. 2003; 423: 534-537.
- Owen AM, Hampshire A, Grahn JA, Stenton R, Dajani S, Burns AS, et al. Putting brain training to the test. Nature. 2010; 465: 775-778.
- Gagnon LG, & Belleville S. Training of attentional control in mild cognitive impairment with executive deficits: Results from a double-blind randomised controlled study. Neuropsychological rehabilitation. 2012; 22: 809-835.
- Gaitán A, Garolera M, Cerulla N, Chico G, Rodriguez-Querol M, & Canela-Soler J. Efficacy of an adjunctive computer-based cognitive training program in amnestic mild cognitive impairment and Alzheimer's disease: a single-blind, randomized clinical trial. International journal of geriatric psychiatry. 2013; 28: 91-99.
- Cerasa A, Gioia MC, Valentino P, Nisticò R, Chiriaco C, Pirritano D, et al. Computer-Assisted Cognitive Rehabilitation of Attention Deficits for Multiple Sclerosis A Randomized Trial With fMRI Correlates. Neurorehabilitation and neural repair. 2013; 27: 284-295.
- Johansson B, Tornmalm M. Working memory training for patients with acquired brain injury: effects in daily life. Scand J Occup Ther. 2012; 19: 176-183.
- Prokopenko SV, Mozheyko EY, Petrova MM, Koryagina TD, Kaskaeva DS, Chernykh TV, et al. Correction of post-stroke cognitive impairments using computer programs. Journal of the neurological sciences. 2013; 325: 148-153.
- Anguera JA, Boccanfuso J, Rintoul JL, Al-Hashimi O, Faraji F, Janowich J, et al. Video game training enhances cognitive control in older adults. Nature. 2013; 501: 97-101.
- Chung CS, Pollock A, Campbell T, Durward BR, Hagen S. Cognitive rehabilitation for executive dysfunction in adults with stroke or other adult non-progressive acquired brain damage. Cochrane Database Syst Rev. 2013; 4: CD008391.
- Shalev L, Tsal Y, Mevorach C. Computerized progressive attentional training (CPAT) program: effective direct intervention for children with ADHD. Child Neuropsychol. 2007; 13: 382-388.
- Fillingham J, Sage K, & Lambon Ralph M. Further explorations and an overview of errorless and errorful therapy for aphasic word-finding difficulties: The number of naming attempts during therapy affects outcome. Aphasiology. 2005; 19: 597-614.
- Robertson IH. Do we need the "lateral" in unilateral neglect? Spatially nonselective attention deficits in unilateral neglect and their implications for rehabilitation. Neuroimage. 2001; 14: S85-S90.
- beck Lh, Bransome Ed Jr, Mirsky Af, Rosvold He, Sarason I . A continuous performance test of brain damage. J Consult Psychol. 1956; 20: 343-350.
- Treisman AM, & Gelade G. A feature-integration theory of attention. Cognitive psychology. 1980; 12: 97-136.
- Davidoff J, Fonteneau E, Fagot J. Local and global processing: observations from a remote culture. Cognition. 2008; 108: 702-709.
- Tsal Y, Shalev L, Mevorach C. The diversity of attention deficits in ADHD: the prevalence of four cognitive factors in ADHD versus controls. J Learn Disabil. 2005; 38: 142-157.
- Mahoney Fi, Barthel Dw . Functional Evaluation: The Barthel Index. Md State Med J. 1965; 14: 61-65.
- Cumming TB, Bernhardt J, Linden T. The montreal cognitive assessment: short cognitive evaluation in a large stroke trial. Stroke. 2011; 42: 2642-2644.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983; 67: 361-370.
- Des Roches CA, Bala chandran I Ascenso, Yorghos EM, Tripodis Y, and Kiran S. Effectiveness of an impairment-based individualized rehabilitation program using an iPad-based software platform. Frontiers in Human Neuroscience. 2015; 8.
- Hwi-Young C, Ki-Tae K, & Jin-Hwa J. Effects of computer assisted cognitive rehabilitation on brain wave, memory and attention of stroke patients: a randomized control trial. Journal of Physiology Therapy Science. 2015; 27: 1029-1032.